Optimizing Cost-Effective Larval Diets for Mass Rearing of Aedes Mosquitoes in Vector Control Programs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Strains
2.2. Study Design
2.3. Formulation and Characteristics of Specialized Diets
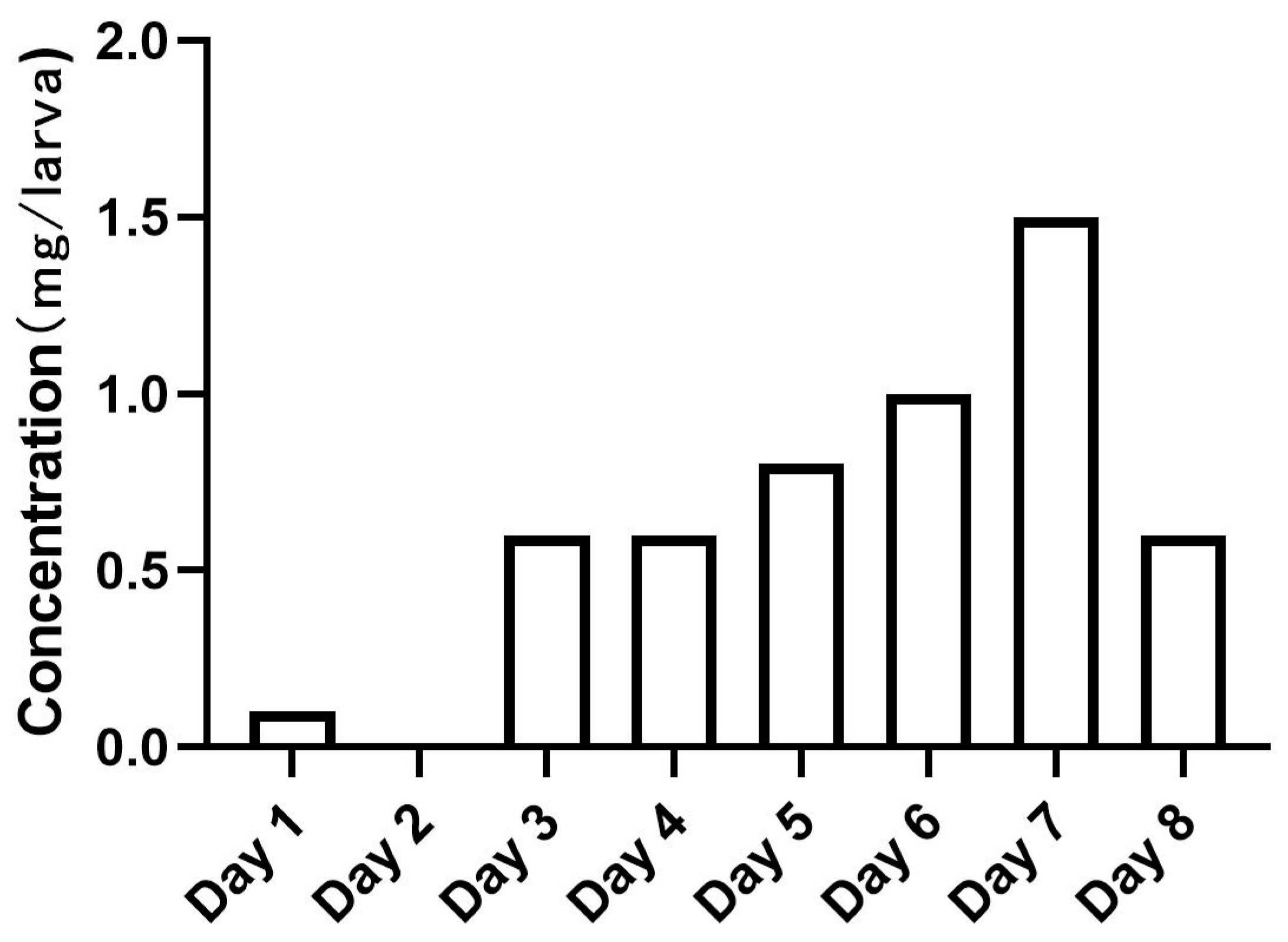
2.4. Rearing Conditions
2.5. Pupation Rate and Male Acquisition Rate
2.6. Pupal Size
2.7. Eclosion Rate
2.8. Wing Length
2.9. Male Flight Ability
2.10. Female Fecundity and Egg Hatch Rate
2.11. Survival
2.12. Statistical Analysis
3. Results
3.1. Pupation Rate
3.2. Male Sex Ratio
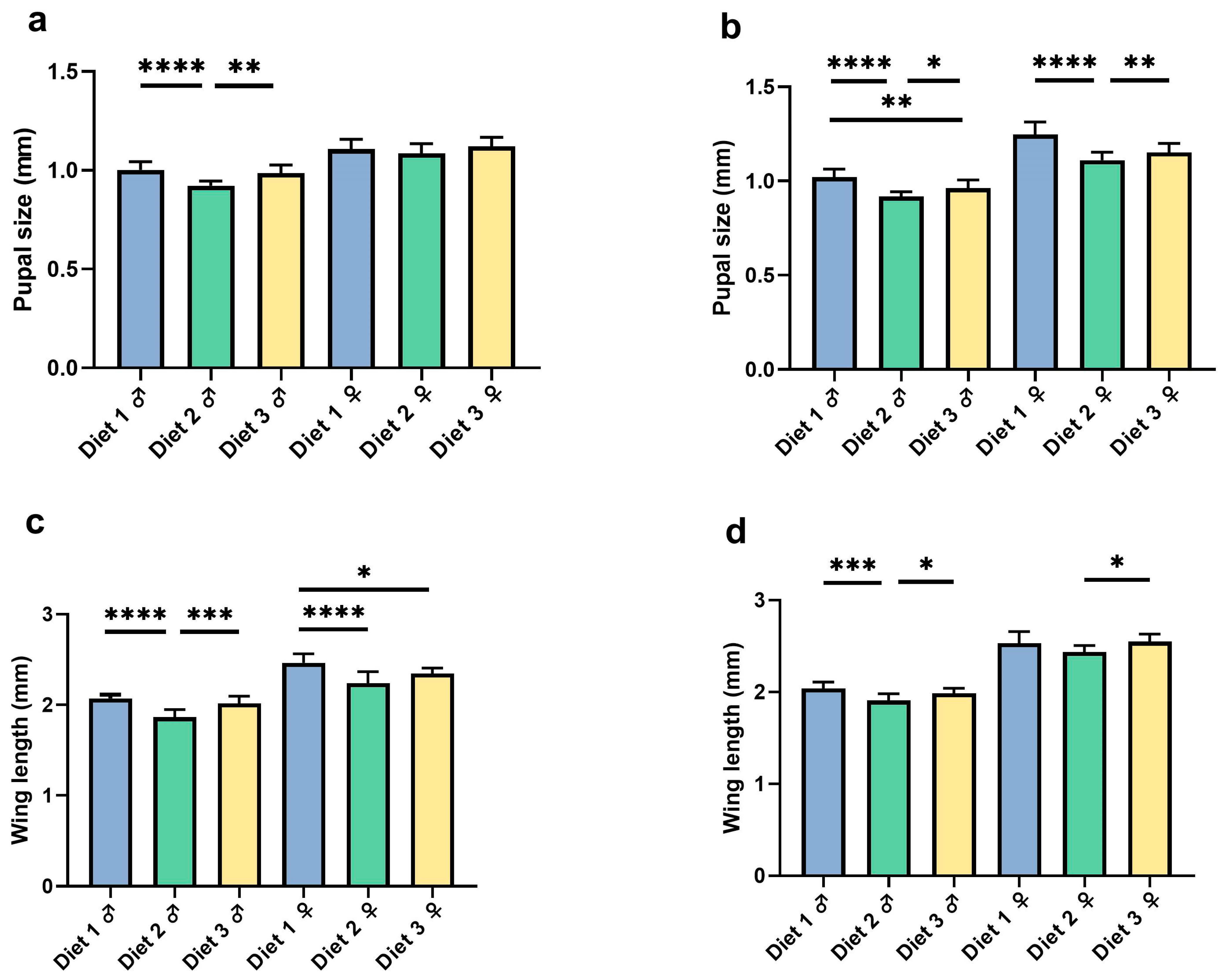
3.3. Pupal Size and Wing Length
3.3.1. Pupal Size
3.3.2. Wing Length
3.4. Eclosion Assessment
3.5. Male Flight Ability
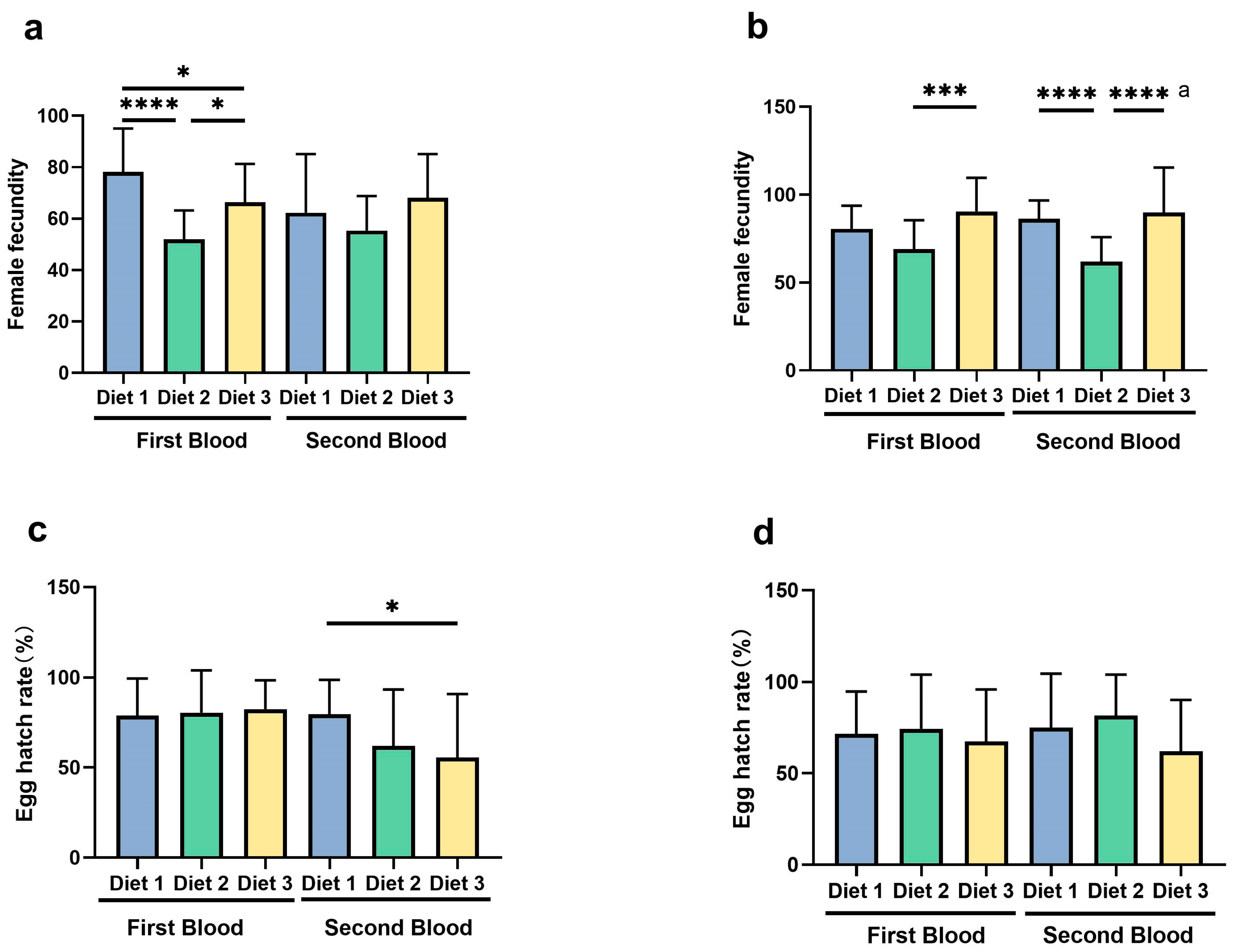
3.6. Female Fecundity and Egg Hatch Rate
3.6.1. Female Fecundity
3.6.2. Egg Hatch Rates
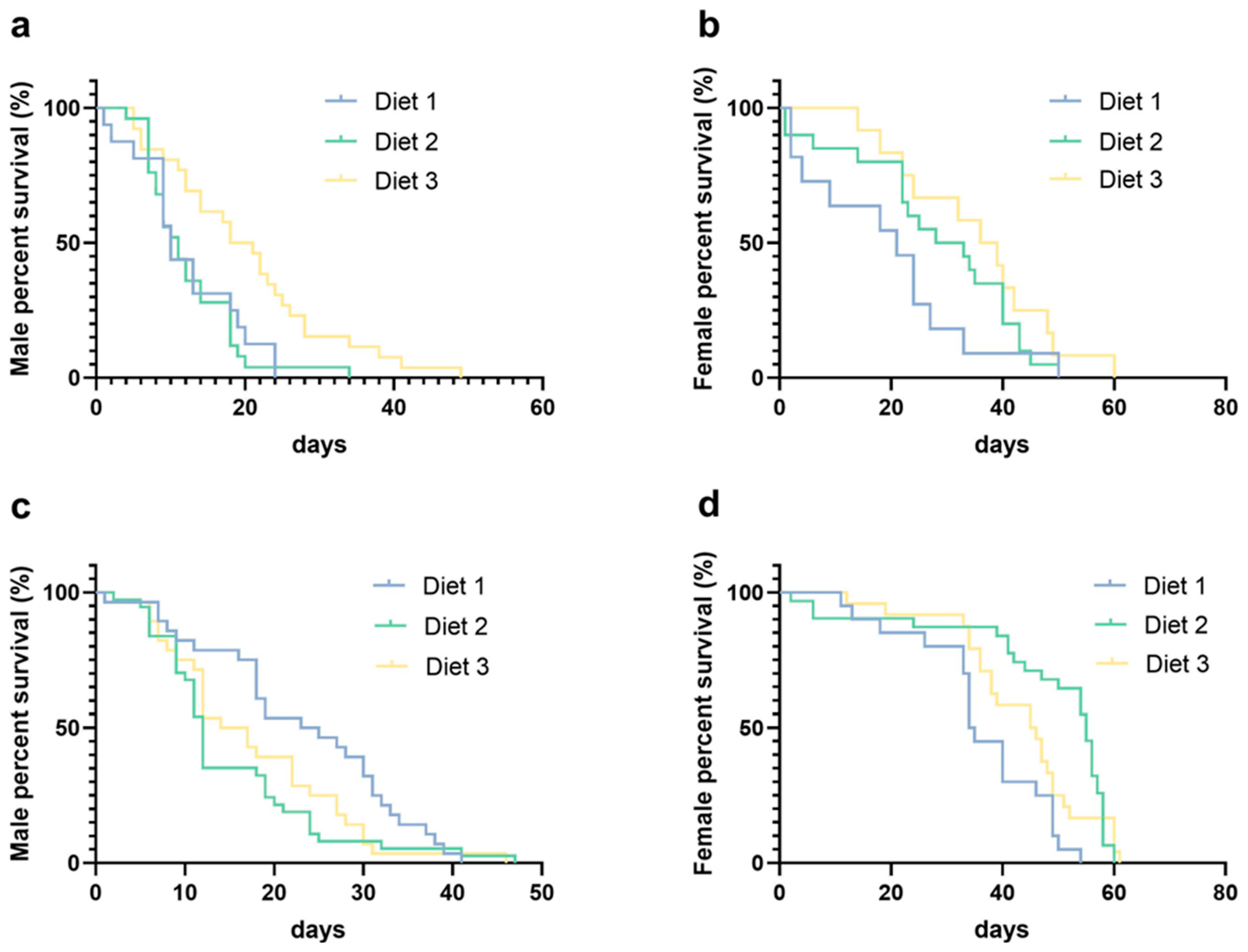
3.7. Longevity Analysis
4. Discussion
4.1. Diet Composition and Developmental Success
4.2. Morphological Traits as Proxies for Fitness
4.3. Flight Ability and Eclosion: Functional Performance
4.4. Reproductive Dynamics and Potential Life History Trade-Offs
4.5. Implication for Vector Control and Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salim, M.; Kamran, M.; Khan, I.; Saljoqi, A.U.R.; Ahmad, S.; Almutairi, M.H.; Sayed, A.A.; Aleya, L.; Abdel-Daim, M.M.; Shah, M. Effect of larval diets on the life table parameters of dengue mosquito, Aedes aegypti (L.) (Diptera: Culicidae) using age-stage two sex life table theory. Sci. Rep. 2023, 13, 11969. [Google Scholar] [CrossRef] [PubMed]
