Environmental Factors Driving the Seasonal Dynamics of Ixodes ricinus and Dermacentor reticulatus in Eastern Poland
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
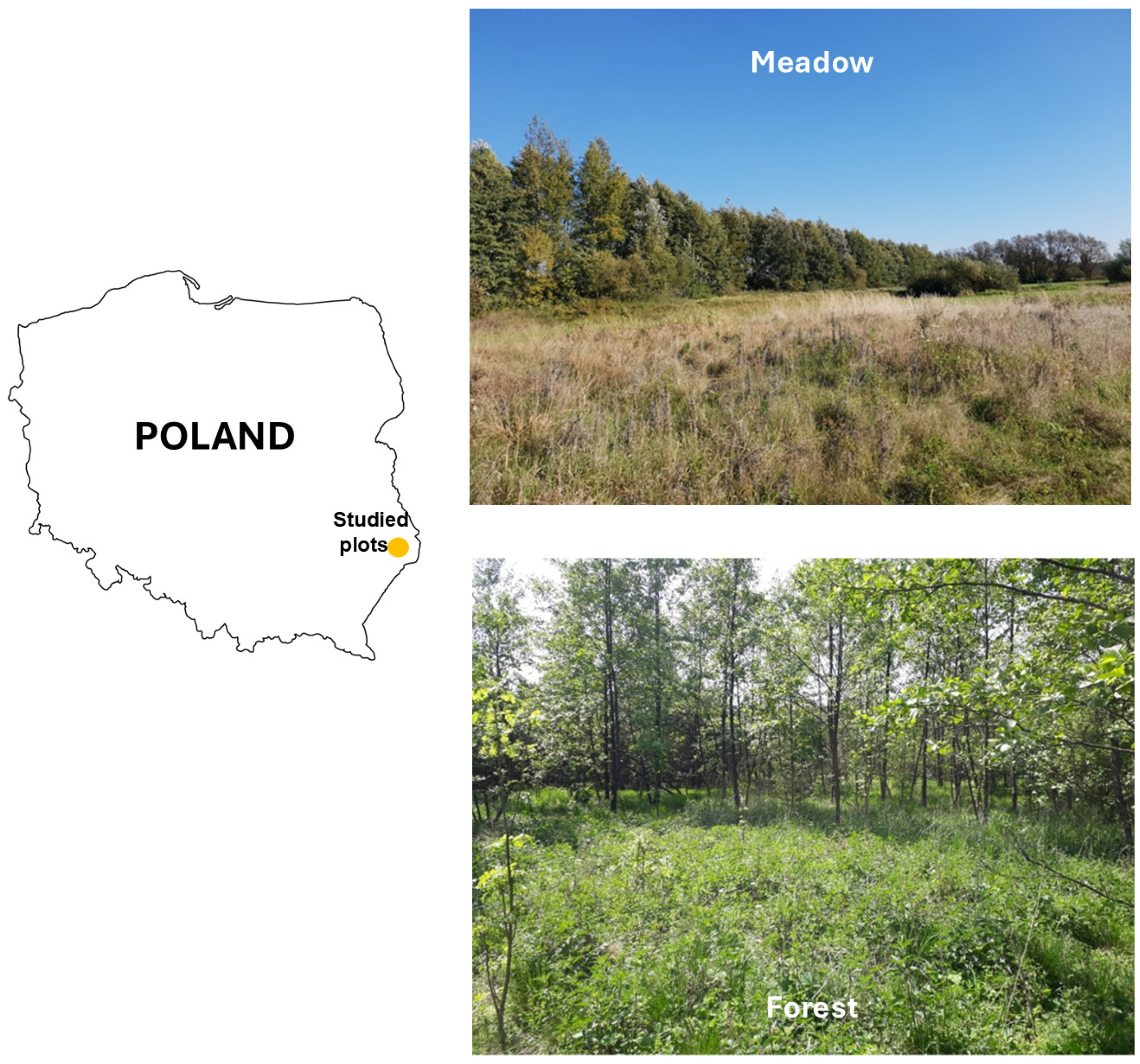
2.1. Study Area
2.2. Tick Surveillance
Tick Collection Sites
2.3. Statistical Analysis
2.3.1. Generalized Additive Model (GAM) Analysis of Tick Activity
2.3.2. Generalized Linear Mixed Model (GLMM) Analysis of Tick Activity in Relation to Habitat Type
3. Results
3.1. Occurrence and Abundance of Ticks
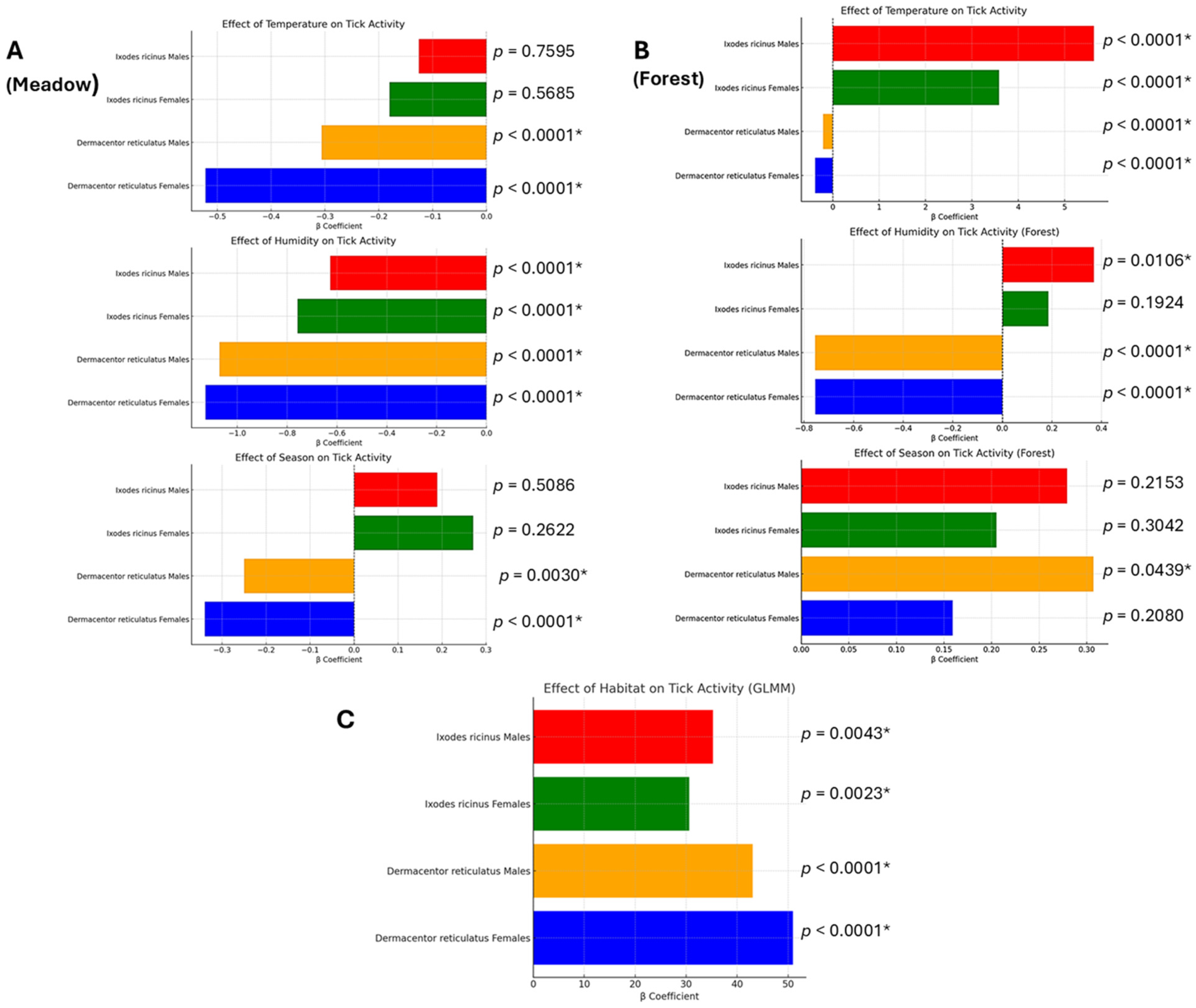
3.2. Impact of Environmental Factors on Tick Activity
3.2.1. Impact of Air Temperature on Tick Activity
3.2.2. Impact of Relative Air Humidity on Tick Activity
3.2.3. Impact of Season on Tick Activity
3.2.4. Impact of Habitat Type on Tick Occurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Földvári, G.; Široký, P.; Szekeres, S.; Majoros, G.; Sprong, H. Dermacentor reticulatus: A Vector on the Rise. Parasites Vectors 2016, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Chmura, M. Fauna kleszczy (Ixodida) Europy Środkowej; Wydawnictwo Naukowe Uniwersytetu Pedagogicznego: Kraków, Poland, 2013. [Google Scholar]
- Noll, M.; Wall, R.; Makepeace, B.L.; Vineer, H.R. Distribution of Ticks in the Western Palearctic: An Updated Systematic Review (2015–2021). Parasites Vectors 2023, 16, 141. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Materna, J.; Honig, V.; Metelka, L.; Danielová, V.; Harcarik, J.; Kliegrová, S.; Grubhoffer, L. Vertical Distribution of the Tick Ixodes ricinus and Tick-Borne Pathogens in the Northern Moravian Mountains Correlated with Climate Warming (Jeseníky Mts., Czech Republic). Cent. Eur. J. Public Health 2009, 17, 139–145. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J. The Ecology of Ticks and Epidemiology of Tick-Borne Viral Diseases. Antivir. Res. 2014, 108, 104–128. [Google Scholar] [CrossRef]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Stanek, G. Lyme Borreliosis: Biology, Epidemiology and Control; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Rizzoli, A.; Hauffe, H.C.; Carpi, G.; Vourc’h, G.I.; Neteler, M.G.; Rosa, R. Lyme Borreliosis in Europe. Eurosurveillance 2011, 16, 19906. [Google Scholar] [CrossRef]
