Environmental Factors Determining the Distribution Pattern of Chironomidae in Different Types of Freshwater Habitats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
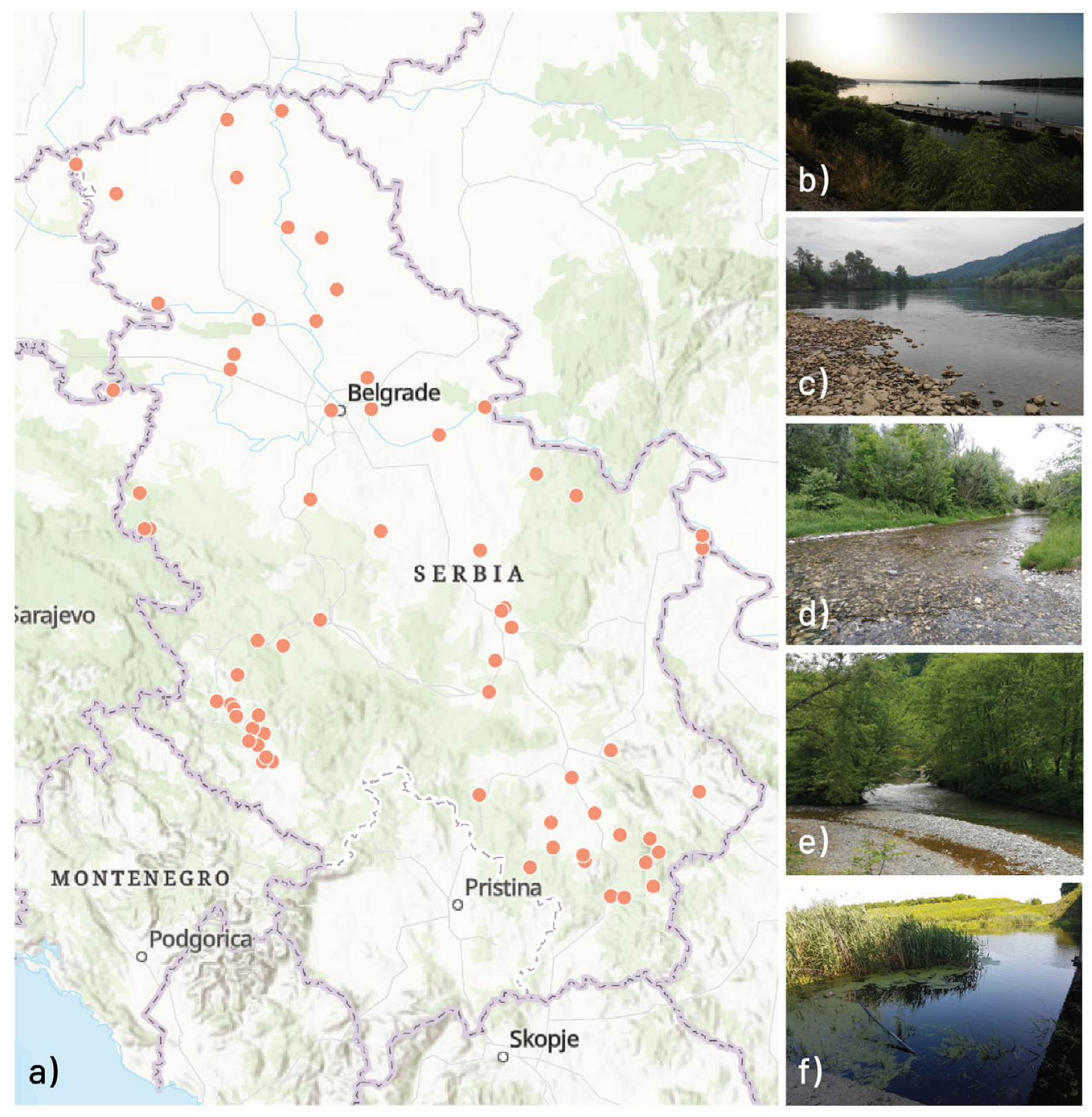
2.1. Study Area
2.2. Field and Laboratory Work
2.3. Data Analyses
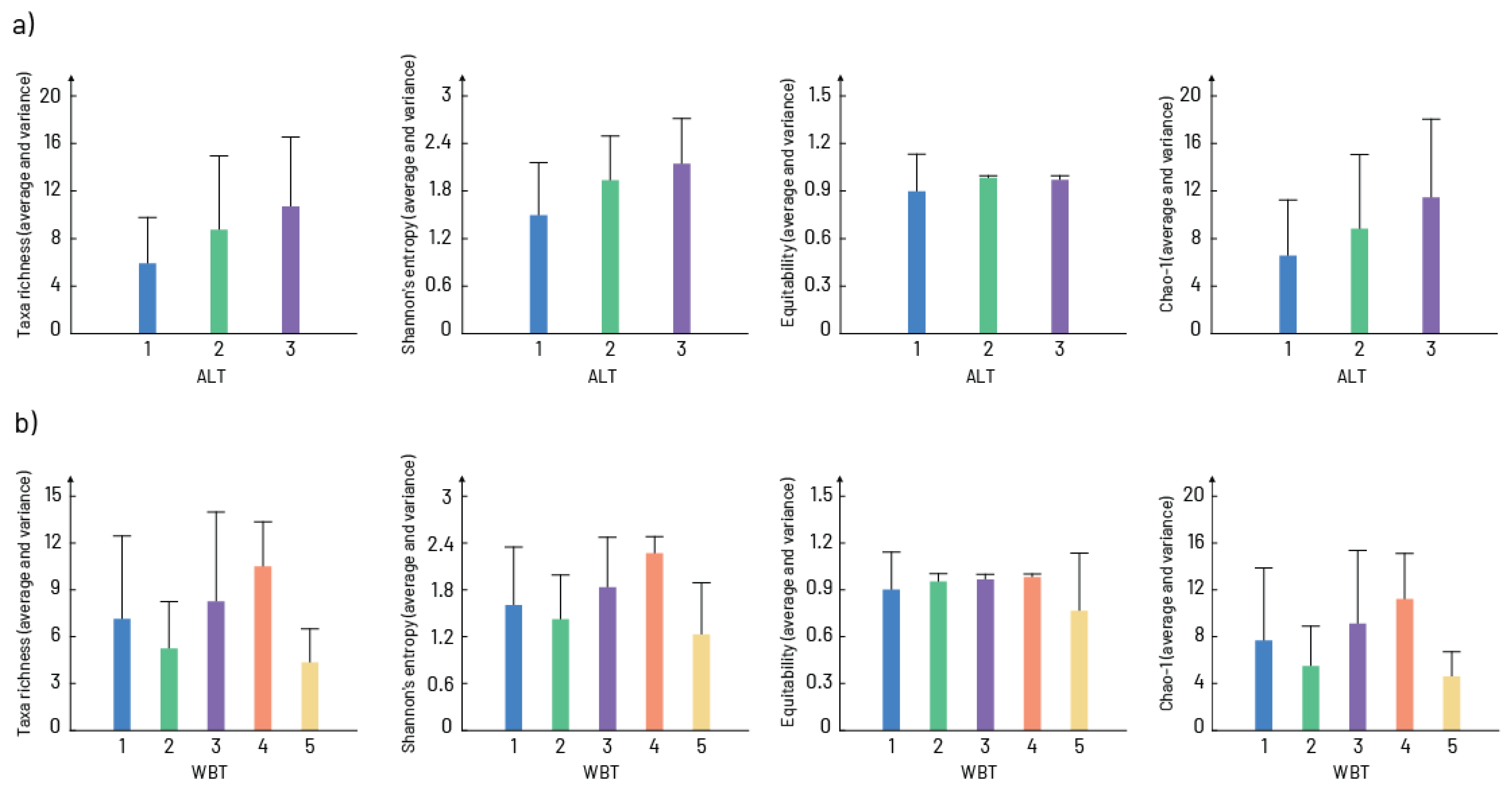
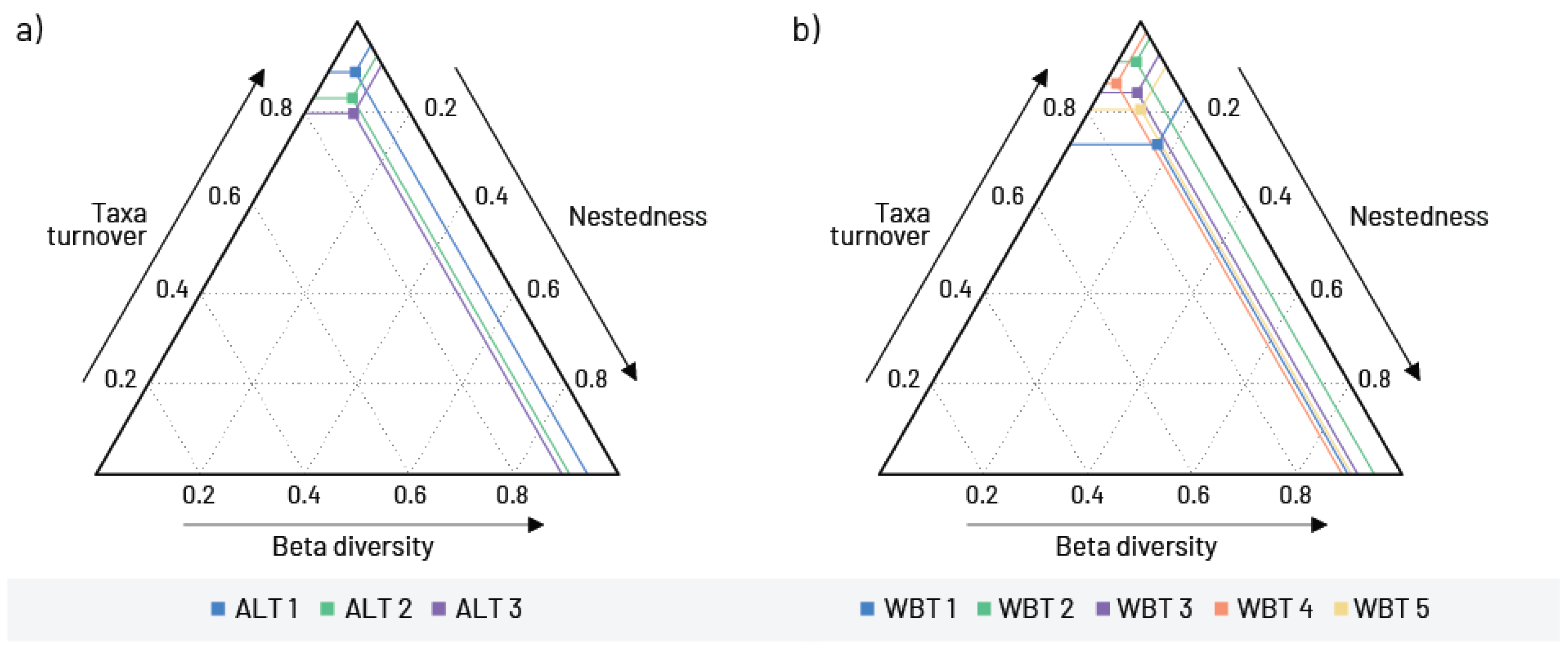
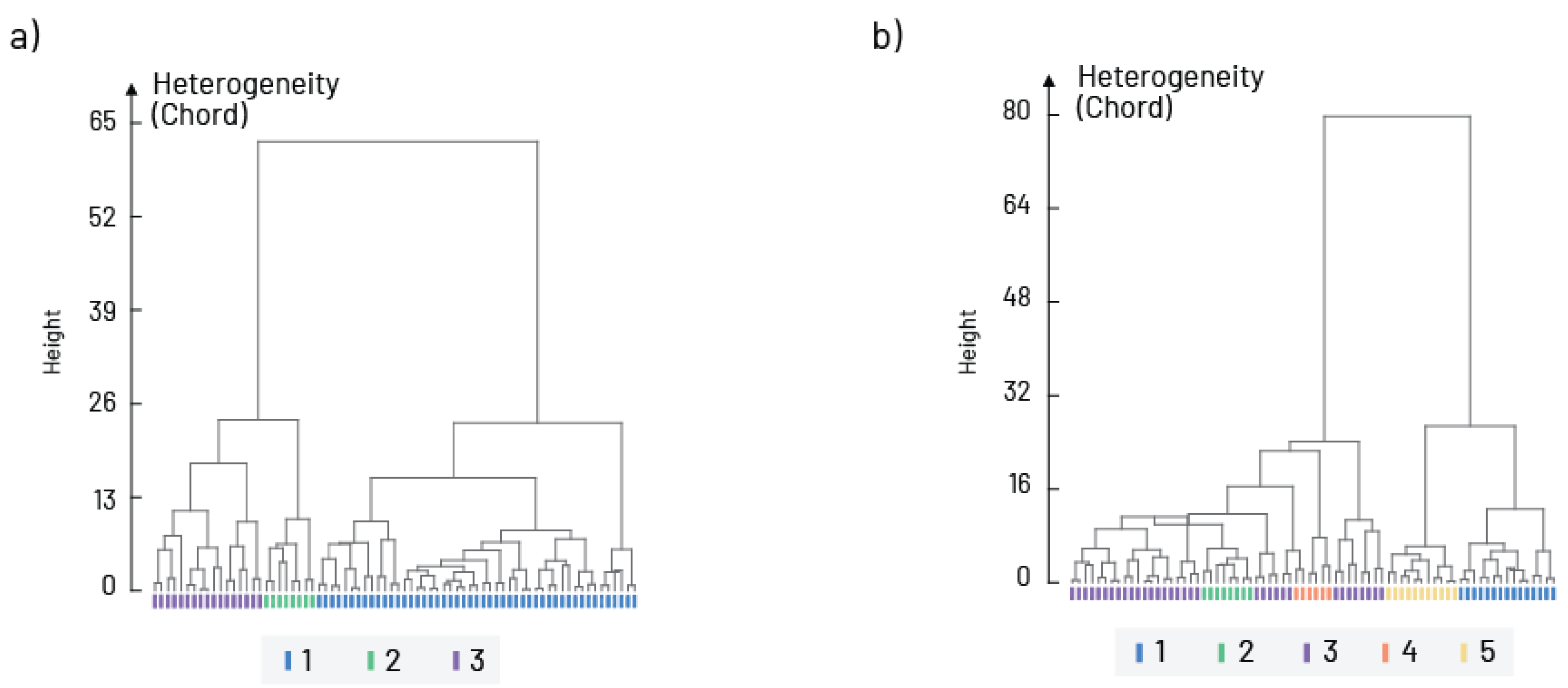
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermoso, V.; Kennard, M.J.; Linke, S. Integrating Multidirectional Connectivity Requirements in Systematic Conservation Planning for Freshwater Systems. Divers. Distrib. 2012, 18, 448–458. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Melo, A.S. Explaining Dissimilarities in Macroinvertebrate Assemblages among Stream Sites Using Environmental Variables. Zoologia 2009, 26, 79–84. [Google Scholar] [CrossRef]
- Leszczyska, J.; Gowacki, L.; Grzybkowska, M. Factors Shaping Species Richness and Biodiversity of Riverine Macroinvertebrate Assemblages at the Local and Regional Scale. Community Ecol. 2017, 18, 227–236. [Google Scholar] [CrossRef]
- Pregun, C.Z. Dynamics of Self-Regulatory Processes in a Lowland River Due to Seasonal Changes in Certain Hydro-Ecological and Water Quality Factors. Ecol. Eng. 2022, 178, 106595. [Google Scholar] [CrossRef]
- Frouz, J.; Matena, J.; Ali, A. Survival Strategies of Chironomids (Diptera: Chironomidae) Living in Temporary Habitats: A Review. Eur. J. Entomol. 2003, 100, 459–466. [Google Scholar] [CrossRef]