- Alves, W.C.L.; Loureiro, E.C.B. Isolated bacteria from hematophagous Culicidae (Diptera: Nematocera) in Belém, Pará State, Brazil. Rev. Pan-Amaz. Saúde 2010, 1, 61–66. [Google Scholar]
- Khan, J.; Adil, M.; Wang, G.; Tsheten, T.; Zhang, D.; Pan, W.; Khan, M.A.; Rehman, I.U.; Zheng, X.; Wu, Z.; et al. A cross-sectional study to assess the epidemiological situation and associated risk factors of dengue fever; knowledge, attitudes, and practices about dengue prevention in Khyber Pakhtunkhwa Province, Pakistan. Front. Public Health 2022, 10, 923277. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Gholizadeh, S.; Zhang, D.; Wang, G.; Guo, Y.; Zheng, X.; Wu, Z.; Wu, Y. Identification of a biological form in the Anopheles stephensi laboratory colony using the odorant-binding protein 1 intron I sequence. PLoS ONE 2022, 17, e0263836. [Google Scholar] [CrossRef]
- Zhang, D.; Maiga, H.; Li, Y.; Bakhoum, M.T.; Wang, G.; Sun, Y.; Damiens, D.; Mamai, W.; Bimbilé Somda, N.S.; Wallner, T.; et al. Mating harassment may boost the effectiveness of the sterile insect technique for Aedes mosquitoes. Nat. Commun. 2024, 15, 1980. [Google Scholar] [CrossRef]
- Yamili, C.P.; Pablo, F.P.J.; Pérez-Carillo, S.; Puerta-Guardo, H.; Geded-Moreno, E.; Correa-Morales, F.; Che-Mendoza, A.; Ayora-Talavera, G.; Vazquez-Prokopec, G.; Martín-Park, A.; et al. Different larval diets for Aedes aegypti (Diptera: Culicidae) under laboratory conditions: In preparation for a mass-rearing system. Biologia 2023, 78, 3387–3399. [Google Scholar] [CrossRef]