- Randolph, S.E. Transmission of Tick-Borne Pathogens between Co-Feeding Ticks: Milan Labuda’s Enduring Paradigm. Ticks Tick Borne Dis. 2011, 2, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, A.; Milewski, R.; Pancewicz, S.; Dunaj, J.; Czupryna, P.; Milewska, A.J.; Moniuszko-Malinowska, A. Comparison of Tick-Borne Pathogen Prevalence in Ixodes ricinus Ticks Collected in Urban Areas of Europe. Sci. Rep. 2020, 10, 6975. [Google Scholar] [CrossRef]
- Kiewra, D.; Stefańska-Krzaczek, E.; Szymanowski, M.; Szczepańska, A. Local-Scale Spatio-Temporal Distribution of Questing Ixodes ricinus L. (Acari: Ixodidae)—A Case Study from a Riparian Urban Forest in Wrocław, SW Poland. Ticks Tick Borne Dis. 2017, 8, 362–369. [Google Scholar] [CrossRef]
- Zając, Z.; Kulisz, J.; Bartosik, K.; Woźniak, A.; Dzierżak, M.; Khan, A. Environmental Determinants of the Occurrence and Activity of Ixodes ricinus Ticks and the Prevalence of Tick-Borne Diseases in Eastern Poland. Sci. Rep. 2021, 11, 15472. [Google Scholar] [CrossRef]
- Karbowiak, G. The Occurrence of Dermacentor reticulatus and D. marginatus in Poland and Their Role as Vectors of Diseases. Ann. Parasitol. 2014, 60, 267–275. [Google Scholar]
- Mysterud, A.; Stigum, V.M.; Seland, I.V.; Herland, A.; Easterday, W.R.; Viljugrein, H. Tick Abundance, Pathogen Prevalence, and Disease Incidence in Relation to Season, Temperature, and Host Density in a Tick-Borne Disease System. Sci. Rep. 2019, 9, 309. [Google Scholar]
- Roe, R.M.; Donahue, K.V.; Khalil, S.M.; Bissinger, B.W.; Zhu, J.I.W.E.I.; Sonenshine, D.E. Hormonal Regulation of Metamorphosis and Reproduction in Ticks. Biol. Ticks 2013, 1, 416. [Google Scholar] [CrossRef][Green Version]