- Milošević, D.; Mančev, D.; Čerba, D.; Piperac, M.S.; Popović, N.; Atanacković, A.; Đuknić, J.; Simić, V.; Paunović, M. The Potential of Chironomid Larvae-Based Metrics in the Bioassessment of Non-Wadeable Rivers. Sci. Total Environ. 2018, 616, 472–479. [Google Scholar] [CrossRef]
- Ríos-Touma, B.; Ramírez, A. Multiple Stressors in the Neotropical Region: Environmental Impacts in Biodiversity Hotspots. In Multiple Stressors in River Ecosystems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–220. [Google Scholar]
- Rossaro, B.; Marziali, L.; Montagna, M.; Magoga, G.; Zaupa, S.; Boggero, A. Factors Controlling Morphotaxa Distributions of Diptera Chironomidae in Freshwaters. Water 2022, 14, 1014. [Google Scholar] [CrossRef]
- Domisch, S.; Araújo, M.B.; Bonada, N.; Pauls, S.U.; Jähnig, S.C.; Haase, P. Modelling Distribution in E Uropean Stream Macroinvertebrates under Future Climates. Glob. Change Biol. 2013, 19, 752–762. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Zhang, X.; Wang, J.; Yang, Y.; Sun, Y.; Guo, X.; Wu, Q.; Nepovimova, E.; Watson, A.E. Biodiversity Conservation in the Context of Climate Change: Facing Challenges and Management Strategies. Sci. Total Environ. 2024, 937, 173377. [Google Scholar] [CrossRef]
- Nieto, C.; Ovando, X.M.C.; Loyola, R.; Izquierdo, A.; Romero, F.; Molineri, C.; Rodríguez, J.; Rueda Martín, P.; Fernández, H.; Manzo, V. The Role of Macroinvertebrates for Conservation of Freshwater Systems. Ecol. Evol. 2017, 7, 5502–5513. [Google Scholar] [CrossRef] [PubMed]
