Simple Summary
Habrobracon hebetor is a biocontrol agent applied against lepidopteran pests of a broad range. It harbors an endosymbiotic bacterium, Wolbachia, which regulates many aspects of the biochemistry and physiology of its insect hosts. To study the effects of Wolbachia, it was eradicated from H. hebetor, and this culture was compared with the initial Wolbachia-positive culture in terms of their ability to paralyze and develop on lepidopteran larvae. Six host species were paralyzed, and successful development of the parasitoid was observed in four of them. In some host species, the indices of parasitism were significantly lower in Wolbachia-negative compared to Wolbachia-positive strains. It can be concluded that Wolbachia improves the ability of the parasitoid wasp to attack and develop on lepidopteran larvae.
Abstract
Habrobracon hebetor is a globally acknowledged larval ectoparasitoid that is widely used to control lepidopteran pests. Wolbachia is a natural endosymbiont that regulates various aspects of the insect host biology. The ability of H. hebetor to paralyze and develop on lepidopteran larvae from five families was tested under laboratory conditions. Two lines of the wasp were used, “W+” containing a naturally occurring Wolbachia from the supergroup B, and “W−”, with the endosymbiont eradicated by antibiotic treatment, followed by propagation of 20 subsequent generations. The proportions of larvae in which host paralysis, as well as parasitoid oviposition, larval, pupal, and adult development were observed, were usually higher in W+ compared to W−. In Loxostege sticticalis, differences in these indices were not statistically significant. In Galleria mellonella, Mamestra brassicae, and Ostrinia nubilalis, some of the parasitism indices were significantly higher in W+ than in W−. In Bombyx mori and Plutella xylostella, H. hebetor could not complete its life cycle, but parasitism levels at the initial steps (from paralysis symptoms to the presence of larvae/pupae of the parasitoid) were 2–5 times lower in W− compared to W+ (p < 0.01). It can be suggested that the presence of Wolbachia is advantageous for H. hebetor, as it increases the success of parasitism in a broad range of lepidopteran hosts.
1. Introduction
Biological pest control using microbial entomopathogens and entomophagous arthropods [,,], as well as their combinations [], is a reliable and healthy alternative to the application of synthetic insecticides. The latter exert numerous adverse effects on ecosystems at local and global scales, including environmental pollution, biodiversity degradation, and pest resistance development [,,,,]. Diverse factors affect the efficiency of biocontrol agents in natural and artificial ecosystems [,,]. In-depth studies of the biology of predatory and parasitic insects are essential to advance biocontrol strategies and programs [].
Parasitoids represent a unique form of symbiosis (“parasitoidism”) that differs from parasites due to peculiar trends in evolutionary history. The evolution of parasitoid–host interactions, unlike those within parasite–host systems, targeted regulation of the host by the parasitoid for the benefit of its progeny []. The most diverse and successful parasitoid group is the parasitoid wasps (Hymenoptera) [].
Wolbachia is an intracellular symbiotic bacterium that is broadly dispersed among major taxa of nematodes and arthropods [,,,,,]. The taxonomy of Wolbachia is yet unclear, as the vast diversity of known isolates has been traditionally attributed to the single species of W. pipientis. The attempts of some researchers to assign the vast diversity of known W. pipientis uncultured isolates into a series of distinct taxa at the species rank based upon their phylogeny [] have not been accepted by the scientific community []. In contrast, several phylogenetically distinct supergroups are recognized, labeled using the Latin capitals A, B, C, D, E, F, etc., up to W []. In different symbiotic systems, Wolbachia’s effects on the host organism and population vary from adverse to beneficial, including reproductive manipulation, such as cytoplasmic incompatibility [,,,], induction of parthenogenesis [], reduced sperm competitive ability [], and altered mating behavior []. Other effects include altered behavior [], improved learning and memory capacity [], prolonged immature periods and adult female longevity [], increased fecundity [], and enhanced resistance to infections [,]. The impact of Wolbachia on the host’s metabolism was demonstrated in a series of works utilizing a comparison of Wolbachia-infected vs. Wolbachia-free laboratory insect cultures. To remove the potential influence of Wolbachia interactions with the intestinal microbiome of the host, these studies were further assisted by the use of metabolomic profiles of Wolbachia-infected vs. uninfected cell cultures. This showed that the endosymbiont caused substantial changes in host-cell metabolome profiles, which may have important fitness consequences [].
Curiously, the year 2024 is the 100th anniversary of the discovery of Wolbachia [], and scientific interest in this bacterium has risen over the decades. Numerous research groups all over the world have contributed to exploration of the diversity of Wolbachia, its interactions with hosts [,,,], and the intrinsic mechanisms of these interactions at the molecular level [,]. Practical implications of this cryptic endosymbiont include the control of arthropod vectors of dangerous infections [,], filarial diseases [,], and agricultural pests [,].
Wolbachia infection is among the key stress factors that have an impact on parasitoid oviposition [] and thus contribute to the overall fitness of parasitoid populations. In one of the most widespread and practically important species of braconid ectoparasitoids, Habrobracon hebetor, the endosymbiont presence showed notable outcomes. In a series of studies using a parasitoid culture originating from Iran, reproductive manipulation strategies were observed, including cytoplasmic incompatibility, altered mating behavior, and increased fitness of the progeny originating from infected females and males. It is concluded that some of these effects may facilitate endosymbiont transmission [,,]. Using another culture of H. hebetor naturally infected with Wolbachia, compared to the culture where the endosymbiont was eradicated, it was discovered that Wolbachia’s presence incurred elevated levels of digestive enzyme activities and lipid accumulation in parasitoid larvae []. Taken together with observations in other Wolbachia–insect symbiotic systems, these data suggest that metabolic alterations are induced by Wolbachia in their hosts to promote the endosymbiont’s survival and proliferation [,].
The goal of the present work is to study the effect of the naturally occurring Wolbachia on the ability of H. hebetor to attack and develop on lepidopteran insect hosts. Our main hypothesis is that the physiological and biochemical changes of the parasitoid organism associated with Wolbachia’s presence [] should be reflected in the parasitism efficiency of H. hebetor.
2. Materials and Methods
2.1. Insect Cultures
2.1.1. The Parasitoid and Its Endosymbiont
A laboratory strain of H. hebetor (Hymenoptera: Braconidae) originating from Berlin, Germany, was cultured on larvae of the greater wax moth Galleria mellonella L. (Lepidoptera: Pyralidae) (see below, Section 2.1.2) for more than 10 years as a permanent laboratory line with stable biological properties and positive for Wolbachia infection (W+). To obtain the line of Wolbachia-free H. hebetor (W−), the antibiotic treatment was applied as follows. The macrocyclic antibiotic rifampicin (RUPE Belmedpreparaty, Minsk, Belarus) was dissolved in sterile saline (0.9% NaCl) and injected individually into the hemocoel of the last instar larvae of G. mellonella using a microinjector at a dosage of ~4 µg per larva. Habrobracon hebetor was reproduced for three consecutive generations using the rifampicin-injected wax moth larvae as the host, then routinely maintained separately from the W+ line on antibiotic-free larvae for another 20 generations to avoid cross-contamination of cultures [].