- Rees, H.H. Hormonal Control of Tick Development and Reproduction. Parasitology 2004, 129, S127–S143. [Google Scholar] [CrossRef] [PubMed]
- Zając, Z.; Obregon, D.; Foucault-Simonin, A.; Wu-Chuang, A.; Moutailler, S.; Galon, C.; Cabezas-Cruz, A. Disparate Dynamics of Pathogen Prevalence in Ixodes ricinus and Dermacentor reticulatus Ticks Occurring Sympatrically in Diverse Habitats. Sci. Rep. 2023, 13, 10645. [Google Scholar] [CrossRef] [PubMed]
- Kulisz, J.; Hoeks, S.; Kunc-Kozioł, R.; Woźniak, A.; Zając, Z.; Schipper, A.M.; Huijbregts, M.A. Spatiotemporal Trends and Covariates of Lyme Borreliosis Incidence in Poland, 2010–2019. Sci. Rep. 2024, 14, 10768. [Google Scholar] [CrossRef]
- Richling, A.; Solon, J.; Macias, A.; Balon, J.; Borzyszkowski, J.; Kistowski, M. Regionalna Geografia Fizyczna Polski: Praca Zbiorowa; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2021. [Google Scholar]
- Elias, S.P.; Gardner, A.M.; Maasch, K.A.; Birkel, S.D.; Anderson, N.T.; Rand, P.W.; Smith, R.P. A Generalized Additive Model Correlating Blacklegged Ticks with White-Tailed Deer Density, Temperature, and Humidity in Maine, USA, 1990–2013. J. Med. Entomol. 2021, 58, 125–138. [Google Scholar] [CrossRef]
- Ledger, K.J.; Keenan, R.M.; Sayler, K.A.; Wisely, S.M. Multi-Scale Patterns of Tick Occupancy and Abundance across an Agricultural Landscape in Southern Africa. PLoS ONE 2019, 14, e0222879. [Google Scholar] [CrossRef]
- Zając, Z.; Kulisz, J.; Woźniak, A.; Bartosik, K.; Khan, A. Seasonal Activity of Dermacentor reticulatus Ticks in the Era of Progressive Climate Change in Eastern Poland. Sci. Rep. 2021, 11, 20382. [Google Scholar] [CrossRef]
- Zając, Z.; Bartosik, K. Factors Influencing the Distribution and Activity of Dermacentor reticulatus (F.) Ticks in an Anthropopressure-Unaffected Area in Central-Eastern Poland. Ann. Agric. Environ. Med. 2016, 23, 2. [Google Scholar] [CrossRef]