- Carvajal-Lago, L.; Ruiz-López, M.J.; Figuerola, J.; Martínez-de la Puente, J. Implications of diet on mosquito life history traits and pathogen transmission. Environ. Res. 2021, 195, 110893. [Google Scholar] [CrossRef]
- Gunathilaka, N.; Upulika, H.; Udayanga, L.; Amarasinghe, D. Effect of larval nutritional regimes on morphometry and vectorial capacity of Aedes aegypti for dengue transmission. BioMed. Res. Int. 2019, 2019, 3607342. [Google Scholar] [CrossRef]
- Khan, I.; Farid, A.; Zeb, A. Development of inexpensive and globally available larval diet for rearing Anopheles stephensi (Diptera: Culicidae) mosquitoes. Parasites Vectors 2013, 6, 90. [Google Scholar] [CrossRef]
- Damiens, D.; Benedict, M.Q.; Wille, M.; Gilles, J.R. An inexpensive and effective larval diet for Anopheles arabiensis (Diptera: Culicidae): Eat like a horse, a bird, or a fish? J. Med. Entomol. 2012, 49, 1001–1011. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Guidelines for Routine Colony Maintenance of Aedes Mosquito Species; Version 1.0; IAEA Insect Pest Control Subprogramme: Vienna, Austria, 2019. [Google Scholar]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R. Combining the Sterile Insect Technique with the Incompatible Insect Technique: Impact of Wolbachia Infection on the Fitness of Triple- and Double-Infected Strains of Aedes albopictus. PLoS ONE 2015, 10, e0121126. [Google Scholar] [CrossRef]