2.1.2. Laboratory Hosts of the Parasitoid
The laboratory culture of G. mellonella was established using larvae captured in bee hives in the Novosibirsk region and the Altai Area (Western Siberia) and maintained for more than 20 years. The insects were bred at 28 °C and in constant darkness in glass jars, and the larvae were fed with a standard artificial diet, as described elsewhere [].
The laboratory culture of the beet webworm Loxostege sticticalis L. (Lepidoptera: Crambidae) stemmed from a group of 30 moths captured in the field in the Krasnoyarsk Area in 2021. The insects were reared at 26 °C with an 18L:6D photoperiod in Petri dishes and plastic containers, and larvae were fed an original artificial diet containing 36.3% soybean meal, 25.9% corn meal, 14.7% wheat bran, 13.0% yeast powder, 6.6% agar-agar, 2.2% ascorbic acid, and 1.3% benzoic acid.
The laboratory culture of the European corn borer Ostrinia nubilalis Hbn (Lepidoptera: Crambidae) was set up utilizing diapausing larvae collected from corn stalks in a maize field in the Krasnodar Area in 2023 and refrigerated for 4 months. After reactivation, the progeny was obtained and maintained at 26 °C with an 18L:6D photoperiod in glass jars, and larvae were fed a standard artificial diet [].
The laboratory culture of the cabbage moth Mamestra brassicae L. (Lepidoptera: Noctuidae) was founded using a batch of larvae obtained from a private cabbage plot in the Novosibirsk region in 2021. The insects were raised at +26 °C with an 18L:6D photoperiod in plastic containers, and larvae were fed an artificial diet modified after Zagorinskiy et al. [] by removing the “powdered dried food plant”.
The laboratory culture of the silkworm Bombyx mori L. (Lepidoptera: Bombycidae) was represented by larvae hatched from eggs acquired at the Scientific Research Station of Sericulture of the North-Caucasian Federal Scientific Agrarian Center (Zheleznovodsk, Stavropol Area) in 2024. The larvae were kept at 26 °C with an 18L:6D photoperiod in plastic containers and fed fresh mulberry leaves cultivated on a hydroponic substrate in an indoor greenhouse.
The laboratory culture of the diamondback moth Plutella xylostella L. (Lepidoptera: Plutellidae) was the progeny of insects sampled from the experimental cabbage plot at the territory of the All-Russian Institute of Plant Protection (St. Petersburg, Russia) in 2024. The insects were grown at 26 °C with an 18L:6D photoperiod in gauze cages containing live cabbage plants grown on a hydroponic substrate in an indoor greenhouse.
The samples of all insect cultures used for the experiments were tested for the presence of Wolbachia (see below, Section 2.2) and were found to be negative.
2.2. Molecular Diagnostics of the Endosymbiont Wolbachia in Insects
For genomic DNA extraction, the simplified protocol of Sambrook et al. [] was used without the addition of phenol. Insect tissues were homogenized and incubated in a lysis buffer for 2 h at +65 °C. DNA was further extracted with chloroform, precipitated with isopropanol, and washed with ethanol. Air-dried DNA pellets were resuspended in ultra-purified water. The primers targeting standard multilocus typing (MLST) loci, i.e., gatB, coxA, hcpA, ftsZ, and fbpA, as well as wsp, were exploited [].
DreamTaq Green PCR Master Mix 2X (Thermo Fisher Scientific, Waltham, MA, USA) was utilized for DNA amplification. The PCR program consisted of initial denaturation (95 °C for 5 min), 35 amplification cycles (denaturation at 95 °C for 1min; annealing at 54 °C for 1 min, elongation at 72 °C for 1 min), and a final extension step (72 °C for 5 min). The PCR products were subjected to 1% agarose gel electrophoresis, and the GeneRuler Ladder Mix molecular weight marker (Thermo Fisher Scientific, Waltham, MA, USA) aided in the detection of amplicons of expected size.
Prior to the parasitism bioassays described below, the wild-type H. hebetor adults (“W+”) showed a positive reaction when subjected to Wolbachia-targeted PCR analysis. On the contrary, the samples of adults from the “W−” line maintained for 20 generations after antibiotic treatment were negative (Figure 1). This result is in agreement with our previous study, where the presence and absence of Wolbachia in W+ and W− H. hebetor cultures were confirmed using conventional PCR, followed by Sanger sequencing and a metagenomic survey [].
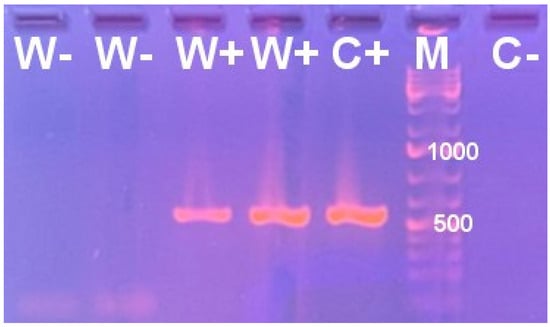
Figure 1.
Electrophoretic profile of PCR products obtained using specific primers targeting the Wolbachia surface protein gene fragment for the genomic DNA samples from Habrobracon hebetor belonging either to the wild-type Wolbachia-positive culture (W+) or to the culture maintained for 20 generations after eradication of Wolbachia. C+, positive control (reference sample of W+ H. hebetor, with Wolbachia diagnosis confirmed using sequencing of six multilocus sequence typing genes); C−, negative control (no genomic DNA), M, molecular weight marker with the positions of the 500 and 1000 bp bands labeled.
The amplicons from the W+ culture sample were further purified using the silica sorption method []. The purified amplicons were sequenced in forward and reverse directions according to Sanger [] using an ABI Prism sequencer, corrected manually in BioEdit, and compared with Genbank entries using the BLAST utility, version 2.16.0. The nucleotide sequences were aligned in BioEdit []. The alignment of six MLST genes, 2050 bp long, was prepared with the addition of PubMLST accessible entries of Wolbachia from the two major supergroups A and B. The most basal Wolbachia strains from Brugiya malayi and Litomosoides sigmodontis were used as an outgroup. The phylogenetic reconstruction was performed using Bayesian inference with Markov’s Monte Carlo chain algorithm in MrBayes version 3.1.2. The evolutionary model was GTR + I + G. The algorithm was run for 800,000 iterations to reach the standard deviation of split frequencies below 0.009. Every 100th generation was sampled, and the first 25% of samples were discarded as a burn-in fraction. Parameter values were summarized, and a consensus tree was constructed.
2.3. Parasitism Assays
Upon emergence of the H. hebetor adults, they were fed with 20% honey syrup and allowed to mate for 48 h. Then, the females were placed individually in ventilated plastic 30 mL containers, supplied with a single last instar lepidopteran larva of a particular species, with the exception of the silkworm, where a fourth instar was provided. The experiment was performed at 26 °C with an 18L:6D photoperiod. The ratios of the paralyzed host larvae and those showing the presence of eggs, larvae, cocoons, and emerged adults of the parasitoid were calculated. The experiments were repeated in two or three repetitions. The numbers of individual replicates of each variant equaled 65 in G. mellonella, 30 in L. sticticalis, 118 in O. nubilalis, 37 in M. brassicae, 30 in P. xylostella, and 40 in B. mori.