- Radović, I.; Mesaroš, G.; Pavićević, D.; Mihajlović Lj Protić Lj, Ć.A. Diverzitet Entomofaune (Insecta) Jugoslavije, Sa Pregledom Vrsta Od Medjunarodnog Značaja. In Biodiverzitet Jugoslavije sa Pregledom Vrsta od Medjunarodnog Značaja; Faculty of Biology and Ecolibri: Belgrade, Serbia, 1995; pp. 371–424. [Google Scholar]
- Stevanović, V.; Vasić, V. Biodiverzitet Jugoslavije: Sa Pregledom Vrsta Od Međunarodnog Značaja; Ecolibri: Belgrade, Serbia, 1995; ISBN 8670780046. [Google Scholar]
- Paunović, M.; Tubić, B.; Kračun-Kolarević, M.; Marković, V.; Simić, V.; Zorić, K.; Atanacković, A. Ecoregions Delineation for the Territory of Serbia. Water Res. Manag. 2012, 2, 65–74. [Google Scholar]
- Milovanović, B. Climate Regionalization of Serbia According to Köppen Climate Classification. Збoрник радoва Геoграфскoг института" Јoван Цвијић" САНУ 2017, 67, 103–114. [Google Scholar] [CrossRef]
- Milovanović, B.; Stanojević, G.; Radovanović, M. Climate of Serbia—The Geography of Serbia: Nature, People, Economy; Manić, E., Nikitović, V., Djurović, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 57–68. ISBN 978-3-030-74701-5. [Google Scholar]
- EN 27828:1994; Water QualityMethods of Biological Sampling—Guidance on Handnet Sampling of Aquatic Benthic Macro-Invertebrates (ISO 7828:1985). European Committee for Standardisation: Brussels, Belgium, 1994.