- Zhang, D.J.; Sun, Y.; Yamada, H.; Wu, Y.; Wang, G.; Feng, Q.D.; Paerhande, D.; Maiga, H.; Bouyer, J.; Qian, J.; et al. Effects of radiation on the fitness, sterility and arbovirus susceptibility of a Wolbachia-free Aedes albopictus strain for use in the sterile insect technique. Pest Manag Sci. 2023, 79, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Mamai, W.; Lobb, L.N.; Bimbilé Somda, N.S.; Maiga, H.; Yamada, H.; Lees, R.S.; Bouyer, J.; Gilles, J.R.L. Optimization of Mass-Rearing Methods for Anopheles arabiensis Larval Stages: Effects of Rearing Water Temperature and Larval Density on Mosquito Life-History Traits. J. Econ. Entomol. 2018, 111, 2383–2390. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Wu, Y.; Gilles, J.R.L.; Yamada, H.; Wu, Z.; Xi, Z.; Zheng, X. Establishment of a medium-scale mosquito facility: Optimization of the larval mass-rearing unit for Aedes albopictus (Diptera: Culicidae). Parasites Vectors 2017, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, S.E.; Briegel, H. Molting and Metamorphosis in Mosquito Larvae: A Morphometric Analysis. Mitt. Schweiz. Entomol. Ges. 1998, 71, 373–387. [Google Scholar]
- Puggioli, A.; Balestrino, F.; Damiens, D.; Lees, R.S.; Soliban, S.M.; Madakacherry, O.; Dindo, M.L.; Bellini, R.; Gilles, J.R. Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.G.; Ramírez-Osorio, A.; Marina, C.F.; Fernández-Salas, I.; Liedo, P.; Dor, A.; Williams, T. Efficiency of two larval diets for mass-rearing of the mosquito Aedes aegypti. PLoS ONE 2017, 12, e0187420. [Google Scholar] [CrossRef]