2.4. Statistical Analysis
For each pairwise comparison procedure, data were pooled from all repetitions of a given experimental variant and presented as a matrix of four values, namely, the total number of insects showing signs of parasitism (symptoms of paralysis, presence of parasitoid eggs, larvae, pupae, or emerged adults) in variant 1 and variant 2, and the total number of insects surviving (no signs of paralysis or parasitoid development until a certain stage) in variant 1 and variant 2. When all the values in the comparison group exceeded a value of 10, data analyses were performed with Pearson’s Chi-squared test []. If any of the values were below 10, Pearson’s Chi-squared test corrected according to Yates [] was applied. Finally, Fisher’s exact test [] was employed in cases when the values were below 5. The data were considered statistically significant at p < 0.01 or p < 0.05.
3. Results
3.1. Molecular Genetic Classification of Wolbachia
For detailed characterization of the Wolbachia genotype, the sequences of the main diagnostic MLST genes were obtained. The set of gatB, coxA, hcpA, ftsZ, and fbpA loci corresponded to that characteristic of the sequence type ST-125. In particular, the three former sequences were identical to those available in the PubMLST database, while the latter two contained only 1–2 point mutations. The wsp gene sequence and its hypervariable regions were more conservative, as their sets coincided in a broad range of sequence types (Table 1). As displayed in the respective phylogenetic reconstruction (Figure 2), the endosymbiont from H. hebetor belonged to the lineage of Wolbachia isolates from lepidopteran hosts of the families of Noctuidae (such as Spodoptera exempta Walker, also harboring Wolbachia ST-125), Nymphalidae, and Pieridae, with an isolate from the parasitoid wasp Eretmocerus emiratus Zolnerowich and Rose (Hymenoptera: Aphelinidae) in a more basal position, with a maximal value for the branch support for this phylogenetic grouping (1.00 posterior probability) This lineage belongs to the supergroup B, which is the largest clade of the molecular phylogenetic tree of Wolbachia, containing predominately endosymbionts of Lepidoptera, as well as other host orders, including Hymenoptera.

Table 1.
A comparison of molecular genetic data used for the classification of Wolbachia sequence type (ST) in Habrobracon hebetor based upon multilocus sequence typing (MLST) and Wolbachia surface protein (wsp).
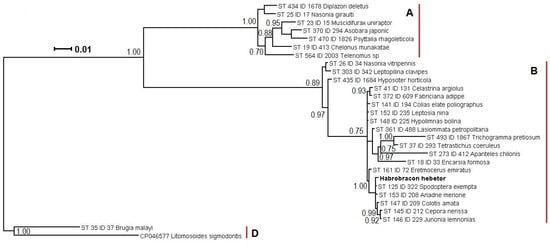
Figure 2.
A phylogenetic reconstruction of Wolbachia isolate from Habrobracon hebetor (in bold) and PubMLST database entries, annotated with sequence type (ST), ID, and host species name. The tree was built using Bayesian inference in MrBayes 3.1.2, using a concatenated sequence of multilocus sequence typing loci (see the Section 2). The numbers at the intermediate nodes (branch support) indicate posterior probability of 0.70 or higher. Capital Latin letters in bold font indicate three respective supergroups of Wolbachia. The scale bar is 0.01 expected changes per site.
3.2. Parasitism Success of the Parasitoid in Six Host Species
The adult females of H. hebetor eagerly attacked the larvae of the six assayed species of lepidopteran hosts. Notably, the rates of host paralysis, oviposition, and larval, pupal, and adult production of the parasitoid were usually about 10–20% higher in W+ compared to the W− parasitoid line. These differences, however, were not always statistically significant. In particular, in the greater wax moth, W+ and W− caused paralysis in 86.15 ± 4.28% and 76.92 ± 1.92% of the larvae, respectively (not significant, χ2 = 1.3, p > 0.05). The proportions of host specimens on which the parasitoid oviposited were 80.00 ± 4.96% and 61.54 ± 6.03% in W+ and W−, respectively (χ2 = 5.4, p < 0.05). Parasitoid larvae were found in 69.23 ± 5.77% and 55.38 ± 6.16% of cases (χ2 = 2.7, p > 0.05), cocoons were formed in 60.00 ± 6.08% and 46.15 ± 6.18% of cases (χ2 = 2.5, p > 0.05), and adults emerged in 56.92 ± 6.14% and 44.62 ± 6.17% of cases (χ2 = 2.0, p > 0.05), respectively, with the differences being not significant (Figure 3).
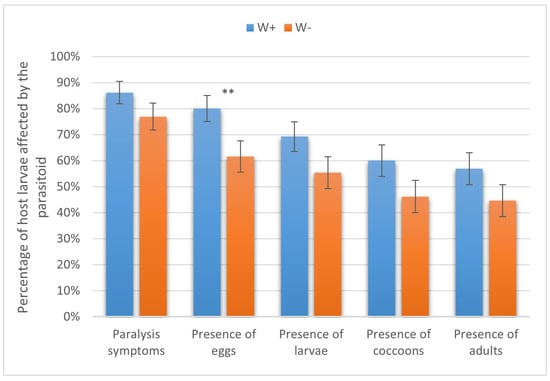
Figure 3.
Parasitism success of Wolbachia-positive (W+) and Wolbachia-negative (W−) lines of Habrobracon hebetor on larvae of the greater wax moth Galleria mellonella. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.05 (**).
In the beet webworm, 100% and 80.00 ± 8.94% of the larvae were paralyzed by W+ and W−, respectively (p = 0.053, Fisher’s test). Eggs of W+ and W− were observed on 85.00 ± 7.89% and 70.00 ± 10.25% of the paralyzed larvae (p = 0.16, Fisher’s test); parasitoid larvae developed on 60.00 ± 10.95% and 50.00 ± 11.18% of the host larvae, respectively (χ2 = 0.1, p > 0.05). Cocoons were formed in 55.00 ± 11.12% and 50.00 ± 11.18% of cases, and W+ and W− adults emerged in 50.00 ± 11.18% and 45.00 ± 11.12% of cases, respectively (χ2 = 0, p > 0.05). Hence, no statistical differences were found in any of these parasitism stages (Figure 4).
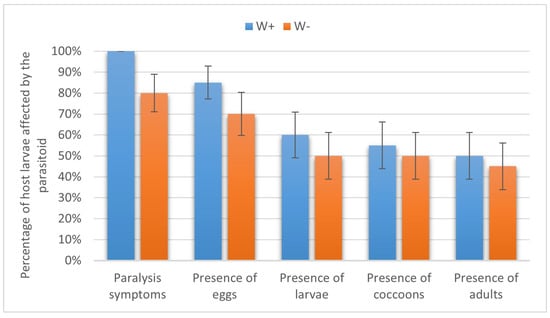
Figure 4.
Parasitism success of Habrobracon hebetor on larvae of the beet webworm Loxostege sticticalis. Indications as in Figure 3.
