Species Richness and Distribution of Calliphoridae Along an Elevation Gradient in Sicily (Italy) and Ecuador
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Procedure
2.2. Study Areas
3. Data Analysis
4. Results
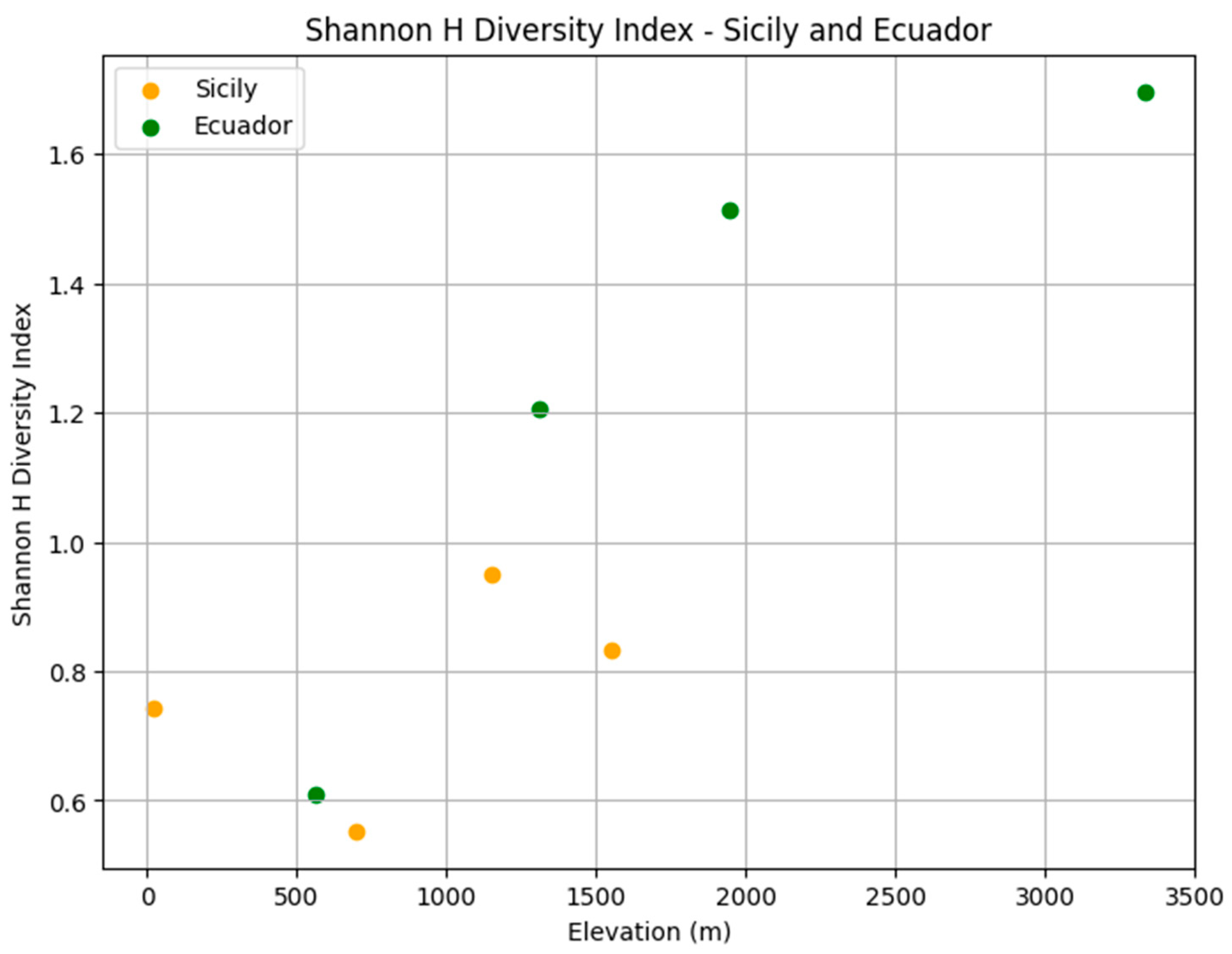
4.1. SICILY
4.2. Ecuador
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grassberger, M.; Frank, C. Initial study of arthropod succession on pig carrion in a central European urban habitat. J. Med. Entomol. 2004, 41, 511–523. [Google Scholar] [CrossRef]
- De Jong, G.D. An annotated checklist of the Calliphoridae (Diptera) of Colorado, with notes on carrion associations and forensic importance. J. Kans. Entomol. Soc. 1994, 67, 378–385. [Google Scholar]
- Gruner, S.V.; Slone, D.H.; Capinera, J.L. Forensically important Calliphoridae (Diptera) associated with pig carrion in rural north-central Florida. J. Med. Entomol. 2007, 44, 509–515. [Google Scholar] [CrossRef]
- De Jong, G.D. Insect Succession on Dog Carcasses in Horsetooth Mountain Park; Unpublished Report to Larimer County (Colorado) Parks Department: Loveland, CO, USA, 1993; Volume 60. [Google Scholar]
- Vogt, W.G.; Morton, R. Estimation of population size and survival of sheep blowfly, Lucilia cuprina, in the field from serial recoveries of marked flies affected by weather dispersal and age-dependent trappability. Popul. Ecol. 1991, 33, 141–163. [Google Scholar] [CrossRef]
- Spradbery, J.P.; Mahon, R.J.; Morton, R.; Tozer, R.S. Dispersal of the Old World screw-worm fly Chrysomya bezziana. Med. Vet. Entomol. 1995, 9, 161–168. [Google Scholar] [CrossRef]
- Williams, K.L.; Barton Browne, L.; van Gerwen, C.M. Quantitative relationships between the ingestion of protein-rich material and ovarian development in the Australian sheep blowfly, Lucilia cuprina (Wied). Int. J. Invertebr. Reprod. 1979, 1, 75–88. [Google Scholar] [CrossRef]
- Norris, K.R. The bionomics of blowflies. Annu. Rev. Entomol. 1965, 10, 47–68. [Google Scholar] [CrossRef]
- Byrd, J.H.; Tomberlin, J.K. (Eds.) Insects of Forensic Importance. In Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2020; pp. 15–62. [Google Scholar]
- Braack, L.E.O. Visitation patterns of principal species of the insect complex at carcasses in the Kruger National Park. Koedoe 1981, 24, 33–49. [Google Scholar] [CrossRef][Green Version]
- Velásquez, Y. A checklist of arthropods associated with rat carrion in a montane locality of northern Venezuela. Forensic Sci. Int. 2008, 174, 68–70. [Google Scholar] [CrossRef]
- Greenberg, B. Flies and Disease Volume I. Ecology, Classification and Biotic Associations; Princeton University Press: Princeton, NJ, USA, 1971. [Google Scholar]
- Greenberg, B. Flies and Disease Volume II. Biology and Disease Transmission; Princeton University Press: Princeton, NJ, USA, 1973. [Google Scholar]
- Wall, R.; French, N.; Morgan, K. Blowfly species composition in sheep myiasis in Britain. Med. Vet. Entomol. 1992, 6, 177–178. [Google Scholar] [CrossRef]
- Howlett, B.G. Hybrid carrot seed crop pollination by the fly Calliphora vicina (Diptera: Calliphoridae). J. Appl. Entomol. 2012, 136, 421–430. [Google Scholar] [CrossRef]
- Faulkner, G.J. Seed production of F1 hybrid Brussels sprouts. Proc. Symp. Seed Probl. Hortic. 1977, 93, 37–42. [Google Scholar] [CrossRef]
- Pérez-Bañón, C.; Petanidou, T.; Marcos-Garcia, A. Pollination in small islands by occasional visitors: The case of Daucus carota subsp. commutatus (Apiaceae) in the Columbretes archipelago, Spain. Plant Ecol. 2007, 192, 133–151. [Google Scholar] [CrossRef]
- Clement, S.L.; Hellier, B.C.; Elberson, L.R.; Staska, R.T.; Evans, M.A. Flies (Diptera: Muscidae: Calliphoridae) are efficient pollinators of Allium ampeloprasum L. (Alliaceae) in field cages. J. Econ. Entomol. 2007, 100, 131–135. [Google Scholar] [CrossRef]
- Carter, D.O.; Yellowlees, D.; Tibbett, M. Cadaver decomposition in terrestrial ecosystems. Die Naturwiss. 2007, 94, 12–24. [Google Scholar] [CrossRef]