- Senevirathna, U.; Udayanga, L.; Ganehiarachchi, G.A.S.M.; Hapugoda, M.; Ranathunge, T.; Silva Gunawardene, N. Development of an Alternative Low-Cost Larval Diet for Mass Rearing of Aedes aegypti Mosquitoes. Biomed Res. Int. 2020, 2020, 1053818. [Google Scholar] [CrossRef]
- Gutiérrez, J.E.H.; Walker, K.R.; Ernst, K.C.; Riehle, M.A.; Davidowitz, G. Size as a Proxy for Survival in Aedes aegypti (Diptera: Culicidae) Mosquitoes. J. Med. Entomol. 2020, 57, 1228–1238. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Nimmo, D.; Naish, N.; McKemey, A.R.; Gray, P.; Wilke, A.B.; Marrelli, M.T.; Virginio, J.F.; Alphey, L.; Capurro, M.L. Mass Production of Genetically Modified Aedes aegypti for Field Releases in Brazil. J. Vis. Exp. 2014, 83, e3579. [Google Scholar]
- Charlwood, J.D.; Smith, T.A.; Kampango, A.; Tomas, E.V.E.; Chitnis, N. Time Series Analysis of Survival and Oviposition Cycle Duration of Anopheles funestus (Giles) in Mozambique. PeerJ 2023, 11, e15230. [Google Scholar] [CrossRef] [PubMed]
- Helinski, M.E.; Harrington, L.C. Male Mating History and Body Size Influence Female Fecundity and Longevity of the Dengue Vector Aedes aegypti. J. Med. Entomol. 2011, 48, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C. Life History Evolution: Successes, Limitations, and Prospects. Naturwissenschaften 2000, 87, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.J.; Yan, J.; Kim, C.H.; Barreaux, A.M.G.; Stone, C.M. Larval diet and temperature alter mosquito immunity and development: Using body size and developmental traits to track carry-over effects on longevity. Parasites Vectors 2023, 16, 434. [Google Scholar] [CrossRef]