In the European corn borer, W+ paralyzed 92.37 ± 2.44% of the host larvae, eggs were laid on 83.90 ± 3.38% of the larvae, and parasitoid larvae, cocoons, and adults were recorded in 72.03 ± 4.13%, 55.93 ± 4.57%, and 46.61 ± 4.59%, respectively. As for W−, 69.49 ± 4.24%, 64.41 ± 4.41%, 53.39 ± 4.59%, 34.75 ± 4.38%, and 32.20 ± 4.30% of cases showed host paralysis, oviposition, larval development, cocoon formation, and adult emergence, respectively. The difference in the percentage of the host larvae on which the parasitoid adults emerged (χ2 = 18.6) was statistically significant at p < 0.05 and, for the other indices (eggs, χ2 = 11.7; larvae, χ2 = 8.8; cocoons, χ2 = 10.7; and adults, χ2 = 18.6), at p < 0.01 (Figure 5).
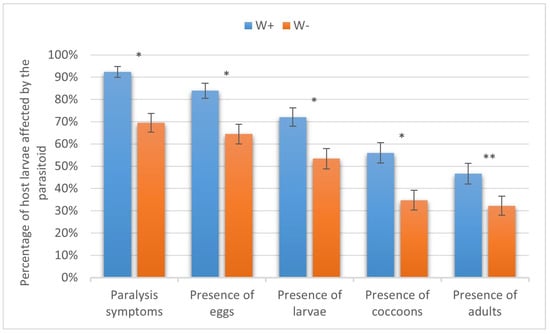
Figure 5.
Parasitism success of Habrobracon hebetor on larvae of the European corn borer Ostrinia nubilalis. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.01 (*) or p < 0.05 (**).
As many as 89.19 ± 5.10% and 83.78 ± 6.06% of the cabbage moth larvae were paralyzed by W+ and W− (p = 0.21, Fisher’s exact test), and eggs were laid on 86.49 ± 5.62% and 70.27 ± 7.51% of the larvae (χ2 = 2.0, p > 0.05), respectively. Differences in these instances were not statistically significant. On the other hand, there were significant differences between W+ and W− in the levels of host larvae on which parasitoid larvae (81.08 ± 6.44% vs. 48.65 ± 7.05%, χ2 = 7.2, p < 0.01), cocoons (59.46 ± 8.07% vs. 35.14 ± 7.85%, χ2 = 4.4, p < 0.05), and adults (59.46 ± 8.07% vs. 35.14 ± 7.85%, χ2 = 4.4, p < 0.05) developed (Figure 6).
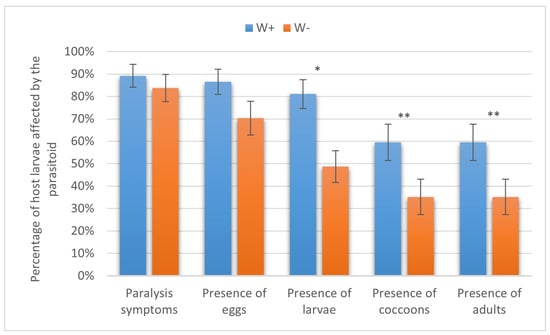
Figure 6.
Parasitism success of Habrobracon hebetor on larvae of the cabbage moth Mamestra brassicae. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.01 (*) or p < 0.05 (**).
In the silkworm, H. hebetor could not complete its life cycle, as the paralyzed host larvae decayed. However, the parasitism levels were about twice as high in W+ than in W− in the initial steps, and the differences were significant at p < 0.01. This included host paralysis (97.50 ± 2.47% vs. 60.00 ± 7.75%, Fisher’s exact text), oviposition (90.00 ± 4.74% vs. 37.50 ± 7.65%, Fisher’s test), and larval development (80.00 ± 6.32% vs. 32.50 ± 7.41%, χ2 = 16.5) (Figure 7).
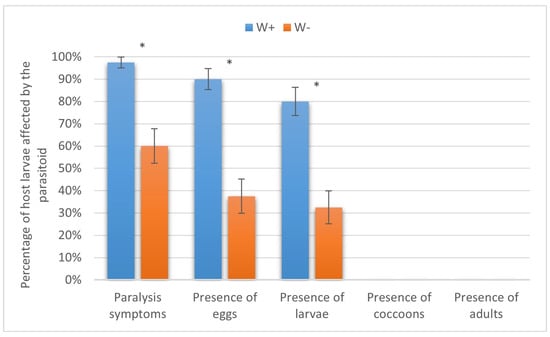
Figure 7.
Parasitism success of Habrobracon hebetor on larvae of the silkworm Bombyx mori. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.01 (*).
Similarly, the larvae of the diamondback moth were not suitable for complete parasitoid development due to their small size. Nevertheless, the parasitism levels in W+ vs. W− at the stages of paralysis (80.00 ± 7.30% vs. 16.67 ± 6.80%) and oviposition (43.33 ± 8.80% vs. 13.33 ± 6.21%) were significantly different (p < 0.01). As for the presence of larvae and cocoons, both indices equaled 10.00 ± 5.48% in W+ and 6.67 ± 4.55% in W− (p = 0.32, Fisher’s test), being not significantly different (Figure 8).
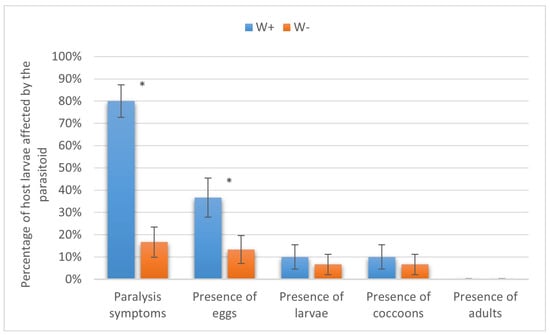
Figure 8.
Parasitism success of Habrobracon hebetor on larvae of the diamondback moth Plutella xylostella. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.01 (*).
4. Discussion
Habrobracon hebetor is an important biocontrol agent utilized against lepidopteran pests of different taxa []. It is routinely accepted that the number of susceptible hosts of H. hebetor is quite high, though modern studies exploring the host range of H. hebetor in detail are scarce []. The host checklists generated for this parasitoid in the past century may be inaccurate, as cryptic species with different biological characteristics may have been encountered []. It is, therefore, important to collect and update the information on the host range of particular species and isolates of parasitoids and to uncover the key factors regulating their efficiency.
Multilocus sequence typing is a powerful tool for the precise delineation of Wolbachia supergroups and the discrimination of closely related genotypes. This helps in understanding how the major endosymbiont–host patterns are distributed among the phylogenetic lineages of the bacterium. Unfortunately, many studies in the past only relied upon a limited set of markers. For example, the effects of infection with a Wolbachia strain were examined in Hypolimnas bolina (L.), showing the male-killing trait in this nymphalid host, and two genetic loci were sequenced, namely ftsZ and wsp []. However, comparison of the available MLST profiles of Wolbachia from Lepidoptera displayed the presence of four different sequence types of Wolbachia in H. bolina, two of which, ST-125 and ST-148, are not distinguishable using only ftsZ and wsp (see Table 1). Therefore, it cannot be determined which sequence type was investigated in that study. Hence, accumulation of genomic and MLST data is essential for future research, especially given that the same sequence types (such as ST-125, found in the present study) may be present in phylogenetically distant, though ecologically related, hosts, as exemplified by the braconid parasitoid (H. hebetor) and its potential lepidopteran hosts (Talicada nyseus (Guerin), S. exempta, and H. bolina).