- Schmid, P.-E. A Key to the Larval Chironomidae and Their Instars from Austrian Danube Region Streams and Rivers with Particular Reference to a Numerical Taxonomic Approach. 1 1; Hrsg.: Bundesamt für Wassergüte, Wien-Kaisermühlen. Schriftenleitung: Werner Kohl. Selbstverlag, 1993. Acta Hydrochim. Hydrobiol. 1994, 22, 191. [Google Scholar]
- Vallenduuk, H.J.; Pillot, H.K.M.M. Chironomidae Larvae, Vol. 1: Tanypodinae: General Ecology and Tanypodinae; Brill: Leiden, The Netherlands, 2014; ISBN 9004278036. [Google Scholar]
- Maschwitz, D.E.; Cook, E.F. Revision of the Nearctic Species of the Genus Polypedilum Kieffer (Diptera: Chironomidae) in the Subgenera P.(Polypedilum) Kieffer and P.(Uresipedilum) Oyewo and Sæether; Ohio Biological Survey, College of Biological Sciences, The Ohio State University: Columbus, OH, USA, 2000; ISBN 0867271302. [Google Scholar]
- Epler, J.H. Identification Guide to the Larvae of the Tribe Tanytarsini (Diptera: Chironomidae) in Florida; Florida Department of Environmental Protection: Tallahassee, FL, USA, 2014.
- Moller Pillot, H.K.M. Chironomidae Larvae, Vol. 2: Chironomini: Biology and Ecology of the Chironomini; BRILL: Leiden, The Netherlands, 2009; ISBN 9004278044. [Google Scholar]
- Orendt, C.; Spies, M. Chironomini:(Diptera: Chironomodae: Chironominae). In Keys to Central European Larvae Using Mainly Macroscopuc Characters; Orendt Hydrobiologie: Leipzig, Germany, 2012; ISBN 3000388427. [Google Scholar]
- Andersen, T.; Sæther, O.A.; Cranston, P.S.; Epler, J.H. The Larvae of Orthocladiinae (Diptera: Chironomidae) of the Holarctic Region-Keys and Diagnoses. Insect Syst. Evol. 2013, 66, 189–385. [Google Scholar]
- Bitušík, P.; Hamerlík, L. Príručka Na Určovanie Lariev Pakomárov (Diptera: Chironomidae) Slovenska. Čast’2. In Tanypodinae. Belianum. Vydav; Univerzity Matej Bela v Banskej Bystrici, Banska Bystrica: Banská Bystrica, Slovakia, 2014. [Google Scholar]
- Vallenduuk, H.J. Chironomini Larvae of Western European Lowlands (Diptera: Chironomidae): Keys with Notes to the Species: With a Redescription of Glyptotendipes (Caulochironomus) Nagorskayae and a First Description of Glyptotendipes (Caulochironomus) Kaluginae New Species; Erik Mauch Verlag: Dinkelscherben, Germany, 2017. [Google Scholar]
- Dodds, W.K.; Bruckerhoff, L.; Batzer, D.; Schechner, A.; Pennock, C.; Renner, E.; Tromboni, F.; Bigham, K.; Grieger, S. The Freshwater Biome Gradient Framework: Predicting Macroscale Properties Based on Latitude, Altitude, and Precipitation. Ecosphere 2019, 10, e02786. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the Turnover and Nestedness Components of Beta Diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Karadžić, B. FLORA: A Software Package for Statistical Analysis of Ecological Data. Water Res. Manag. 2013, 3, 45–54. [Google Scholar]
- Ward Jr, J.H.; Hook, M.E. Application of an Hierarchical Grouping Procedure to a Problem of Grouping Profiles. Educ. Psychol. Meas. 1963, 23, 69–81. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities. An approach to Stat. Anal. Interpret. 2001, 2, 1–168. [Google Scholar]
- Stoichev, S. On the Chironomid Fauna from Bulgarian Inland Waters. Lauterbornia 1996, 25, 117–123. [Google Scholar]
- Spies, M.; Sæther, O.A. Chironomidae. Fauna Europaea. In Fauna Europaea: Diptera, Nematocera. Fauna Europaea. Version 2.6; Beuk, P., Pape, T., Eds.; Leibniz-Institut für Evolutions- und Biodiversitätsforschung: Berlin, Germany, 2013; Available online: http://www.faunaeur.org (accessed on 15 January 2025).