- Zając, Z.; Woźniak, A.; Kulisz, J. Density of Dermacentor reticulatus Ticks in Eastern Poland. Int. J. 2020, 17, 2814. [Google Scholar] [CrossRef]
- Asman, M.; Bartosik, K.; Jakubas-Zawalska, J.; Świętek, A.; Witecka, J. A New Endemic Locality of Dermacentor reticulatus in Central–Southern Poland and Its Potential Epidemiological Implications. Insects 2024, 15, 580. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, E.J.; Alsarraf, M.; Behnke, J.M.; Bajer, A. The Effect of Changes in Agricultural Practices on the Density of Dermacentor reticulatus Ticks. Vet. Parasitol. 2015, 211, 259–265. [Google Scholar] [CrossRef]
- Boehnke, D.; Gebhardt, R.; Petney, T.; Norra, S. On the Complexity of Measuring Forest Microclimate and Interpreting Its Relevance in Habitat Ecology: The Example of Ixodes ricinus Ticks. Parasites Vectors 2017, 10, 549. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Bednarska, M.; Hamera, A.; Religa, E.; Poryszewska, M.; Mierzejewska, E.J.; Welc-Falęciak, R. Long-Term Study of Borrelia and Babesia Prevalence and Co-Infection in Ixodes ricinus and Dermacentor reticulatus Ticks Removed from Humans in Poland, 2016–2019. Parasites Vectors 2021, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, A.; Dunaj-Małyszko, J.; Pancewicz, S.; Czupryna, P.; Milewski, R.; Majewski, P.; Moniuszko-Malinowska, A. Prevalence of Tick-Borne Pathogens in Questing Ixodes ricinus and Dermacentor reticulatus Ticks Collected from Recreational Areas in Northeastern Poland with Analysis of Environmental Factors. Pathogens 2022, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Cunze, S.; Glock, G.; Kochmann, J.; Klimpel, S. Ticks on the Move—Climate Change-Induced Range Shifts of Three Tick Species in Europe: Current and Future Habitat Suitability for Ixodes ricinus in Comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 2022, 121, 2241–2252. [Google Scholar] [CrossRef]