In the present work, six species of lepidopteran insects turned out to be susceptible to attacks by H. hebetor. The greater wax moth is not only a standard pest for laboratory cultivation and large-scale production of the parasitoid [,,] but also a target pest of bee hives and stored products, which can be controlled using H. hebetor. Its performance in this host is well studied [,]. The beet webworm is a dangerous pest for multiple agricultural crops, with eruptive population dynamics and high migratory activity []. Our search for records of the beet webworm as a host for H. hebetor was not successful. However, congeneric species, such as H. nigricans Szepligeti [] and H. concolorans Marshal [], are listed among the parasitoids of this notorious pest. Another crambid moth, the European corn borer, is minacious to maize and some other crops. It is known for its concealed larval development, making entomophagous insects with active host-seeking behavior an essential solution for biocontrol [,,]. It is a recognized host for parasitoid wasps, such as H. hebetor and H. brevicornis []. The former is capable of causing a population depression of O. nubilalis under natural conditions []. As for the cabbage moth, the dangerous pest of vegetables [,,], papers on parasitism by H. hebetor were not found. Nevertheless, our observations correspond to reports describing its interactions with and biocontrol applications against other important noctuid pests, such as the cotton bollworm, the tobacco cutworm, and the tobacco budworm [,,].
The diamondback moth turned out to be an unsuitable host for H. hebetor development. Similarly, only a single adult of the congeneric parasitoid H. brevicornis Wesmael completed development on the fourth instar larva of P. xylostella during observation from May to July in South Africa, though other parasitoids were abundant []. However, our laboratory assay showed high levels of paralysis, indicating that H. hebetor could be an effective biocontrol agent against this pest in the field. As for the silkworm, though this domesticated insect exploited in sericulture is not a target for biocontrol of harmful arthropods, it might be a promising laboratory model for various applications [,,] due to its large body size and established technologies of mass production. Under the conditions of individual rearing, the rate of host larvae with symptoms of paralysis reached almost 100%, indicating their high susceptibility to venom injected by this minute wasp. Meanwhile, the individual venom portion was not sufficient to block the decay processes in the huge larvae of this host species.
Lowered values of parasitism indices in the parasitoid deprived of naturally occurring Wolbachia compared to the wild type suggest a positive effect of this endosymbiont on H. hebetor performance. Since the sequence type of the endosymbiont identified herein demonstrates attribution to the clade of predominantly lepidopteran isolates, it is logical to assume that Wolbachia was acquired by hymenopteran parasitoids from their lepidopteran hosts. This is consistent with the concept of horizontal transmission of Wolbachia to predatory and parasitic insects from their victims/hosts []. The acquisition of an endosymbiont with a positive influence benefits species survival and favors the evolutionary establishment of new symbiotic systems.
The results of the present study have two important outcomes. First, enhanced levels of successful parasitism imply better efficiency of the biocontrol agent employed against a broad range of lepidopteran pests of stored products and agricultural crops. Interestingly, the most prominent differences in the paralysis proportions between W+ and W− were seen in two hosts unsuitable for complete development of the parasitoid, namely, the diamondback moth and the silkworm (Figure 9). Second, the increased number of cases with successful completion of the life cycle also makes the Wolbachia-positive H. hebetor culture more appropriate for different biocontrol release programs, as it extends the range of suitable hosts both in large-scale production and in the field for augmentative and introductory releases (Figure 10).
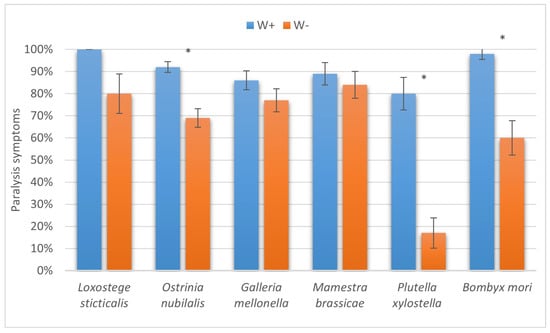
Figure 9.
The proportion of larvae of six species of lepidopteran hosts with paralysis symptoms due to attack from Habrobracon hebetor. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.01 (*).
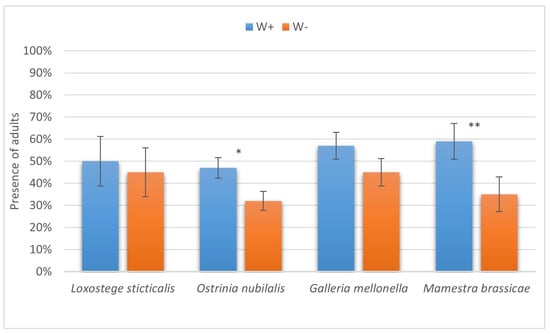
Figure 10.
The proportion of larvae of six species of lepidopteran hosts on which Habrobracon hebetor development was completed till the adult stage. Whiskers show standard errors. Asterisks denote values showing significant differences at p < 0.01 (*) or p < 0.05 (**).
In addition to the simple discovery of phenomena like those described in the present study, it is very important to uncover the specific mechanisms underlying these interactions. The endosymbiont–parasitoid–host systems are complex biological assemblages, and many of their intrinsic processes are unclear. We find it logical to suppose that cultivation of the parasitoid for more than 20 generations after the antibiotic treatment was discontinued should result in stabilization of the intestinal microbial community. We have shown before [] that after the eradication of Wolbachia from larvae of H. hebetor, gut bacteria of the genera Enterococcus and Pseudomonas, as well as unclassified Enterobacteriaceae, become prevalent. These bacteria are seemingly acquired by the parasitoid from the host cuticle and haemolymph, as Enterococcus is dominant in Galleria mellonella, while different Enterobacteriaceae and Pseudomonas spp. may also be (sub)dominant in the host coelom [,]. Similarly, other studies [,] indicated that the microbiome of parasitoids was formed by bacteria originating from their hosts. These components of the bacterial community may significantly contribute to the fitness of parasitic wasps [,]. The effects of Wolbachia removal on parasitoid survival can be both direct, due to regulation of the metabolism of amino acids, lipids, sugars, vitamins, and cofactors [,], and indirect, because of the microbiome rearrangement, which should also influence the fitness of the host, its resilience to pathogens, and food digestion.
Moreover, Wolbachia infection may influence the synthesis of neurohormones and neuromodulators [], and this phenomenon might be connected with behavior modulation and possibly the search for new host species. Overall, Wolbachia may positively contribute to the population dynamics of H. hebetor, as it increases the number of host larvae on which it can develop. The presence of such an endosymbiont may become an evolutionary advantage, increasing the chances of survival of the parasitoid.