- Johansen, H.; Solum, M.; Knudsen, G.K.; Hagvar, E.B.; Norli, H.R.; Aak, A. Blow fly responses to semiochemicals produced by decaying carcasses. Med. Vet. Entomol. 2014, 28, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Die Naturwiss. 2004, 91, 51–65. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; VanLaerhoven, S.L. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef]
- Kneidel, K.A. Competition and disturbance in communities of carrion-breeding Diptera. J. Anim. Ecol. 1984, 53, 849–865. [Google Scholar] [CrossRef]
- Ives, A.R. Aggregation and coexistence in a carrion fly community. Ecol. Monogr. 1991, 61, 75–94. [Google Scholar] [CrossRef]
- Weidner, L.M.; Jennings, D.E.; Tomberlin, J.K.; Hamilton, G.C. Seasonal and geographic variation in biodiversity of forensically important blow flies (Diptera: Calliphoridae) in New Jersey, USA. J. Med. Entomol. 2015, 52, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Kuusela, S.; Hanski, I. The structure of carrion fly communities: The size and the type of carrion. Holartic Ecol. 1982, 5, 337–348. [Google Scholar] [CrossRef]
- Kuusela, S. Community structure of carrion flies along an island gradient. Holarc. Ecol. 1983, 6, 372–380. [Google Scholar] [CrossRef]
- Hanski, I. Carrion fly community dynamics: Patchiness, seasonality and coexistence. Ecol. Entomol. 1987, 12, 257–266. [Google Scholar] [CrossRef]
- Davies, L. Species composition and larval habitats of blowfly (Calliphoridae) populations in upland areas in England and Wales. Med. Vet. Entomol. 1990, 4, 61–68. [Google Scholar] [CrossRef]
- Feddern, N.; Amendt, J.; Schyma, C.; Jackowski, C.; Tschui, J. A preliminary study about the spatiotemporal distribution of forensically important blow flies (Diptera: Calliphoridae) in the area of Bern, Switzerland. Forensic Sci. Int. 2018, 289, 57–66. [Google Scholar] [CrossRef]
- Buncho, N.; Sukontason, K.; Sanit, S.; Chidburee, P.; Kurahashi, H.; Sukontason, K.L. Occurrence of blow fly species (Diptera: Calliphoridae) in Phitsanulok Province, Northern Thailand. Trop. Biomed. 2012, 29, 532–543. [Google Scholar]
- Tumrasvin, W.; Sucharit, S.; Kano, R. Studies on medically important flies in Thailand. IV. Altitudinal distribution on flies belonging to Muscidae and Calliphoridae in Doi Indhanondh Mountain, Chiengmai, in early summer season. Bull. Tokyo Med. Dent. Univ. 1978, 25, 77–81. [Google Scholar]
- Baumgartner, D.; Greenberg, B. Distribution and medical ecology of the blow flies (Diptera: Calliphordae) of Peru. Ann. Entomol. Soc. Am. 1985, 78, 565–587. [Google Scholar] [CrossRef]
- Baz, A.; Cifrián, B.; Díaz-äranda, L.M.; Martín-Vega, D. The distribution of adult blow-flies (Diptera: Calliphoridae) along an altitudinal gradient in Central Spain. Ann. Soc. Entomol. Fr. 2007, 43, 289–296. [Google Scholar] [CrossRef]
- Moophayak, K.; Klong-Klaew, T.; Sukontason, K.; Kurahashi, H.; Tomberlin, J.K.; Sukontason, K.L. Species composition of carrion blow flies in northern Thailand: Altitude appraisal. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Hodecek, J.; Jakubec, P. Spatio-temporal distribution and habitat preference of necrophagous Calliphoridae based on 160 real cases from Switzerland. Int. J. Leg. Med. 2022, 136, 923–934. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Princeton University Press: Princeton, NJ, USA, 1984. [Google Scholar]
- Stevens, G.C. The elevational gradient in altitudinal range: An extension of Rapoport’s latitudinal rule to altitude. Am. Nat. 1992, 140, 893–911. [Google Scholar] [CrossRef]
- Whittaker, R.H. A vegetation bibliography for the Northeastern State. Ecology 1960, 41, 245–246. [Google Scholar] [CrossRef]
- Janzen, D.H. Sweep samples of tropical foliage insects: Effects of seasons, vegetation types, elevation, time of day and insularity. Ecology 1973, 54, 687–708. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Niering, W.A. Vegetation of the Santa Catalina Mountains, Arizona. V. Biomass, production, and diversity along the elevation gradient. Ecology 1975, 56, 771–790. [Google Scholar] [CrossRef]