- Gilbert, L.; Aungier, J.; Tomkins, J.L. Climate of Origin Affects Tick (Ixodes ricinus) Host-Seeking Behavior in Response to Temperature: Implications for Resilience to Climate Change. Ecol. Evol. 2014, 4, 1186–1198. [Google Scholar] [CrossRef]
- Probst, J.; Springer, A.; Topp, A.K.; Bröker, M.; Williams, H.; Dautel, H.; Strube, C. Winter Activity of Questing Ticks (Ixodes ricinus and Dermacentor reticulatus) in Germany—Evidence from Quasi-Natural Tick Plots, Field Studies, and a Tick Submission Study. Ticks Tick Borne Dis. 2023, 14, 102225. [Google Scholar] [CrossRef]
- Kubiak, K.; Szymańska, H.; Dziekońska-Rynko, J.; Tylkowska, A.; Dmitryjuk, M.; Dzika, E. Tick-Borne Pathogens in Questing Adults Dermacentor reticulatus from the Eastern European Population (Northeastern Poland). Sci. Rep. 2024, 14, 698. [Google Scholar] [CrossRef]
- Dwużnik-Szarek, D.; Mierzejewska, E.J.; Rodo, A.; Goździk, K.; Behnke-Borowczyk, J.; Kiewra, D.; Bajer, A. Monitoring the Expansion of Dermacentor reticulatus and Occurrence of Canine Babesiosis in Poland in 2016–2018. Parasites Vectors 2021, 14, 267. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, A.; Zając, Z.; Kulisz, J. Environmental Factors Driving the Seasonal Dynamics of Ixodes ricinus and Dermacentor reticulatus in Eastern Poland. Insects 2025, 16, 490. https://doi.org/10.3390/insects16050490
Woźniak A, Zając Z, Kulisz J. Environmental Factors Driving the Seasonal Dynamics of Ixodes ricinus and Dermacentor reticulatus in Eastern Poland. Insects. 2025; 16(5):490. https://doi.org/10.3390/insects16050490
Chicago/Turabian StyleWoźniak, Aneta, Zbigniew Zając, and Joanna Kulisz. 2025. "Environmental Factors Driving the Seasonal Dynamics of Ixodes ricinus and Dermacentor reticulatus in Eastern Poland" Insects 16, no. 5: 490. https://doi.org/10.3390/insects16050490
APA StyleWoźniak, A., Zając, Z., & Kulisz, J. (2025). Environmental Factors Driving the Seasonal Dynamics of Ixodes ricinus and Dermacentor reticulatus in Eastern Poland. Insects, 16(5), 490. https://doi.org/10.3390/insects16050490