- Čerba, D.; Koh, M.; Ergović, V.; Mihaljević, Z.; Milošević, D.; Hamerlík, L. Chironomidae (Diptera) of Croatia with Notes on the Diversity and Distribution in Various Habitat Types. Zootaxa 2020, 4780, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Tatole, V. An Updated Checklist of Chironomid Species (Diptera, Chironomidae) from Romania. Trav. du Muséum Natl. d’Histoire Nat. “Grigore Antipa” 2023, 66, 17–105. [Google Scholar] [CrossRef]
- Moller Pillot, H.K.M. Chironomidae Larvae, Vol. 3: Orthocladiinae: Biology and Ecology of the Aquatic Orthocladiinae; KNNV Publishing: Zeist, The Netherlands, 2013; Volume 3, ISBN 9004278052. [Google Scholar]
- Bazzanti, M. Ecological Requirements of Chironomids (Diptera: Chironomidae) on the Soft Bottom of the River Arrone, Central Italy. J. Freshw. Ecol. 2000, 15, 397–409. [Google Scholar] [CrossRef]
- Armitage, P.D.; Patrick, D.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae: Biology and Ecology of Non-Biting Midges; Springer: Berlin/Heidelberg, Germany, 1995; Volume 572. [Google Scholar]
- Rossaro, B.; Marziali, L.; Magoga, G.; Montagna, M. Corrections and Additions to Descriptions of Some Species of the Subgenus Corrections and Additions to Descriptions of Some Species of the Subgenus Orthocladius s. Str. (Diptera, Chironomidae, Orthocladiinae). Insects 2022, 13, 51. [Google Scholar] [CrossRef]
- Villamarín, C.; Villamarín-Cortez, S.; Salcido, D.-M.; Herrera-Madrid, M.; Ríos-Touma, B. Drivers of Diversity and Altitudinal Distribution of Chironomids (Diptera: Chironomidae) in the Ecuadorian Andes. Rev. Biol. Trop. 2021, 69, 113–126. [Google Scholar] [CrossRef]
- Syrovátka, V.; Schenková, J.; Brabec, K. The Distribution of Chironomid Larvae and Oligochaetes within a Stony-Bottomed River Stretch: The Role of Substrate and Hydraulic Characteristics. Fundam. Appl. Limnol. 2009, 174, 43–62. [Google Scholar] [CrossRef]
- Lencioni, V.; Marziali, L.; Rossaro, B. Diversity and Distribution of Chironomids (Diptera, Chironomidae) in Pristine Alpine and Pre-Alpine Springs (Northern Italy). J. Limnol. 2011, 70, 106–121. [Google Scholar] [CrossRef]
- Přidalová, M.S.; Hamerlík, L.; Novikmec, M.; Slobodníková, V.; Veselská, M.; Bitušík, P.; Svitok, M. Diversity and Distribution of Chironomids in Central European Ponds. Ecol. Evol. 2024, 14, e11354. [Google Scholar] [CrossRef]
- Spies, M.; Sæther, O.A. Notes and Recommendations on Taxonomy and Nomenclature of Chironomidae (Diptera). Zootaxa 2004, 752, 1–90. [Google Scholar] [CrossRef]
- Hamerlík, L.; Bitušík, P. The Distribution of Littoral Chironomids along an Altitudinal Gradient in High Tatra Mountain Lakes: Could They Be Used as Indicators of Climate Change? Ann. Limnol. Int. J. Lim. 2009, 45, 145–156. [Google Scholar] [CrossRef]
- Moller Pillot, H.K.M. 2 General Aspects of the Systematics, Biology and Ecology of the Aquatic Orthocladiinae. In Chironomidae Larvae, Vol. 3: Orthocladiinae; KNNV Publishing: Zeist, The Netherlands, 2013; pp. 7–21. ISBN 9004278052. [Google Scholar]