| Variable | Diet 1 | Diet 2 | Diet 3 | |
|---|---|---|---|---|
| Proportion | P:S:Y = 6:3:1 a | B:S:Y = 6:3:1 b | Tortoise food c | |
| Price | ≈12.3 dollars/kg | ≈17.8 dollars/kg | ≈5.5 dollars/kg | |
| Color | Light brown | Dark brown | Dark green | |
| Texture | Powder | Powder | Particle (3 × 7 mm) e | |
| Physicochemical properties of the main component | Proteins | ≈20% | ≥50% | ≥38% |
| Fats | ≈3.5% | ≤8% | ≥4.0% | |
| Dietary fiber | - d | - d | ≤8.0% | |
| Ca2+ | - d | - d | ≤4.5% | |
| Total phosphorus | - d | - d | ≥1.5% | |
| Diet | Rep | Pupation Rate on the 8th Day from L1 | Pupation Rate on the 10th Day from L1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GT | AEG | GT | AEG | ||||||
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| Diet 1 | 4 | 0.36 ± 0.04 a | 0.14 ± 0.02 a | 0.25 ± 0.08 b | 0.04 ± 0.03 b | 0.46 ± 0.05 a | 0.30 ± 0.04 a | 0.50 ± 0.07 a | 0.29 ± 0.03 a |
| Diet 2 | 4 | 0.17 ± 0.01 b | 0.03 ± 0.01 b | 0.08 ± 0.02 c | 0.02 ± 0.02 b | 0.33 ± 0.03 b | 0.12 ± 0.01 b | 0.25 ± 0.02 b | 0.11 ± 0.04 b |
| Diet 3 | 4 | 0.35 ± 0.08 a | 0.15 ± 0.02 a | 0.37 ± 0.03 a | 0.10 ± 0.03 a | 0.44 ± 0.06 a | 0.34 ± 0.05 a | 0.56 ± 0.02 a | 0.33 ± 0.03 a |
| Strain | Diet | Rep | On the 8th Day | On the 10th Day | ||
|---|---|---|---|---|---|---|
| Total Pupa Collected (n) | Recovery of Male Pupae | Total Pupa Collected (n) | Recovery of Male Pupae | |||