- Shmida, A.V.I.; Wilson, M.V. Biological determinants of species diversity. J. Biogeogr. 1985, 12, 1–20. [Google Scholar] [CrossRef]
- McCoy, E.D. The distribution of insects along elevational gradients. Oikos 1990, 58, 313–332. [Google Scholar] [CrossRef]
- Lieberman, D.; Lieberman, M.; Peralta, R.; Hartshorn, G.S. Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. J. Ecol. 1996, 84, 137–152. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern? Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Rahbek, C. The relationship among area, elevation, and regional species richness in neotropical birds. Am. Nat. 1997, 149, 875–902. [Google Scholar] [CrossRef]
- Fleishman, E.; Austin, G.T.; Weiss, A. An empirical test of Rapoport’s rule: Elevational gradients in montane butterfly communities. Ecology 1998, 79, 2472–2483. [Google Scholar]
- Raviraja, N.S.; Sridhar, K.R.; Barlocher, F. Fungal species richness in Western Ghat streams (southern India): Is it related to pH, temperature or altitude. Fungal Diver. 1998, 1, 179–191. [Google Scholar]
- Bruun, H.H.; Moen, J.; Virtanen, R.; Grytnes, J.A.; Oksanen, L.; Angerbjörn, A. Effects of altitude and topography on species richness of vascular plants, bryophytes and lichens in alpine communities. J. Veg. Sci. 2006, 17, 37–46. [Google Scholar] [CrossRef]
- Terborgh, J. Bird species diversity on an Andean elevational gradient. Ecology 1977, 58, 1007–1019. [Google Scholar] [CrossRef]
- Owen, J.G. Patterns of mammalian species richness in relation to temperature, productivity, and variance in elevation. J. Mammal. 1990, 71, 1–13. [Google Scholar] [CrossRef]
- Lawton, J.H.; MacGarvin, M.; Heads, P.A. Effects of altitude on the abundance and species richness of insect herbivores on bracken. J. Animal Ecol. 1987, 56, 147–160. [Google Scholar] [CrossRef]
- Wolda, H. Altitude, habitat and tropical insect diversity. Biol. J. Linn. Soc. 1987, 30, 313–323. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Price, P.W. Biogeographical gradients in galling species richness. Oecologia 1988, 76, 161–167. [Google Scholar] [CrossRef]
- Kearns, C.A. Anthophilous fly distribution across an elevation gradient. Am. Midl. Nat. 1992, 127, 172–182. [Google Scholar] [CrossRef]
- Olson, D.M. The distribution of leaf litter invertebrates along a neotropical altitude gradient. J. Trop. Ecol. 1994, 10, 129–150. [Google Scholar] [CrossRef]
- Sparrow, H.R.; Sisk, T.D.; Ehrlich, P.R.; Murphy, D.D. Techniques and guidelines for monitoring neotropical butterflies. Conserv. Biol. 1994, 8, 800–809. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, J.F.; Baz, A. The effects of elevation on the butterfly communities of a Mediterranean mountain, Sierra de Javalmbre, central Spain. J. Lepid. Soc. 1995, 49, 192–207. [Google Scholar]
- Sanders, N.J. Elevational gradients in ant species richness: Area, geometry, and Rapoport’s rule. Ecography 2002, 25, 25–32. [Google Scholar] [CrossRef]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
- Sanders, N.J.; Moss, J.; Wagner, D. Patterns of ant species richness along elevational gradients in an arid ecosystem. Glob. Ecol. Biogeogr. 2003, 12, 93–102. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Vetaas, O.R.; Grytnes, J.A. Fern species richness along a central Himalayan elevational gradient, Nepal. J. Biogeogr. 2004, 31, 389–400. [Google Scholar] [CrossRef]
- Grytnes, J.A.; Vetaas, O.R. Species richness and altitude: A comparison between null models and interpolated plant species richness along the Himalayan altitudinal gradient, Nepal. Am. Nat. 2002, 159, 294–304. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Future Climate Change, Risks, and Long-Term Responses; IPCC: Geneva, Switzerland, 2023; p. 12. [Google Scholar]
- IPCC. Climate Change 2001: The Scientific Basis; Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2001; p. 881. [Google Scholar]