- Płociennik, M.; Karaouzas, I. The Chironomidae (Diptera) Fauna of Greece: Ecological Distributions and Patterns, Taxalist and New Records. Ann. Limnol. Int. J. Lim. 2014, 50, 19–34. [Google Scholar] [CrossRef]
- Puntí, T.; Rieradevall, M.; Prat, N. Environmental Factors, Spatial Variation, and Specific Requirements of Chironomidae in Mediterranean Reference Streams. J. North Am. Benthol. Soc. 2009, 28, 247–265. [Google Scholar] [CrossRef]
- Schöll, F.; Haybach, A. Typology of Large European Rivers According to Their Chironomidae Communities (Insecta: Diptera). Ann. Limnol. Int. J. Lim. 2004, 40, 309–316. [Google Scholar] [CrossRef]
- Haase, P.; Hering, D.; Jähnig, S.C.; Lorenz, A.W.; Sundermann, A. The Impact of Hydromorphological Restoration on River Ecological Status: A Comparison of Fish, Benthic Invertebrates, and Macrophytes. Hydrobiologia 2013, 704, 475–488. [Google Scholar] [CrossRef]
- Popović, N.; Marinković, N.; Čerba, D.; Raković, M.; Đuknić, J.; Paunović, M. Diversity Patterns and Assemblage Structure of Non-Biting Midges (Diptera: Chironomidae) in Urban Waterbodies. Diversity 2022, 14, 187. [Google Scholar] [CrossRef]
- Marziali, L.; Rossaro, B. Response of Chironomid Species (Diptera, Chironomidae) to Water Temperature: Effects on Species Distribution in Specific Habitat. J. Entomol. Acarol. Res. 2013, 45, 73–89. [Google Scholar] [CrossRef]
- Fesl, C. Niche-oriented Species–Abundance Models: Different Approaches of Their Application to Larval Chironomid (Diptera) Assemblages in a Large River. J. Anim. Ecol. 2002, 71, 1085–1094. [Google Scholar] [CrossRef]
- Grzybkowska, M.; Witczak, J. Distribution and Production of Chironomidae (Diptera) in the Lower Course of the Grabia River (Central Poland). Freshw. Biol. 1990, 24, 519–531. [Google Scholar] [CrossRef]
- Chaib, N.; Bouhala, Z.; Fouzari, L.; Marziali, L.; Samraoui, B.; Rossaro, B. Environmental Factors Affecting the Distribution of Chironomid Larvae of the Seybouse Wadi, Northeastern Algeria. J. Limnol. 2013, 72, 203–214. [Google Scholar] [CrossRef]
- Leszczyńska, J.; Głowacki; Grzybkowska, M.; Przybylski, M. Chironomid Riverine Assemblages at the Regional Temperate Scale–Compositional Distance and Species Diversity. Eur. Zool. J. 2021, 88, 731–748. [Google Scholar] [CrossRef]
- Karaouzas, I.; Płóciennik, M. Spatial Scale Effects on Chironomidae Diversity and Distribution in a Mediterranean River Basin. Hydrobiologia 2016, 767, 81–93. [Google Scholar] [CrossRef]
- Rossaro, B.; Marziali, L.; Magoga, G.; Montagna, M.; Boggero, A. Corrections and Additions to Descriptions of Some Species of the Subgenus Orthocladius s. Str.(Diptera, Chironomidae, Orthocladiinae). Insects 2022, 13, 51. [Google Scholar] [CrossRef]
- Epele, L.B.; Miserendino, M.L.; Brand, C. Does Nature and Persistence of Substrate at a Mesohabitat Scale Matter for Chironomidae Assemblages? A Study of Two Perennial Mountain Streams in Patagonia, Argentina. J. Insect Sci. 2012, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Malmqvist, B. Aquatic Invertebrates in Riverine Landscapes. Freshw. Biol. 2002, 47, 679–694. [Google Scholar] [CrossRef]