| GT | Diet 1 | 4 | 35.75 ± 4.19 a | 0.72 ± 0.04 b | 45.50 ± 4.93 a | 0.60 ± 0.05 a |
| Diet 2 | 4 | 17.00 ± 1.41 b | 0.86 ± 0.03 a | 33.25 ± 2.99 b | 0.74 ± 0.04 b | |
| Diet 3 | 4 | 35.25 ± 8.14 a | 0.70 ± 0.08 b | 43.75 ± 5.50 a | 0.57 ± 0.06 a | |
| AEG | Diet 1 | 4 | 24.75 ± 8.38 a | 0.89 ± 0.05 a | 50.25 ± 6.65 a | 0.63 ± 0.06 a |
| Diet 2 | 4 | 7.50 ± 2.38 a | 0.84 ± 0.13 a | 24.75 ± 1.89 a | 0.70 ± 0.06 a | |
| Diet 3 | 4 | 37.00 ± 3.27 a | 0.78 ± 0.06 a | 56.25 ± 1.71 a | 0.63 ± 0.03 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wei, T.; Sun, Y.; Khan, J.; Zhang, D. Optimizing Cost-Effective Larval Diets for Mass Rearing of Aedes Mosquitoes in Vector Control Programs. Insects 2025, 16, 483. https://doi.org/10.3390/insects16050483
Li Q, Wei T, Sun Y, Khan J, Zhang D. Optimizing Cost-Effective Larval Diets for Mass Rearing of Aedes Mosquitoes in Vector Control Programs. Insects. 2025; 16(5):483. https://doi.org/10.3390/insects16050483
Chicago/Turabian StyleLi, Qianqian, Tongxin Wei, Yan Sun, Jehangir Khan, and Dongjing Zhang. 2025. "Optimizing Cost-Effective Larval Diets for Mass Rearing of Aedes Mosquitoes in Vector Control Programs" Insects 16, no. 5: 483. https://doi.org/10.3390/insects16050483
APA StyleLi, Q., Wei, T., Sun, Y., Khan, J., & Zhang, D. (2025). Optimizing Cost-Effective Larval Diets for Mass Rearing of Aedes Mosquitoes in Vector Control Programs. Insects, 16(5), 483. https://doi.org/10.3390/insects16050483