- Hulme, M.; Jenkins, G. Climate Change Scenarios for the United Kingdom Scientific Report; Oxford University Research Archive: Oxford, UK, 1998. [Google Scholar]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kubler, K.; Bissolli, P.; Braslavska, O.G.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Root, T.L.; MacMynowski, D.P.; Mastrandrea, M.D.; Schneider, S.H. Human- modified temperatures induce species changes: Joint attribution. Proc. Natl. Acad. Sci. USA 2005, 102, 7465–7469. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–358. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hillk, J.K.; Thomas, C.D.; Descimon, H.; Huntleyk, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Casassa, G.; Karoly, D.J.; Imeson, A.; Liu, C.; Menzel, A.; Rawlins, S.; Root, T.L.; Seguin, B.; Tryjanowski, P. Assessment of observed changes and responses in natural and managed systems. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hansen, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 79–131. [Google Scholar]
- Tüzün, N.; Stoks, R. Evolution of geographic variation in thermal performance curves in the face of climate change and implications for biotic interactions. Curr. Opin. Insect Sci. 2018, 29, 78–84. [Google Scholar] [CrossRef]
- Shah, A.A.; Dillon, M.E.; Hotaling, S.; Woods, H.A. High elevation insect communities face shifting ecological and evolutionary landscapes. Curr. Opin. Insect Sci. 2020, 41, 1–6. [Google Scholar] [CrossRef]
- Huntley, B. Species distribution and environmental change: Considerations from the site to the landscape scale. In Ecosystem Management: Questions for Science and Society; Maltby, E., Holdgate, M., Acreman, M., Weir, A., Eds.; Royal Holloway Institute for Environmental Research; Royal Holloway, University of London: London, UK, 1999; pp. 115–129. [Google Scholar]
- Richards, C.S.; Price, B.W.; Villet, M. Thermal ecophysiology of seven carrion-feeding blowflies in Southern Africa. Entomol. Exp. Appl. 2009, 131, 11–19. [Google Scholar] [CrossRef]
- Amendt, J. Insect Decline—A Forensic Issue? Insects 2021, 12, 324. [Google Scholar] [CrossRef]
- Fremdt, H.; Amendt, J. Species composition of forensically important blow flies (Diptera: Calliphoridae) and flesh flies (Diptera: Sarcophagidae) through space and time. Forensic Sci. Int. 2014, 236, 1–9. [Google Scholar] [CrossRef]
- de Carvalho Moretti, T.; Godoy, W.A.C. Spatio-Temporal Dynamics and Preference for Type of Bait in Necrophagous Insects, Particularly Native and Introduced Blow Flies (Diptera: Calliphoridae). J. Med. Entomol. 2013, 50, 415–424. [Google Scholar] [CrossRef]
- Szpila, K. Key for Identification of European and Mediterranean Blowflies (Diptera, Calliphoridae) of medical and Veterinary Importance-adult Flies. Int. J. Leg. Med. 2017, 132, 831–842. [Google Scholar]
- de Carvalho, C.J.B.; de Mello-Patui, C.A. Key to the adults of the most common forensic species of Diptera in South America. Rev. Bras. Entomol. 2008, 52, 390–406. [Google Scholar] [CrossRef]
- Amat, E.; Velex, M.C.; Wolff, M. [Clave ilustrada para la identificación de los géneros y las especies de califóridos (Diptera: Calliphoridae) de Colombia]. Illustrated key for identification to genera and species of blowflies (Diptera: Calliphoridae) of Colombia. Caldasia 2008, 30, 231–244. [Google Scholar]
- Whitworth, T.L. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of the West Indies and description of a new species of Lucilia Robineau-Desvoidy. Zootaxa 2010, 2663, 1–35. [Google Scholar] [CrossRef]
- Erzinclioglu, Z. Blowflies; Richmond Publishing Co. Ltd.: Slough, UK, 1996; Volume 23, p. 71. [Google Scholar]
- Monaco, C.; Catalano, S.; De Guidi, G.; Gresta, S.; Langer, H.; Tortorici, L. The geological map of the urban area of Catania (Eastern Sicily): Morphotectonic and seismotectonic implications. Memor. Soc. Geol. Ital. 2000, 55, 425–438. [Google Scholar]
- Morales, K.; Vinicius, T. Amazon rainforest: Biodiversity and biopiracy. Student BMJ 2003, 13, 386–387. [Google Scholar]