- Costa, S.S.; Melo, A.S. Beta Diversity in Stream Macroinvertebrate Assemblages: Among-Site and among-Microhabitat Components. Hydrobiologia 2008, 598, 131–138. [Google Scholar] [CrossRef]
- Lindegaard, C. Chironomidae (Diptera) of European Cold Springs and Factors Influencing Their Distribution. J. Kansas Entomol. Soc. 1995, 68, 108–131. [Google Scholar]
- Rossaro, B.; Marziali, L.; Boggero, A. Response of Chironomids to Key Environmental Factors: Perspective for Biomonitoring. Insects 2022, 13, 911. [Google Scholar] [CrossRef]
- Lencioni, V.; Rossaro, B. Microdistribution of Chironomids (Diptera: Chironomidae) in Alpine Streams: An Autoecological Perspective. Hydrobiologia 2005, 533, 61–76. [Google Scholar] [CrossRef]
- Rossaro, B.; Lencioni, V.; Boggero, A.; Marziali, L. Chironomids from Southern Alpine Running Waters: Ecology, Biogeography*. Hydrobiologia 2006, 562, 231–246. [Google Scholar] [CrossRef]
- Acosta, R.; Prat, N. Chironomid Assemblages in High Altitude Streams of the Andean Region of Peru. Fundam. Appl. Limnol. 2010, 177, 57. [Google Scholar] [CrossRef]
- Tejerina, E.G.; Malizia, A. Chironomidae (Diptera) Larvae Assemblages Differ along an Altitudinal Gradient and Temporal Periods in a Subtropical Montane Stream in Northwest Argentina. Hydrobiologia 2012, 686, 41–54. [Google Scholar] [CrossRef]
- Ferrington, L.C. Global Diversity of Non-Biting Midges (Chironomidae; Insecta-Diptera) in Freshwater. Freshw. Anim. Divers. Assess. 2008, 595, 447–455. [Google Scholar] [CrossRef]
- Marziali, L.; Armanini, D.G.; Cazzola, M.; Erba, S.; Toppi, E.; Buffagni, A.; Rossaro, B. Responses of Chironomid Larvae (Insecta, Diptera) to Ecological Quality in Mediterranean River Mesohabitats (South Italy). River Res. Appl. 2010, 26, 1036–1051. [Google Scholar] [CrossRef]
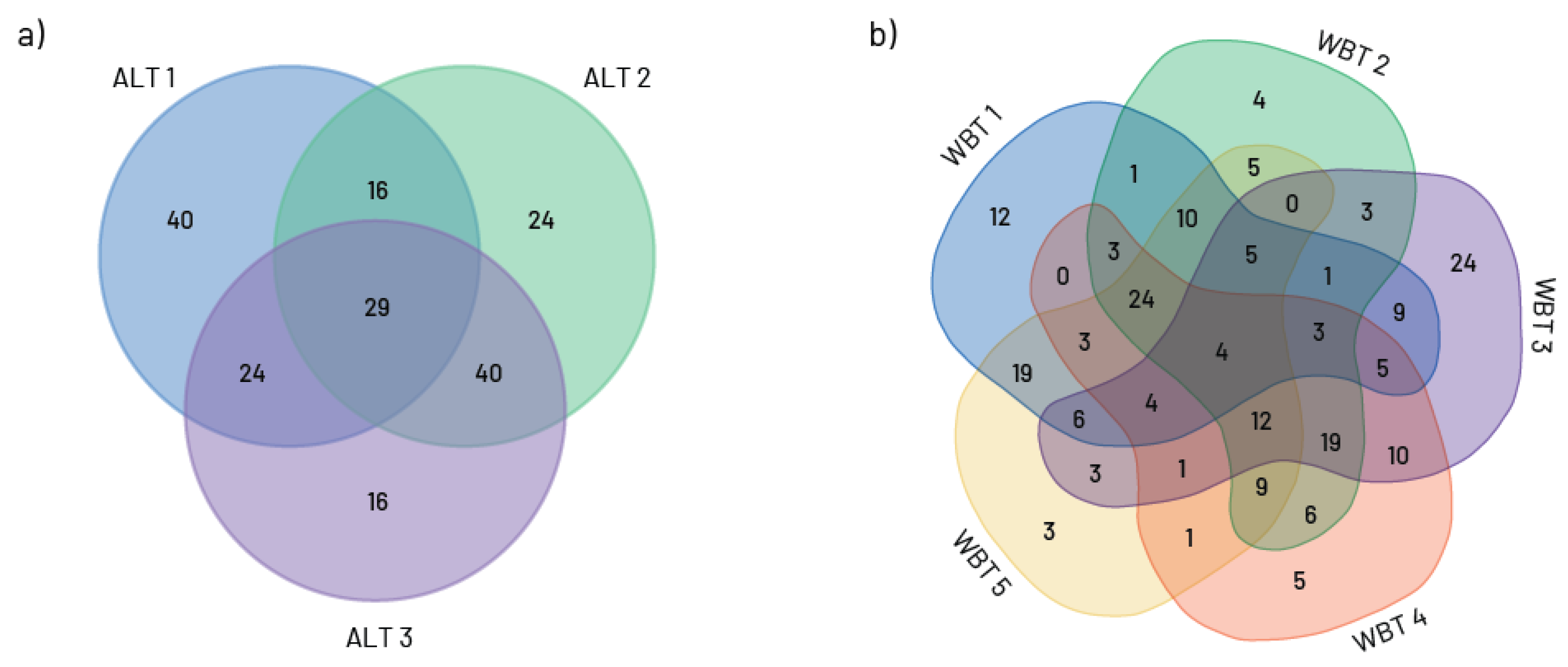

| ALT 1 | A | B | Stat | p Value |
|---|---|---|---|---|
| Macropelopia nebulosa | 1.0000 | 0.2500 | 0.500 | 0.011 * |
| Odontomesa fulva | 0.9199 | 0.2500 | 0.480 | 0.047 * |
| Rheocricotopus effusus | 0.8947 | 0.2500 | 0.473 | 0.023 * |
| Paratanytarsus dissimilis | 0.4762 | 0.3750 | 0.423 | 0.033 * |
| ALT 3 | ||||
| Tanytarsus spp. | 0.8710 | 0.7647 | 0.816 | 0.004 ** |
| Conchapelopia agg. | 0.8775 | 0.5294 | 0.682 | 0.010 ** |
| Tvetenia calvescens agg. | 0.9813 | 0.4706 | 0.680 | 0.003 ** |
| Microtendipes pedellus agg. | 0.9563 | 0.3529 | 0.581 | 0.015 * |
| Corynoneura lobata | 0.9890 | 0.2941 | 0.539 | 0.010 ** |
| Cladotanytarsus sp. | 0.7862 | 0.3529 | 0.527 | 0.044 * |
| Apsectrotanypus trifascipennis | 1.0000 | 0.1765 | 0.420 | 0.038 * |
| Dicrotendipes notatus | 1.0000 | 0.1765 | 0.420 | 0.029 * |
| Xenopelopia sp. | 1.0000 | 0.1765 | 0.420 | 0.040 * |
| ALT 2 and 3 | ||||
| Orthocladius sp. | 0.9785 | 0.4800 | 0.685 | 0.015 * |
| Prodiamesa olivacea | 0.9746 | 0.4400 | 0.655 | 0.008 ** |
| Paratrissocladius excerptus | 0.9992 | 0.2400 | 0.490 | 0.028 * |
| Paracladius conversus | 0.9944 | 0.2400 | 0.489 | 0.028 * |
| WBT 1 | A | B | Stat | p Value |
|---|---|---|---|---|
| Polypedilum gr. scalaenum | 0.9536 | 0.5333 | 0.713 | 0.003 ** |
| Microchironomus tener | 1.0000 | 0.3333 | 0.577 | 0.010 ** |
| Dicrotendipes nervosus | 0.7904 | 0.4000 | 0.562 | 0.020 * |