Another important question arises from the observations herein, namely, why the drastic differences in the parasitism levels of W+ vs. W− parasitoids are markedly displayed only in some of the host species and not in the others. The interactions of parasitoids with their hosts are complex, and the presence of endosymbionts evidently adds to this complexity. The laboratory models provided in the present study allow for further examination of these phenomena and related mechanisms. Interestingly, the most marked differences in parasitism success parameters are found in two insect species in which H. hebetor could not complete its development—B. mori and P. xylostella, i.e., the most unsuitable hosts. It can be supposed that under unfavorable circumstances, the positive effects of Wolbachia are more prominent. Similarly, a more noticeable influence of Wolbachia on host-cell metabolite profiles was demonstrated under conditions of heat stress [].
5. Conclusions
The presence of Wolbachia belonging to the supergroup B, sequence type 125, is beneficial for H. hebetor efficiency as a parasitoid of lepidopteran pests and may have important consequences for its survival and field efficacy. We recommend checking the endosymbiont status in Habrobracon cultures considered for practical usage.
Author Contributions
Conceptualization, Y.S.T. and N.A.K.; methodology, J.M.M., N.A.K. and E.A.C.; validation, Y.S.T. and N.A.K.; investigation, A.M.U. and J.M.M.; resources, Y.S.T. and E.A.C.; data curation, J.M.M., A.M.U. and Y.S.T.; writing—original draft preparation, A.M.U. and Y.S.T.; writing—review and editing, Y.S.T., J.M.M. and N.A.K.; visualization, J.M.M. and A.M.U.; supervision, Y.S.T.; project administration, Y.S.T.; funding acquisition, Y.S.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research is supported by the Russian Science Foundation grant #23-16-00262.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The Authors are indebted to Ken Smith, (Santo Domingo, Dominican Republic), for English grammar and style correction of the manuscript. Molecular genetic identification was performed using the equipment of the Core Centrum “Innovative plant protection technologies” at the All-Russian Institute of Plant Protection (St. Petersburg, Russia).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hajek, A.E.; Gardescu, S.; Delalibera, I. Summary of classical biological control introductions of entomopathogens and nematodes for insect control. BioControl 2021, 66, 167–180. [Google Scholar] [CrossRef]
- Cusumano, A.; Fatouros, N. Editorial overview: Parasites/parasitoids/biological control (2023)—Understanding parasitoid ecology and evolution to advance biological control programs. Curr. Opin. Insect Sci. 2023, 58, 101050. [Google Scholar] [CrossRef]
- Saabna, N.; Keasar, T. Parasitoids for biological control in dryland agroecosystems. Curr. Opin. Insect Sci. 2024, 64, 101226. [Google Scholar] [CrossRef]
- Zhu, H.; Kim, J.J. Target-oriented dissemination of Beauveria bassiana conidia by the predators, Harmonia axyridis (Coleoptera: Coccinellidae) and Chrysoperla carnea (Neuroptera: Chrysopidae) for biocontrol of Myzus persicae. Biocontrol Sci. Technol. 2012, 22, 393–406. [Google Scholar] [CrossRef]
- Dwivedi, S.A.; Sonawane, V.K.; Pandit, T.R. Review on the impact of insecticides utilization in crop ecosystem: Their prosperity and threats. In Insecticidesi; Ranz, R., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Cross, P. Pesticide hazard trends in orchard fruit production in Great Britain from 1992 to 2008: A time-series analysis. Pest Manag. Sci. 2013, 69, 768–774. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Cusumano, A.; Bin, F.; Polaszek, A.; van Lenteren, J.C. How to escape from insect egg parasitoids: A review of potential factors explaining parasitoid absence across the Insecta. Proc. R. Soc. B 2020, 287, 20200344. [Google Scholar] [CrossRef]
- Litwin, A.; Nowak, M.; Różalska, S. Entomopathogenic fungi: Unconventional applications. Rev. Environ. Sci. Biotechnol. 2020, 19, 23–42. [Google Scholar] [CrossRef]
- Hatcher, M.J.; Dick, J.T.A.; Dunn, A.M. Parasites that change predator or prey behavior can have keystone effects on community composition. Biol. Lett. 2014, 10, 20130879. [Google Scholar] [CrossRef]
- Thilagam, P.; Sharanappa, C.H.; Roy, S.; Deb, L.; Padhan, S.; Srividhya, S.; Awadhiya, P. A review on advances in biocontrol techniques for managing insect pests in sustainable agriculture. Int. J. Environ. 2023, 13, 2114–2125. [Google Scholar] [CrossRef]
- Vinson, S.B.; Iwantsch, G.F. Host regulation by insect parasitoids. Q. Rev. Biol. 1980, 55, 143–165. [Google Scholar] [CrossRef]
- Polaszek, A.; Vilhelmsen, L. Biodiversity of hymenopteran parasitoids. Curr. Opin. Insect Sci. 2023, 56, 101026. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Cusumano, A.; Poelman, E.H. Microbial symbionts of parasitoids. Annu. Rev. Entomol. 2020, 65, 171–190. [Google Scholar] [CrossRef]
- Verhulst, E.C.; Pannebakker, B.A.; Geuverink, E. Variation in sex determination mechanisms may constrain parthenogenesis-induction by endosymbionts in haplodiploid systems. Curr. Opin. Insect Sci. 2023, 56, 101023. [Google Scholar] [CrossRef]
- Gibson, C.M.; Hunter, M.S. Inherited fungal and bacterial endosymbionts of a parasitic wasp and its cockroach host. Microb. Ecol. 2009, 57, 542–549. [Google Scholar] [CrossRef]
- Pekas, A.; Tena, A.; Peri, E.; Colazza, S.; Cusumano, A. Competitive interactions in insect parasitoids: Effects of microbial symbionts across tritrophic levels. Curr. Opin. Insect Sci. 2023, 55, 101001. [Google Scholar] [CrossRef]
- Deepak, C.; Patel, H.C.; Patel, H.K. Microbial symbionts of hymenopteran parasitoids: An effective tool for next-generation crop protection. Symbiosis 2024, 93, 153–162. [Google Scholar] [CrossRef]
- Huigens, M.E.; Hohmann, C.L.; Luck, R.F.; Gort, G.; Stouthamer, R. Reduced competitive ability due to Wolbachia infection in the parasitoid wasp Trichogramma kaykai. Entomol. Exp. Appl. 2004, 112, 115–123. [Google Scholar] [CrossRef]