- Kruskal, W.H.; Wallis, A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 582–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biol. Skr. 1948, 5, 1–34. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. 2016 Program and User’s Guide. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/inext-online/ (accessed on 12 March 2025).
- Hurlbert, S.H. The Nonconcept of Species Diversity: A Critique and Alternative Parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef]
- Sanders, H.L. Marine Benthic Diversity: A Comparative Study. Am. Nat. 1978, 102, 243–282. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; p. 300. [Google Scholar]
- R Development Core Team. Data from: R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Google. Google Colab. Available online: https://colab.research.google.com/ (accessed on 12 March 2025).
- Rogers, P.C.; Šebesta, J. Past management spurs differential plant communities within a giant single-clone aspen forest. Forests 2019, 10, 1118–1134. [Google Scholar] [CrossRef]
- Gemmellaro, M.D.; Bucolo, C.; Musumeci, E.; Hamilton, G.C.; Weidner, L.M. First observations of initial blow fly (Diptera: Calliphoridae) activity on lava fields and in subterranean environments in Sicily in cool temperatures. J. Med. Entomol. 2018, 55, 1622–1626. [Google Scholar] [CrossRef]
- Smith, C.A.; Poirier, L.M.; Anderson, G.S. The effect of season and urbanisation on Calliphoridae (Diptera) diversity in British Columbia, Canada, using baited traps. Can. Entomol. 2023, 155, e24. [Google Scholar] [CrossRef]
- Reiter, C. Zum Wachtstumsverhalten der Maden der blauen Schmeissfliege Calliphora vicina. Z. Rechtsmed. 1984, 91, 295–308. [Google Scholar] [CrossRef]
- Haskell, N.H.; Hall, R.D.; Cervenka, V.J.; Clark, M.A. On the body: Insects’ life stage presence and their postmortem artifacts. In Forensic Taphonomy. The Postmortem Fate of Human Remains; Haglund, W.D., Sorg, M.H., Eds.; CRC: Boca Raton, FL, USA, 1997; pp. 415–448. [Google Scholar]
- Martinez-Sanchez, A.; Rojo, S.; Marcos-Garcia, M.A. Annual and spatial activity of dung flies and carrion in a Mediterranean holm-oak pasture ecosystem. Med. Vet. Entomol. 2000, 14, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Lawrence, B.R. The distribution of Calliphora species in Britain and Ireland (Dipt., Calliphoridae). Entomol. Mon. Mag. 1990, 128, 207–213. [Google Scholar]
- Nuorteva, P. Synanthropy of blowflies (Diptera: Calliphoridae) in Finland. Ann. Entomol. Fenn. 1963, 29, 1–49. [Google Scholar]
- Chu, H.; Rosati, J.; Paucar, Y. The diversity of forensically important flies in Manhattan. In Proceedings of the Entomological Society of America, Denver, CO, USA, 5–8 November 2017. [Google Scholar]
- Gemmellaro, D.M. Food preferences of adult male and female Calliphora vicina (Diptera: Calliphoridae). 2025; Manuscript submitted for publication. [Google Scholar]
- Hamilton, W.D. Extraordinary sex ratios. Science 1967, 156, 477–488. [Google Scholar] [CrossRef]
- Iritani, R.; West, S.A.; Abe, J. Cooperative interactions among females can lead to even more extraordinary sex ratios. Evol. Lett. 2021, 5, 370–384. [Google Scholar] [CrossRef]
- Lees, D.C.; Kremen, C.; Andriamampianina, L. A null model for species richness gradients: Bounded range overlap of butterflies and other rainforest endemics in Madagascar. Biol. J. Linn. Soc. 1999, 67, 529–584. [Google Scholar] [CrossRef]
- Ngoen-klan, R.; Moophayak, K.; Klong-klaew, T.; Irvine, K.N.; Sukontason, K.L.; Prangkio, C.; Somboon, P.; Sukontason, K. Do climatic and physical factors affect populations of the blow fly Chrysomya megacephala and house fly Musca domestica? Parasitol. Res. 2011, 109, 1279–1292. [Google Scholar] [CrossRef]
- Jin, B.L.; Jaal, Z. Temporal changes in the abundance of Musca domestica Linn (Diptera: Muscidae) in poultry farms in Penang, Malaysia. Trop. Biomed. 2009, 26, 140–148. [Google Scholar]