| Chironomus acutiventris | 1.0000 | 0.2000 | 0.447 | 0.043 * |
| Parachironomus gr. gracilior | 1.0000 | 0.2000 | 0.447 | 0.050 * |
| Paralauterborniella nigrohalteralis | 1.0000 | 0.2000 | 0.447 | 0.046 * |
| WBT 4 | ||||
| Tvetenia calvescens agg. | 0.8754 | 0.5000 | 0.662 | 0.010 ** |
| Rheotanytarsus sp. | 0.9977 | 0.3333 | 0.577 | 0.010 ** |
| Thienemanniella sp. | 0.9905 | 0.3333 | 0.575 | 0.020 * |
| Micropsectra sp. | 0.9784 | 0.3333 | 0.571 | 0.006 ** |
| Micropsectra bidentata | 0.9749 | 0.3333 | 0.570 | 0.014 * |
| Odontomesa fulva | 0.9699 | 0.3333 | 0.569 | 0.010 ** |
| Rheocricotopus effusus | 0.9577 | 0.3333 | 0.565 | 0.011 * |
| Rheocricotopus chalybeatus | 0.8641 | 0.3333 | 0.537 | 0.011 * |
| WBT 5 | ||||
| Parachironomus gr. gracilior | 0.9907 | 0.4545 | 0.671 | 0.004 ** |
| Ablabesmyia longistyla | 0.7314 | 0.3636 | 0.516 | 0.033 * |
| Polypedilum nubifer | 1.0000 | 0.1818 | 0.426 | 0.037 * |
| Source | df | SS | MS | Pseudo-F | p Value | Unique Perms |
|---|---|---|---|---|---|---|
| WBT | 4 | 27,437 | 6859.3 | 1.7234 | 0.001 | 994 |
| ALT | 2 | 10,725 | 5362.5 | 1.3474 | 0.033 | 998 |
| WBT × ALT | 4 | 19,840 | 4960.1 | 1.2462 | 0.013 | 997 |
| WBT3 | WBT 2 | WBT 4 | WBT 5 | WBT 1 | |
|---|---|---|---|---|---|
| WBT 3 | 0.0460 * | 0.0055 * | 0.0767 | 0.0002 * | |
| WBT 2 | 0.0460 * | 0.0006 * | 0.6706 | 0.0014 * | |
| WBT 4 | 0.0055 * | 0.0006 * | 0.0150 * | 0.0001 * | |
| WBT 5 | 0.0767 * | 0.6706 | 0.0150 * | 0.0021 * | |
| WBT 1 | 0.0002 * | 0.0014 * | 0.0001 * | 0.0021 * |
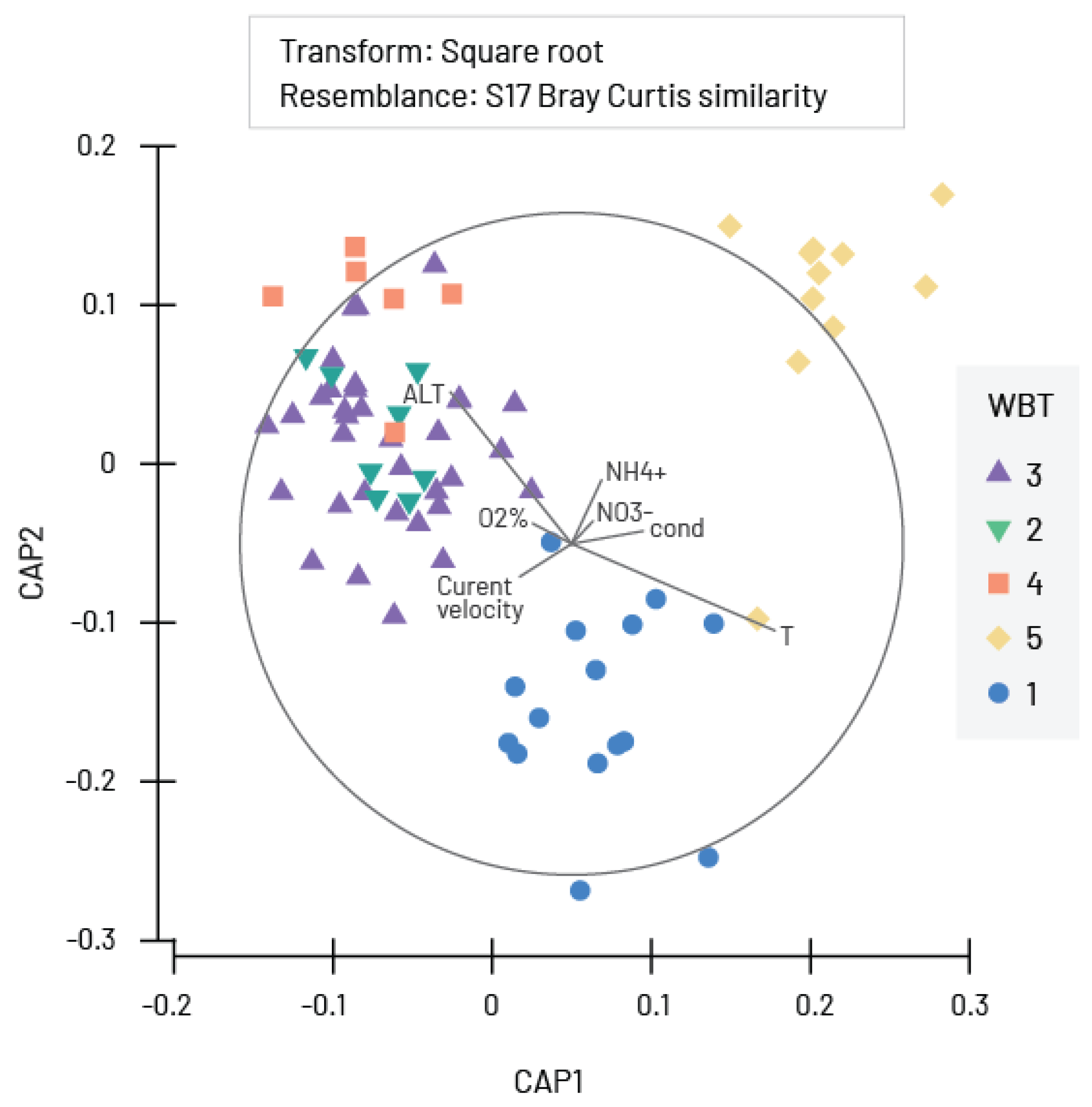
| Model | p Value | Rho | Best Combination of Environmental Parameters |
|---|---|---|---|
| 1 | 0.01 | 0.224 | O2%, conductivity, NH4+, NO3− |
| 2 | 0.01 | 0.222 | O2%, conductivity, pH, NH4+, NO3− |
| 3 | 0.01 | 0.216 | T, O2%, conductivity, NH4+, NO3− |
| 4 | 0.01 | 0.215 | O2%, conductivity, pH, NO3− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popović, N.; Đuknić, J.; Marinković, N.; Tubić, B.; Atanacković, A.; Milošević, D.; Raković, M. Environmental Factors Determining the Distribution Pattern of Chironomidae in Different Types of Freshwater Habitats. Insects 2025, 16, 501. https://doi.org/10.3390/insects16050501
Popović N, Đuknić J, Marinković N, Tubić B, Atanacković A, Milošević D, Raković M. Environmental Factors Determining the Distribution Pattern of Chironomidae in Different Types of Freshwater Habitats. Insects. 2025; 16(5):501. https://doi.org/10.3390/insects16050501
Chicago/Turabian StylePopović, Nataša, Jelena Đuknić, Nikola Marinković, Bojana Tubić, Ana Atanacković, Djuradj Milošević, and Maja Raković. 2025. "Environmental Factors Determining the Distribution Pattern of Chironomidae in Different Types of Freshwater Habitats" Insects 16, no. 5: 501. https://doi.org/10.3390/insects16050501
APA StylePopović, N., Đuknić, J., Marinković, N., Tubić, B., Atanacković, A., Milošević, D., & Raković, M. (2025). Environmental Factors Determining the Distribution Pattern of Chironomidae in Different Types of Freshwater Habitats. Insects, 16(5), 501. https://doi.org/10.3390/insects16050501