- Ramírez-Puebla, S.T.; Servín-Garcidueñas, L.E.; Ormeño-Orrillo, E.; de León, A.V.P.; Rosenblueth, M.; Delaye, L.; Martínez-Romero, E. Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’, ‘Candidatus Wolbachia collembolicola’, and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups. Syst. Appl. Microbiol. 2015, 38, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.R.; Bordenstein, S.R.; Newton, I.L.; Rasgon, J.L. Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al., “Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’, ‘Candidatus Wolbachia collembolicola’ and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups”. Syst. Appl. Microbiol. 2016, 39, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Beliavskaia, A.; Tan, K.K.; Sinha, A.; Husin, N.A.; Lim, F.S.; Loong, S.K.; Bell-Sakyi, L.; Carlow, C.K.; Abubakar, S.; Darby, A.C.; et al. Metagenomics of culture isolates and insect tissue illuminate the evolution of Wolbachia, Rickettsia and Bartonella symbionts in Ctenocephalides spp. fleas. Microb. Genom. 2023, 9, 001045. [Google Scholar] [CrossRef] [PubMed]
- Sinkins, S.P.; Braig, H.R.; O’Neill, S.L. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. R. Soc. Lond. B Biol. Sci. 1995, 261, 325–330. [Google Scholar] [CrossRef]
- Sinkins, S.P. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 723–729. [Google Scholar] [CrossRef]
- Ilinsky, Y.Y.; Zakharov, I.K. Cytoplasmic incompatibility in Drosophila melanogaster is caused by different Wolbachia genotypes. Russ. J. Genet. Appl. Res. 2011, 1, 458–462. [Google Scholar] [CrossRef]
- Sicard, M.; Bonneau, M.; Weill, M. Wolbachia prevalence, diversity, and ability to induce cytoplasmic incompatibility in mosquitoes. Curr. Opin. Insect Sci. 2019, 34, 12–20. [Google Scholar] [CrossRef]
- Tulgetske, G.M.; Stouthamer, R. Characterization of intersex production in Trichogramma kaykai infected with parthenogenesis-inducing Wolbachia. Naturwissenschaften 2012, 99, 143–152. [Google Scholar] [CrossRef]
- Champion de Crespigny, F.E.; Wedell, N. Wolbachia infection reduces sperm competitive ability in an insect. Proc. R. Soc. B. 2006, 273, 1455–1458. [Google Scholar] [CrossRef]
- Richard, F.J. Symbiotic bacteria influence the odor and mating preference of their hosts. Front. Ecol. Evol. 2017, 5, 143. [Google Scholar] [CrossRef]
- Chen, M.Y.; Li, D.; Wang, Z.N.; Xu, F.Z.; Feng, Y.W.; Yu, Q.L.; Wang, Y.Y.; Zhang, S.; Wang, Y.F. Infection by virulent wMelPop Wolbachia improves learning and memory capacity in Drosophila melanogaster. Anim. Behav. 2024, 212, 101–112. [Google Scholar] [CrossRef]
- Wangkeeree, J.; Suwanchaisri, K.; Roddee, J.; Hanboonsong, Y. Effect of Wolbachia infection states on the life history and reproductive traits of the leafhopper Yamatotettix flavovittatus Matsumura. J. Invertebr. Pathol. 2020, 177, 107490. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ji, R.; Zi, H.; Sun, W.; Zhang, Y.; Wu, X.; Yang, Y. Life history parameters of Ectropis grisescens (Lepidoptera: Geometridae) in different Wolbachia infection states. J. Econ. Entomol. 2024, 117, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, D.Y.; Goryacheva, I.I.; Andrianov, B.V.; Reznik, N.L.; Lazebny, O.E.; Kulikov, A.M. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Russ. J. Genet. 2007, 43, 1066–1069. [Google Scholar] [CrossRef]
- Glaser, R.L.; Meola, M.A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zhang, Y.Y.; Wang, X.Y.; Yin, Y.; Du, Y.Z. Wolbachia modify host cell metabolite profiles in response to short-term temperature stress. Environ. Microbiol. Rep. 2024, 16, e70013. [Google Scholar] [CrossRef]
- Hertig, M.; Wolbach, S.B. Studies on Rickettsia-like microorganisms in insects. J. Med. Res. 1924, 44, 329–374. [Google Scholar]
- Zug, R.; Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 2015, 90, 89–111. [Google Scholar] [CrossRef]
- Ramalho, M.D.O.; Kim, Z.; Wang, S.; Moreau, C.S. Wolbachia across social insects: Patterns and Implications. Ann. Entomol. Soc. Am. 2021, 114, 206–218. [Google Scholar] [CrossRef]
- Sanaei, E.; Charlat, S.; Engelstädter, J. Wolbachia host shifts: Routes, mechanisms, constraints and evolutionary consequences. Biol. Rev. 2021, 96, 433–453. [Google Scholar] [CrossRef]
- Henry, L.P.; Fernandez, M.; Wolf, S.; Abhyankar, V.; Ayroles, J.F. Wolbachia impacts microbiome diversity and fitness-associated traits for Drosophila melanogaster in a seasonally fluctuating environment. Ecol. Evol. 2024, 14, e70004. [Google Scholar] [CrossRef] [PubMed]
- Negri, I.; Pellecchia, M.; Dubey, R. Sex steroids in insects and the role of the endosymbiont Wolbachia: A new perspective. In Sex Hormones; Dubey, R., Ed.; InTech: London, UK, 2012; pp. 353–374. [Google Scholar]
- Kaur, R.; Meier, C.J.; McGraw, E.A.; Hillyer, J.F.; Bordenstein, S.R. The mechanism of cytoplasmic incompatibility is conserved in Wolbachia-infected Aedes aegypti mosquitoes deployed for arbovirus control. PLoS Biol. 2024, 22, e3002573. [Google Scholar] [CrossRef]
- Lambrechts, L.; Ferguson, N.M.; Harris, E.; Holmes, E.C.; McGraw, E.A.; O’Neill, S.L.; Ooi, E.E.; Ritchie, S.A.; Ryan, P.A.; Scott, T.W.; et al. Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect. Dis. 2015, 15, 862–866. [Google Scholar] [CrossRef]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef]
- Turner, J.D.; Marriott, A.E.; Hong, D.; O’Neill, P.; Ward, S.A.; Taylor, M.J. Novel anti-Wolbachia drugs, a new approach in the treatment and prevention of veterinary filariasis? Vet. Parasitol. 2020, 279, 109057. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Hong, W.D.; Turner, J.D.; O’Neill, P.M.; Ward, S.A.; Taylor, M.J. Anti-Wolbachia drugs for filariasis. Trends Parasitol. 2021, 37, 1068–1081. [Google Scholar] [CrossRef]
- Gong, J.T.; Li, T.P.; Wang, M.K.; Hong, X.Y. Wolbachia-based strategies for control of agricultural pests. Curr. Opin. Insect Sci. 2023, 57, 101039. [Google Scholar] [CrossRef]
- Hyder, M.; Lodhi, A.M.; Wang, Z.; Bukero, A.; Gao, J.; Mao, R. Wolbachia interactions with diverse insect hosts: From reproductive modulations to sustainable pest management strategies. Biology 2024, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Borges, R.M. Oviposition decisions under environment-induced physiological stress in parasitoids. Curr. Opin. Insect Sci. 2024, 65, 101240. [Google Scholar] [CrossRef]
- Bagheri, Z.; Talebi, A.A.; Asgari, S.; Mehrabadi, M. Wolbachia induce cytoplasmic incompatibility and affect mate preference in Habrobracon hebetor to increase the chance of its transmission to the next generation. J. Invertebr. Pathol. 2019, 163, 1–7. [Google Scholar] [CrossRef]