- Hawkins, B.A.; Felizola Diniz-Filho, J.A. ‘Latitude’ and geographic patterns in species richness. Ecography 2004, 27, 268–272. [Google Scholar] [CrossRef]
- Greenberg, B.; Szyska, M.L. Immature stages and biology of fifteen species of Peruvian Calliphoridae (Diptera). Ann. Entomol. Soc. Am. 1984, 77, 488–517. [Google Scholar] [CrossRef]
- Tsuda, Y.; Hayashi, T.; Higa, Y.; Hoshino, K.; Kasai, S.; Tomita, T.; Kurahashi, H.; Kobayashi, M. Dispersal of a blow fly, Calliphora nigribasis, in relation to the dissemination of highly pathogenic avian influenza virus. Jap. J. Infect. Dis. 2009, 62, 294–297. [Google Scholar] [CrossRef]
- Weidner, L.M.; Gemmellaro, M.D.; Tomberlin, J.K.; Hamilton, G.C. Evaluation of bait traps as a means to predict initial blow fly (Diptera: Calliphoridae) communities associated with decomposing swine remains in New Jersey, USA. Forensic Sci. Int. 2017, 278, 95–100. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, K.; Boudreau, D.R.; Moreau, G. Small bait traps may not accurately reflect the composition of necrophagous Diptera associated to remains. Insects 2021, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Vogt, W.G.; Woodburn, T.L. Effects of bait age on the number, sex, and age composition of Lucilia cuprina (Wiedemann)(Diptera: Calliphoridae) in Western Australian blowfly traps. Aust. J. Exp. Agric. 1994, 34, 595–600. [Google Scholar] [CrossRef]
- Farinha, A.; Dourado, C.G.; Centeio, N.; Oliveira, A.R.; Dias, D.; Rebelo, M.T. Small bait traps as accurate predictors of dipteran early colonizers in forensic studies. J. Insect Sci. 2014, 14, 77. [Google Scholar] [CrossRef]

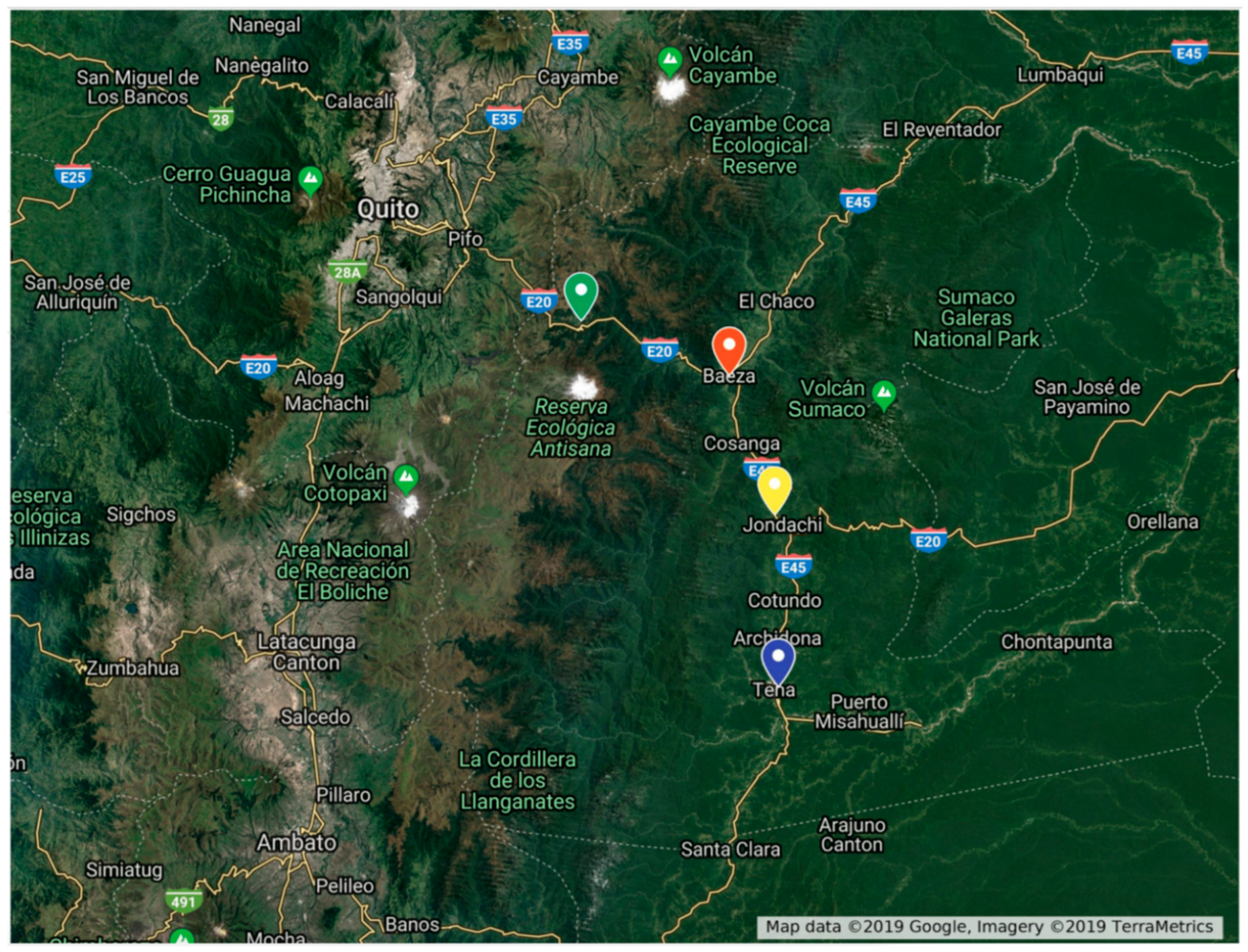
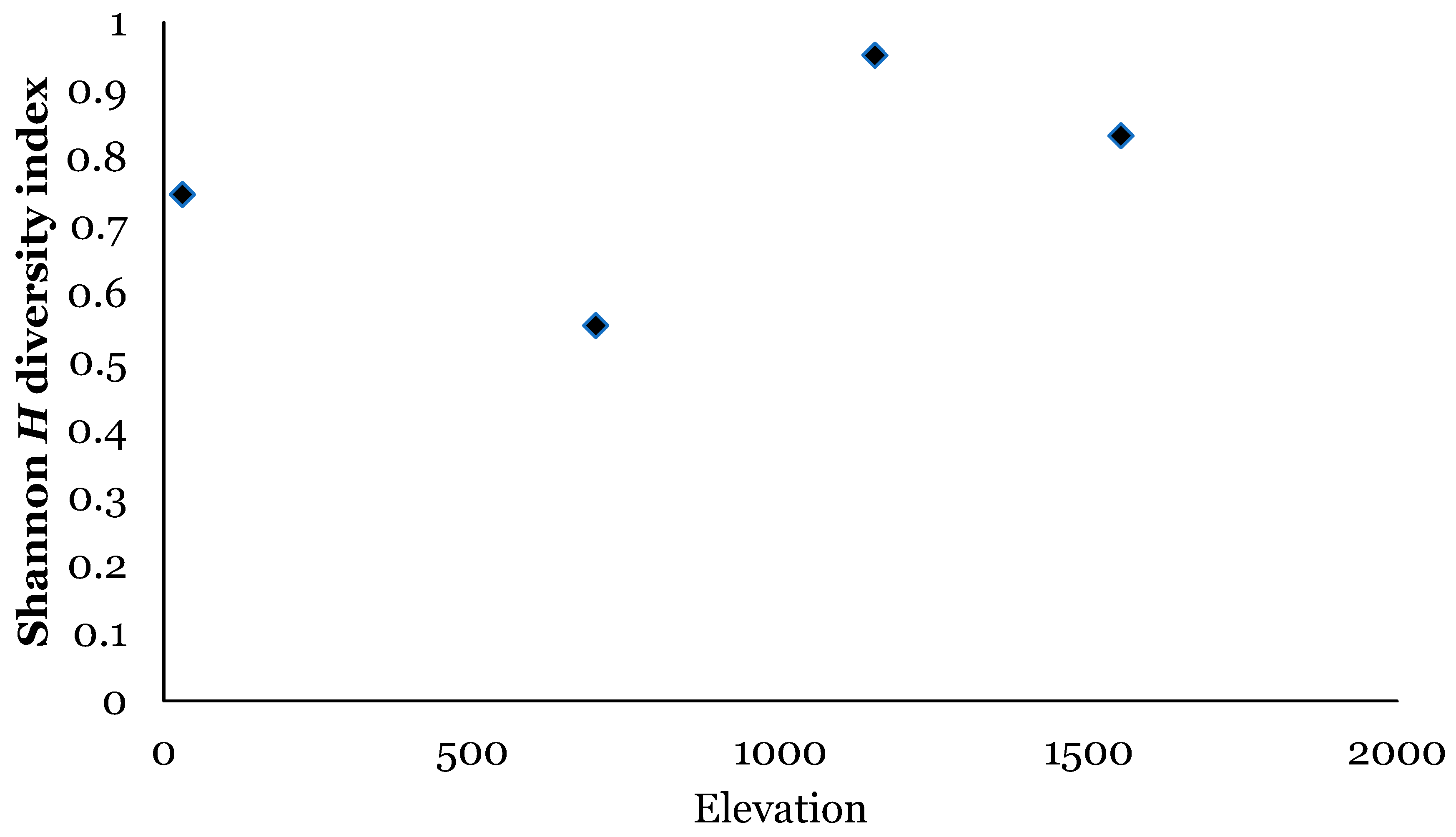
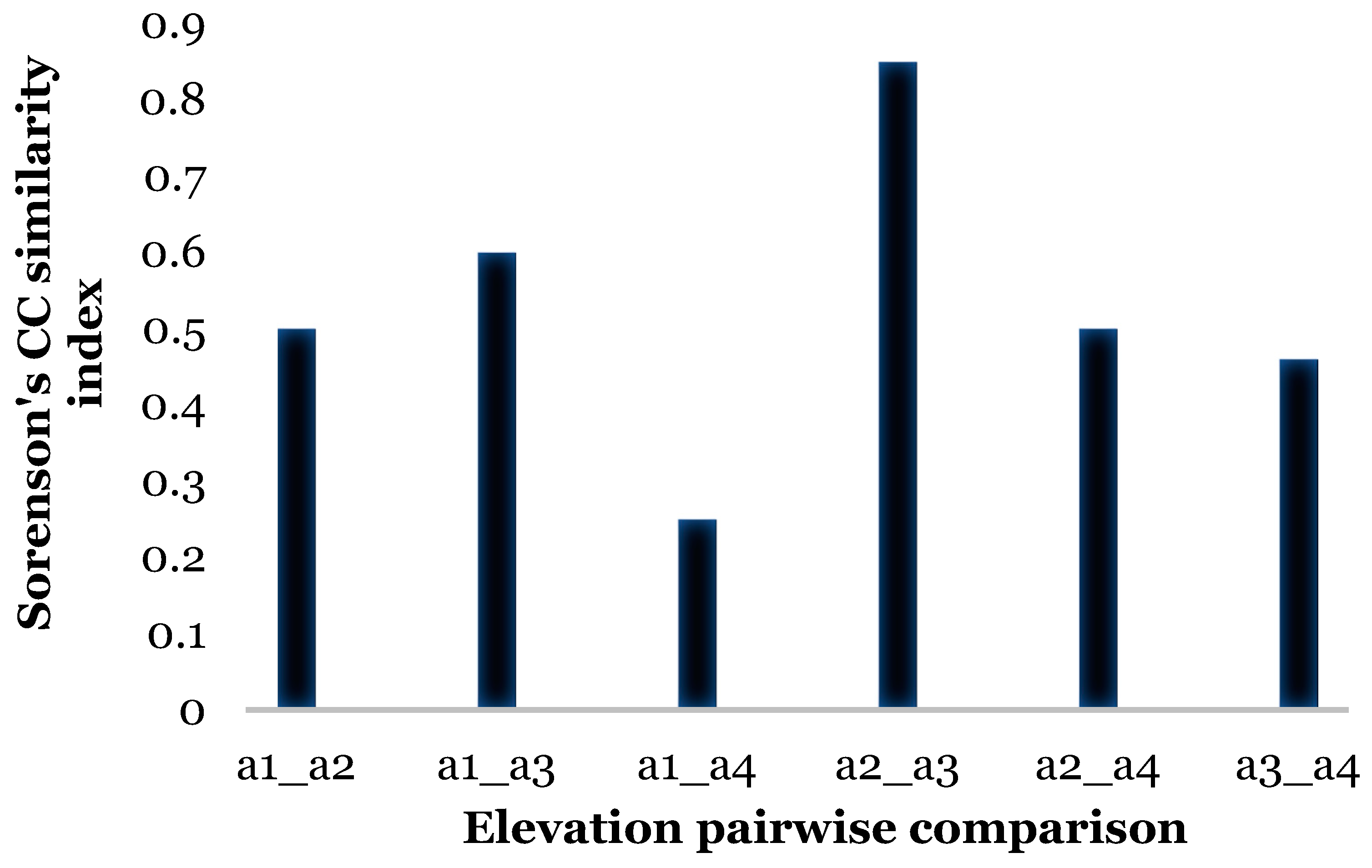
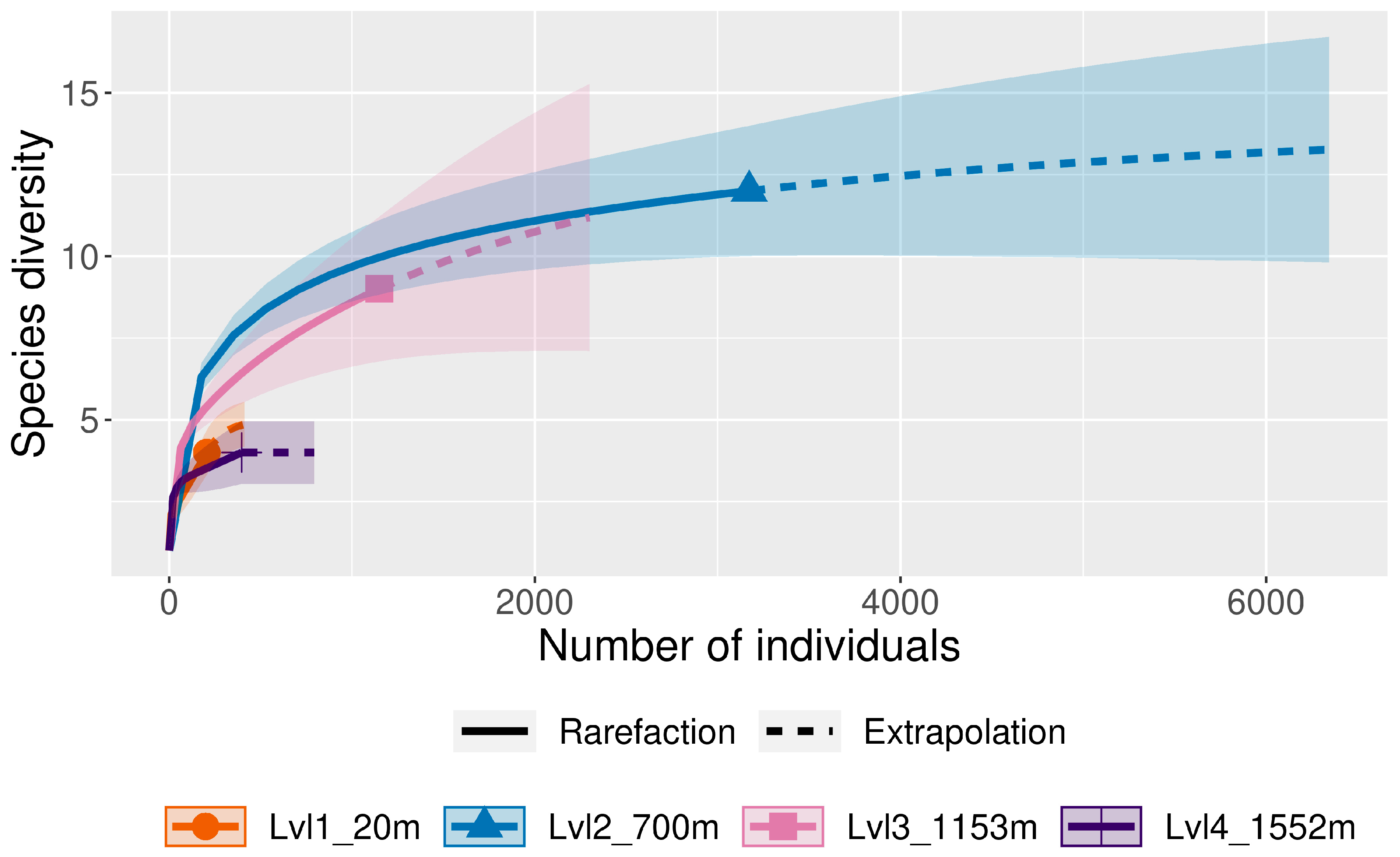


| Location | Level | Elevation (m) | Coordinates |
|---|---|---|---|
| Sicily | |||
| Catania | 1 | 20 | 37.5427° N, 15.0843° E |
| San Teodoro | 2 | 700 | 37.8071° N, 14.6994° E |
| Contrada Casale Nuovo | 3 | 1153 | 37.8521° N, 14.6919° E |
| Monte Soro | 4 | 1552 | 37.9258° N, 14.6702° E |
| Ecuador | |||
| Tena | 1 | 561 | 0.5938° S, 77.4818° W |
| Sarayacu | 2 | 1312 | 0.4208° S, 77.4844° W |
| Baeza | 3 | 1948 | 0.2759° S, 77.5321° W |
| Papallacta | 4 | 3336 | 0.2225° S, 78.0825° W |
| Location | Elevation Level | Elevation(m) | Temperature (°C) (±SE) | Humidity (%) (±SE) | Precipitation |
|---|---|---|---|---|---|
| Sicily | |||||
| Catania | 1 | 20 | 25.23 ± 0.64 | 64.13 ±1.24 | 0.3 mm |
| San Teodoro | 2 | 700 | 22.46 ± 0.83 | 62.06 ± 1.11 | 7 mm |
| Contrada Casale Nuovo | 3 | 1153 | 19.72 ± 0.42 | 61.14 ± 0.87 | 8 mm |
| Monte Soro | 4 | 1552 | 16.32 ± 0.13 | 62.2 ± 1.54 | 8.2 mm |
| Ecuador | |||||
| Tena | 1 | 561 | 23.28 ± 1.2 | 99.52 ± 0.44 | 4330 mm |
| Sarayacu | 2 | 1312 | 19.47 ± 0.66 | 94.57 ± 2.43 | 1013 mm |
| Baeza | 3 | 1948 | 17.07 ± 0.87 | 89.37 ± 1.56 | 2220 mm |
| Papallacta | 4 | 3336 | 11.29 ± 0.11 | 93.41 ± 1.32 | 1281 mm |
| SICILY | ||||||
|---|---|---|---|---|---|---|
| Species | Elevation 20 m | Elevation 700 m | Elevation 1153 m | Elevation 1552 m | Total | Rel. Ab. |
| Lucilia sericata | 42.23% | 89.80% | 43.22% | - | 3375 | 68.50% |
| Calliphora vicina | 56.79% | 5.44% | 50.60% | 40.80% | 1030 | 21.00% |
| Calliphora vomitoria | - | <1% | 2.60% | 55.16% | 251 | 5.09% |
| Lucilia ampullacea | - | 2.83% | 2.78% | <1% | 121 | 2.40% |
| Lucilia silvarum | <1% | 2.09% | <1% | - | 67 | 1.36% |
| Chrysomya albiceps | - | <1% | <1% | - | 27 | 0.55% |
| Calliphora subalpina | - | <1% | - | 3.78% | 19 | 0.38% |
| Lucilia caesar | - | <1% | <1% | - | 14 | 0.28% |
| Lucilia illustris | - | <1% | - | - | 12 | 0.24% |
| Lucilia cuprina | <1% | <1% | <1% | - | 7 | 0.14% |
| Calliphora loewi | - | <1% | <1% | - | 2 | 0.04% |
| Cynomia mortuorum | - | <1% | - | - | 1 | 0.02% |
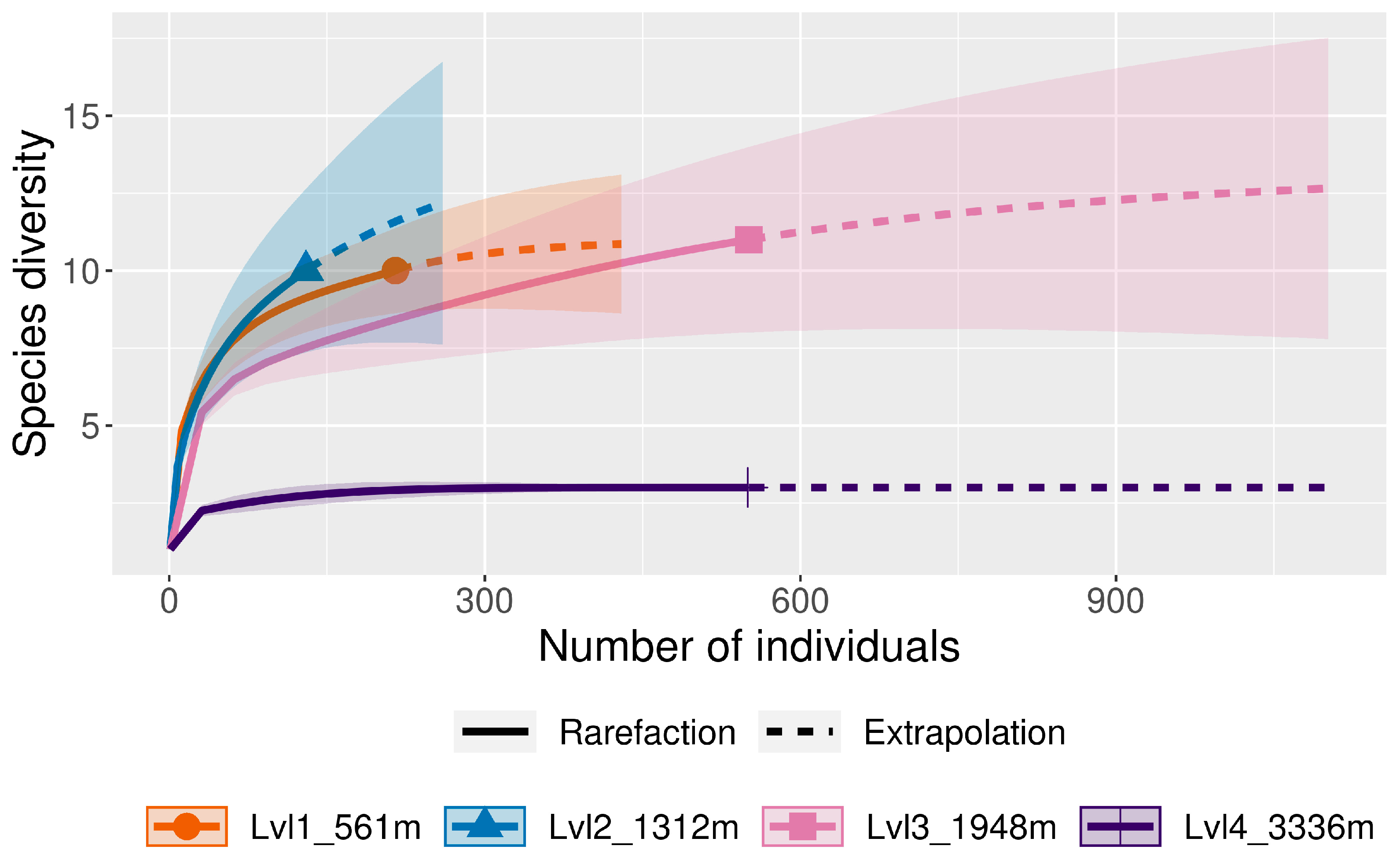
| Number of Species | 4 | 12 | 9 | 4 | ||
| Number of Specimens | 206 | 3108 | 1150 | 397 | 4244 | 100.00% |
| Species | Elevation 20 m | Elevation 700 m | Elevation 1153 m | Elevation 1552 m | Total Males | Tot Females | M/F Ratio |
|---|---|---|---|---|---|---|---|
| Lucilia sericata | 13M/74F | 147M/2644F | 16M/481F | 176 | 3199 | 0.05 | |
| Calliphora vicina | 44M/73F | 72M/97F | 195M/387F | 49M/113F | 360 | 670 | 0.54 |
| Calliphora vomitoria | 2F | 13M/17F | 55M/164F | 68 | 183 | 0.37 | |
| Lucilia ampullacea | 38M/50F | 7M/25F | 1F | 45 | 75 | 0.6 | |
| Lucilia silvarum | 1F | 14M/51F | 1F | 14 | 53 | 0.26 | |
| Chrysomya albiceps | 26F | 1F | 0 | 27 | |||
| Calliphora subalpina | 4F | 6M/9F | 6 | 13 | 0.46 | ||
| Lucilia caesar | 3M/7F | 4M | 7 | 7 | 1 | ||
| Lucilia illustris | 4M/8F | 4 | 8 | 0.5 | |||
| Lucilia cuprina | 1M | 4F | 2F | 1 | 6 | 0.16 | |
| Calliphora loewi | 1F | 1F | 0 | 2 | |||
| Cynomia mortuorum | 1F | 0 | 1 |
| Species | Kruskal–Wallis | Dunn | |||||
|---|---|---|---|---|---|---|---|
| 1 v 2 | 1 v 3 | 1 v 4 | 2 v 3 | 2 v 4 | 3 v 4 | ||
| L. silvarum | 6.771 (0.080) | - | - | - | - | - | - |
| L. sericata | 13.224 (0.004) | 0.014 | 0.211 | 0.670 | 0.210 | 0.007 | 0.114 |
| L. ampullacea | 7.313 (0.063) | - | - | - | - | - | - |
| L. cuprina | 1.187 (0.756) | - | - | - | - | - | - |
| L. illustris | 6.400 (0.094) | - | - | - | - | - | - |
| L. caesar | 3.845 (0.279) | - | - | - | - | - | - |
| C. vicina | 7.318 (0.062) | - | - | - | - | - | - |
| C. vomitoria | 2.522 (0.471) | - | - | - | - | - | - |
| C. subalpina | 6.450 (0.090) | - | - | - | - | - | - |
| C. loewi | 2.143 (0.543) | - | - | - | - | - | - |
| C. albiceps | 4.393 (0.222) | - | - | - | - | - | - |
| C. mortuorum | 4.393 (0.222) | - | - | - | - | - | - |
| Species | Level | Elevation | Value | p Value |
|---|---|---|---|---|
| Lucilia sericata | 2 | 700 m | 82.70 | 0.004 |
| Lucilia silvarum | 2 | 700 m | 72.76 | 0.033 |
| Calliphora vicina | 3 | 1153 m | 56.50 | 0.026 |
| Elevation 20 m | Elevation 700 m | Elevation 1152 m | |
|---|---|---|---|
| Elevation 700 m | A: 0.1982; Obs. Delta: 0.5097; Exp. Delta: 0.6357 p = 0.13 | ||
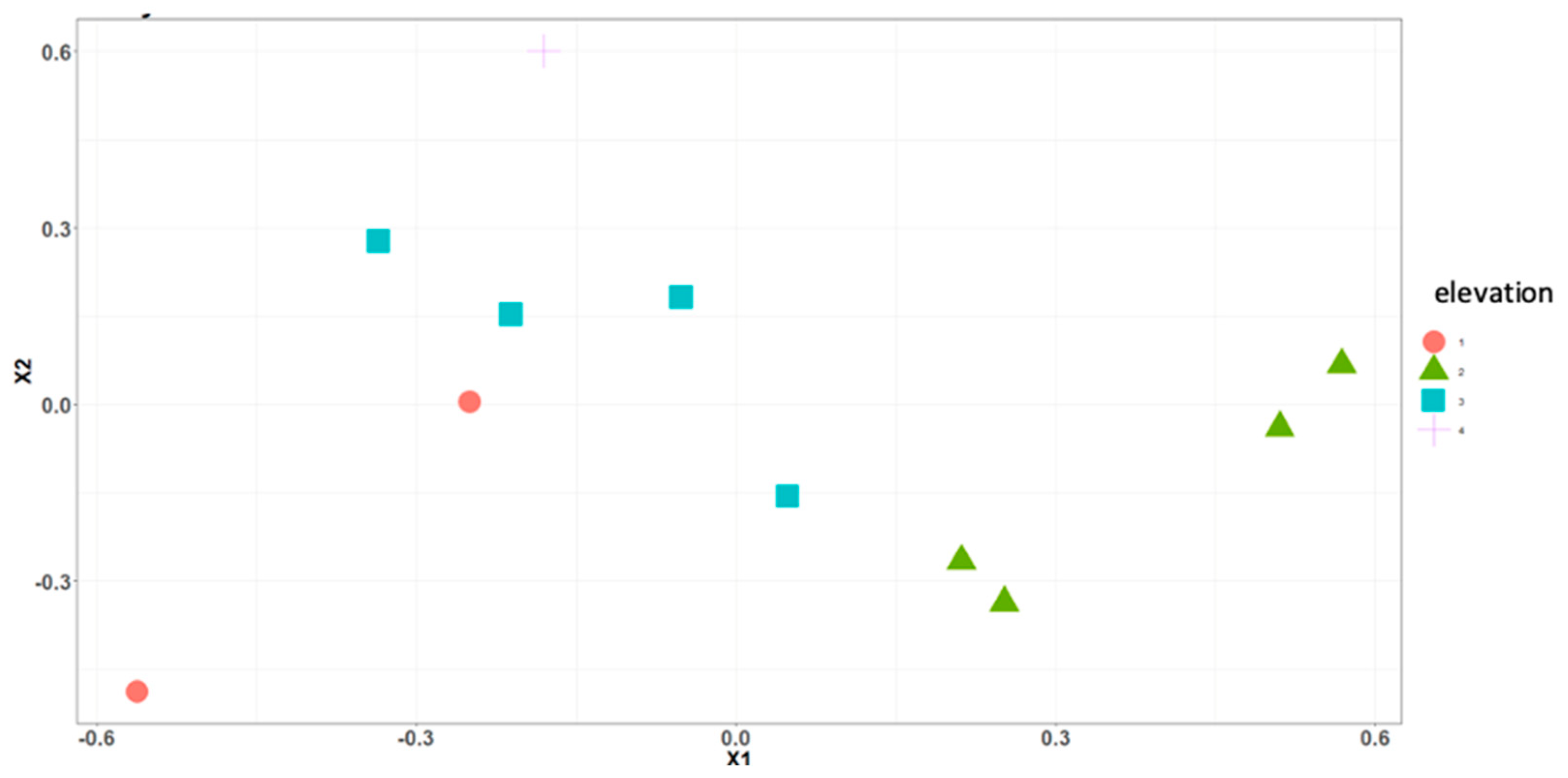
| Elevation 1152 m | A: 0.0042; Obs. Delta: 0.5053; Exp. Delta: 0.5075 p = 0.6 | A: 0.3035; Obs. Delta: 0.3681; Exp. Delta: 0.5285 p = 0.035 | |
| Elevation 1553 m | A: −0.002; Obs. Delta: 0.7864; Exp. Delta: 0.7848 p = 0.66 | A: 0.3732; Obs. Delta: 0.2714; Exp. Delta: 0. 5925 p = 0.2 | A: 0.1968; Obs. Delta: 0.3648; Exp. Delta: 0. 4541 p = 0.2 |
| Species | Elevation 561 m | Elevation 1312 m | Elevation 1948 m | Elevation 3336 m | Total | Rel. Ab. |
|---|---|---|---|---|---|---|
| Consomyiops verena | - | - | 65.27% | 74.90% | 771 | 51.67% |
| Calliphora nigribasis | - | - | - | 24.18% | 138 | 9.25% |
| Lucilia eximia | 25.12% | 27.69% | 2.72% | - | 112 | 7.50% |
| Chrysomya albiceps | 20.46% | 43.85% | <1% | <1% | 108 | 7.24% |
| Paralucilia sp. | 31.63% | 15.38% | - | - | 88 | 5.90% |
| Lucilia purpurescens | - | 2.30% | 13.82% | - | 79 | 5.30% |
| Hemilucilia semidiaphana | 12.09% | 3.08% | 5.09% | - | 58 | 3.90% |
| Chrysomya megacephala | 3.72% | - | 7.81% | - | 51 | 3.42% |
| Hemilucilia segmentaria | 2.79% | <1% | <1% | - | 43 | 2.90% |
| Lucilia ibis | - | 1.54% | 4.00% | - | 24 | 1.60% |
| Chloroprocta idiodea | - | 4.61% | - | - | 6 | 0.40% |
| Lucilia sp. | 1.86% | - | <1% | - | 5 | 0.33% |
| Cochliomyia macellaria | 1.4% | <1% | - | - | 4 | 0.27% |
| Lucilia albofusca | <1% | <1% | - | - | 2 | 0.14% |
| Cochliomyia hominivorax | <1% | - | - | - | 1 | 0.06% |
| Chrysomya sp. | - | - | <1% | - | 1 | 0.06% |
| Lucilia ochicornis | - | - | <1% | - | 1 | 0.06% |
| Number of Species | 10 | 10 | 11 | 3 | ||
| Number of Specimens | 215 | 130 | 550 | 550 | 1492 | 100.00% |
| Species | Elevation 561 m | Elevation 1312 m | Elevation 1948 m | Elevation 3336 m | Tot Male | Tot Female | M/F Ratio |
|---|---|---|---|---|---|---|---|
| Consomyiops verena | 72M/287F | 91M/321F | 163 | 608 | 0.27 | ||
| Calliphora nigribasis | 53M/80F | 53 | 80 | 0.66 | |||
| Lucilia eximia | 8M/46F | 3M/32F | 15F | 19 | 93 | 0.12 | |
| Chrysomya albiceps | 8M/36F | 13M/44F | 2F | 5F | 21 | 87 | 0.24 |
| Paralucilia sp. | 13M/55F | 20F | 13 | 75 | 0.17 | ||
| Lucilia purpurescens | 3F | 7M/69F | 7 | 72 | 0.09 | ||
| Hemilucilia semidiaphana | 4M/22F | 4F | 5M/23F | 9 | 49 | 0.15 | |
| Chrysomya megacephala | 1M/7F | 6M/37F | 7 | 44 | 0.16 | ||
| Hemilucilia segmentaria | 1M/5F | 1F | 2F | 9 | 34 | 0.12 | |
| Lucilia Ibis | 2F | 3M/19F | 3 | 21 | 0.14 | ||
| Chloroprocta idiodea | 6F | 0 | 6 | ||||
| Lucilia sp. | 4F | 1F | 0 | 5 | |||
| Cochliomyia macellaria | 3F | 1F | 0 | 4 | |||
| Lucilia albofusca | 1F | 1F | 0 | 2 | |||
| Cochliomyia hominivorax | 1F | 0 | 1 | ||||
| Chrysomya sp. | 1F | 0 | 1 | ||||
| Lucilia ochicornis | 1F | 0 | 1 |
| Species | Kruskal–Wallis | Dunn | |||||
|---|---|---|---|---|---|---|---|
| 1 v 2 | 1 v 3 | 1 v 4 | 2 v 3 | 2 v 4 | 3 v 4 | ||
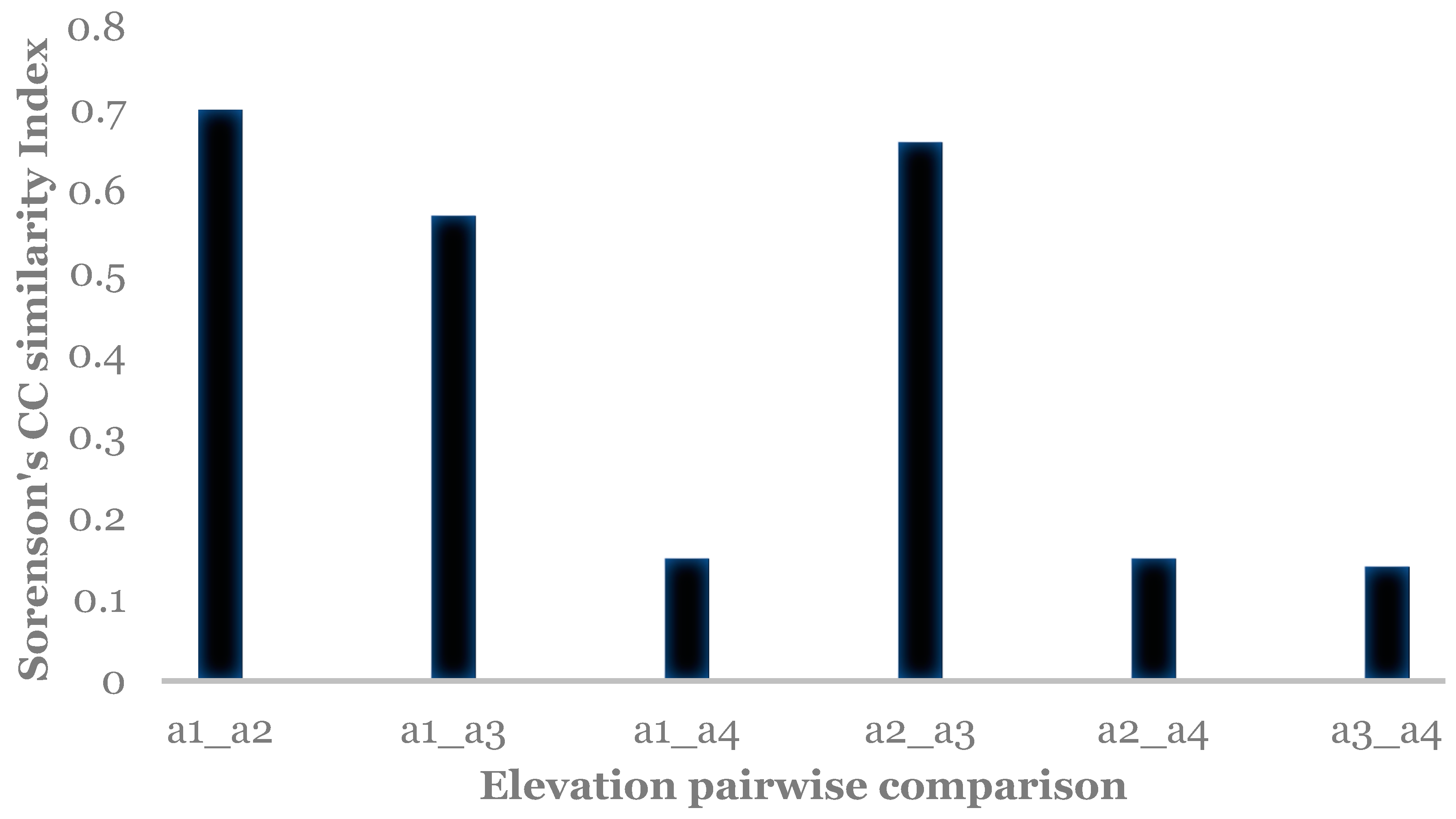
| C. nigribasis | 10.253(0.020) | 1.0 | 1.0 | 0.017 | 1.0 | 0.026 | 0.054 |
| C. megacephala | 5.025 (0.170) | - | - | - | - | - | - |
| C. idiodea | 3.00 (0.392) | - | - | - | - | - | - |
| C. albiceps | 11.061 (0.011) | 0.848 | 0.038 | 0.047 | 0.067 | 0.057 | 1.0 |
| Chrysomya sp. | 3.00 (0.392) | - | - | - | - | - | - |
| C. hominivorax | 3.00 (0.392) | - | - | - | - | - | - |
| C. macellaria | 4.393 (0.222) | - | - | - | - | - | - |
| C. verena | 10.923 (0.010) | 1.0 | 0.074 | 0.026 | 0.099 | 0.053 | 0.615 |
| H. segmentaria | 2.987 (0.394) | - | - | - | - | - | - |
| H. semidiaphana | 8.754 (0.030) | 0.124 | 0.937 | 0.115 | 0.111 | 0.760 | 0.071 |
| L. albofusca | 2.143 (0.543) | - | - | - | - | - | - |
| L. eximia | 5.67 (0.129) | - | - | - | - | - | - |
| L. ibis | 8.137 (0.040) | 0.288 | 0.044 | 1.0 | 0.411 | 0.360 | 0.088 |
| L. ochicornis | 3.00 (0.392) | - | - | - | - | - | - |
| L. purporsecens | 12.913 (<0.001) | 0.706 | 0.006 | 1.0 | 0.023 | 0.882 | 0.013 |
| Lucilia sp. | 7.702 (0.053) | - | - | - | - | - | - |
| Paralucilia sp. | 11.331 (0.010) | 0.441 | 0.018 | 0.036 | 0.098 | 0.130 | 1.0 |
| Species | Level | Elevation | Value | p Value |
|---|---|---|---|---|
| Paralucilia sp. | 1 | 560 m | 77.27 | 0.021 |
| Lucilia purpurescens | 3 | 1948 m | 96.20 | 0.003 |
| Lucilia ibis | 3 | 1948 m | 68.75 | 0.028 |
| Calliphora nigribasis | 4 | 3336 m | 75.00 | 0.033 |
| Elevation 561 m | Elevation 1312 m | Elevation 1948 m | |
|---|---|---|---|
| Elevation 1312 m | A: 0.0108; Obs. Delta: 0.5555; Exp. Delta: 0.5616 p = 0.39 | ||
| Elevation 1948 m | A: 0.2893; Obs. Delta: 0.5465; Exp. Delta: 0.7689 p = 0.027 | A: 0.3161; Obs. Delta: 0.5185; Exp. Delta: 0.7581 p = 0.025 | |
| Elevation 3336 m | A: −0.2747; Obs. Delta: 0.586; Exp. Delta: 0.808 p = 0.04 | A: 0.2977; Obs. Delta: 0.558; Exp. Delta: 0. 7945 p = 0.028 | A: 0.1031; Obs. Delta: 0.5489; Exp. Delta: 0.612 p = 0.083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gemmellaro, M.D.; Anderson, G.S.; Hamilton, G.C.; Domínguez-Trujillo, M.; Weidner, L.M. Species Richness and Distribution of Calliphoridae Along an Elevation Gradient in Sicily (Italy) and Ecuador. Insects 2025, 16, 498. https://doi.org/10.3390/insects16050498
Gemmellaro MD, Anderson GS, Hamilton GC, Domínguez-Trujillo M, Weidner LM. Species Richness and Distribution of Calliphoridae Along an Elevation Gradient in Sicily (Italy) and Ecuador. Insects. 2025; 16(5):498. https://doi.org/10.3390/insects16050498
Chicago/Turabian StyleGemmellaro, M. Denise, Gail S. Anderson, George C. Hamilton, Mariela Domínguez-Trujillo, and Lauren M. Weidner. 2025. "Species Richness and Distribution of Calliphoridae Along an Elevation Gradient in Sicily (Italy) and Ecuador" Insects 16, no. 5: 498. https://doi.org/10.3390/insects16050498
APA StyleGemmellaro, M. D., Anderson, G. S., Hamilton, G. C., Domínguez-Trujillo, M., & Weidner, L. M. (2025). Species Richness and Distribution of Calliphoridae Along an Elevation Gradient in Sicily (Italy) and Ecuador. Insects, 16(5), 498. https://doi.org/10.3390/insects16050498