- Nasehi, S.F.; Fathipour, Y.; Mehrabadi, M. Wolbachia and cytoplasmic incompatibility in Habrobracon hebetor (Hym.: Braconidae). Plant Pest Res. 2021, 11, 53–66. [Google Scholar] [CrossRef]
- Bagheri, Z.; Talebi, A.A.; Asgari, S.; Mehrabadi, M. Wolbachia promotes successful sex with siblings in the parasitoid Habrobracon hebetor. Pest Manag. Sci. 2022, 78, 362–368. [Google Scholar] [CrossRef]
- Kryukova, N.A.; Kryukov, V.Y.; Polenogova, O.V.; Chertkova, E.A.; Tyurin, M.V.; Rotskaya, U.N.; Alikina, T.; Kabilov, M.R.; Glupov, V.V. The endosymbiotic bacterium Wolbachia (Rickettsiales) alters larval metabolism of the parasitoid Habrobracon hebetor (Hymenoptera: Braconidae). Arch. Insect Biochem. Physiol. 2023, 114, e22053. [Google Scholar] [CrossRef]
- Jiménez, N.E.; Gerdtzen, Z.P.; Olivera-Nappa, Á.; Salgado, J.C.; Conca, C. A systems biology approach for studying Wolbachia metabolism reveals points of interaction with its host in the context of arboviral infection. PLoS Negl. Trop. Dis. 2019, 13, e0007678. [Google Scholar] [CrossRef]
- Zhang, H.B.; Cao, Z.; Qiao, J.X.; Zhong, Z.Q.; Pan, C.C.; Liu, C.; Zhang, L.M.; Wang, Y.F. Metabolomics provide new insights into mechanisms of Wolbachia-induced paternal defects in Drosophila melanogaster. PLoS Pathog. 2021, 17, e1009859. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.N.; Berim, M.N.; Grushevaya, I.V. Rearing of trilobed male uncus Ostrinia species in laboratory for experimental purposes. Plant Prot. News 2019, 3, 58–62. [Google Scholar] [CrossRef]
- Zagorinskii, A.A.; Gorbunov, O.G.; Sidorov, A.V. An experience of rearing some hawk moths (Lepidoptera, Sphingidae) on artificial diets. Entomol. Rev. 2013, 93, 1107–1115. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; Volume 3, pp. 154–196. [Google Scholar]
- Baldo, L.; Dunning Hotopp, J.C.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef]
- Vogelstein, B.; Gillespie, D. Preparative and analytical purification of DNA from agarose. Proc. Natl. Acad. Sci. USA 1979, 76, 615–619. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Hall, T.A. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Pearson, K.X. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Phil. Mag. Ser 5 1900, 50, 157–175. [Google Scholar] [CrossRef]
- Yates, F. Contingency table involving small numbers and the χ2 test. Suppl. J. Roy. Stat. Soc. 1934, 1, 217–235. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver Boyd: Edinburgh, UK, 1954. [Google Scholar]
- Saadat, D.; Bandani, A.R.; Dastranj, M. Comparison of the developmental time of Bracon hebetor (Hymenoptera: Braconidae) reared on five different lepidopteran host species and its relationship with digestive enzymes. Eur. J. Entomol. 2014, 111, 495. [Google Scholar] [CrossRef]
- Ghimire, M.N.; Phillips, T.W. Suitability of different Lepidopteran host species for development of Bracon hebetor (Hymenoptera: Braconidae). Environ. Entomol. 2010, 39, 449–458. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Antolin, M.F.; Franqui, R.A.; Strand, M.R. Reproductive isolation and genetic variation between two “strains” of Bracon hebetor (Hymenoptera: Braconidae). Biol. Control 1997, 9, 149–156. [Google Scholar] [CrossRef]
- Dyson, E.; Kamath, M.; Hurst, G. Wolbachia infection associated with all-female broods in Hypolimnas bolina (Lepidoptera: Nymphalidae): Evidence for horizontal transmission of a butterfly male killer. Heredity 2002, 88, 166–171. [Google Scholar] [CrossRef]
- Solà, M.; Castañé, C.; Lucas, E.; Riudavets, J. Optimization of a banker box system to rear and release the parasitoid Habrobracon hebetor (Hymenoptera: Braconidae) for the control of stored-product moths. J. Econ. Entomol. 2018, 111, 2461–2466. [Google Scholar] [CrossRef]
- Hasan, M.M.; Yeasmin, L.; Athanassiou, C.G.; Bari, M.A.; Islam, M.S. Using gamma irradiated Galleria mellonella L. and Plodia interpunctella (Hübner) larvae to optimize mass rearing of parasitoid Habrobracon hebetor (Say) (Hymenoptera: Braconidae). Insects 2019, 10, 223. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I. (Eds.) Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Adly, D.; Marzouk, W.M. Efficacy of the larval parasitoid, Bracon hebetor Say. (Hymenoptera: Braconidae) on the greater wax moth larvae, Galleria mellonella (L.) (Lepidoptera: Pyralidae) under laboratory and field conditions. Egypt J. Biol. Pest Control 2019, 29, 87. [Google Scholar] [CrossRef]
- Alam, M.S.; Alam, M.Z.; Alam, S.N.; Miah, M.R.U.; Mian, M.I.H.; Hossain, M.M. Biology of Bracon hebetor reared on wax moth (Galleria mellonella) larvae. Persian Gulf Crop Prot. 2014, 3, 54–62. [Google Scholar]
- Chen, X.; Zhai, B.; Gong, R.; Yin, M.; Zhang, Y.; Zhao, K. Source area of spring population of meadow moth, Loxostege sticticalis L. (Lepidoptera: Pyralidae), in Northeast China. Acta Ecol. Sin. 2008, 28, 1521–1535. [Google Scholar] [CrossRef]
- Beyarslan, A. Checklist of Braconinae species of Turkey (Hymenoptera: Braconidae). Zootaxa 2014, 3790, 201–242. [Google Scholar] [CrossRef]
- Loni, A.; Samartsev, K.G.; Scaramozzino, P.L.; Belokobylskij, S.A.; Lucchi, A. Braconinae parasitoids (Hymenoptera, Braconidae) emerged from larvae of Lobesia botrana (Denis & Schiffermüller) (Lepidoptera, Tortricidae) feeding on Daphne gnidium L. ZooKeys 2016, 587, 125. [Google Scholar] [CrossRef]
- Camerini, G.; Maini, S.; Riedel, M. Ostrinia nubilalis parasitoids in Northern Italy: Past and present. Biol. Control 2018, 122, 76–83. [Google Scholar] [CrossRef]
- Mahdavi, V.; Saber, M.; Rafiee-Dastjerdi, H.; Mehrvar, A. Comparative study of the population level effects of carbaryl and abamectin on larval ectoparasitoid Habrobracon hebetor Say (Hymenoptera: Braconidae). BioControl 2011, 56, 823–830. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, S.; Lyu, B.; Wu, X.; Lu, H.; Tang, J.; Su, H. Influence of temperature on the development and reproduction of Habrobracon hebetor (Say), as parasitoid of Opisina arenosella Walker. Int. J. Pest Manag. 2022, 70, 1445–1452. [Google Scholar] [CrossRef]
- Lettmann, J.; Mody, K.; Kursch-Metz, T.; Blüthgen, N.; Wehner, K. Bracon wasps for ecological pest control–a laboratory experiment. PeerJ 2021, 9, e11540. [Google Scholar] [CrossRef]
- Frolov, A.N. Biotic factors suppressing the European corn borer, Ostrinia nubilalis. Plant Prot. News 2004, 2, 37–47. [Google Scholar]
- Jankowska, B. The occurrence of some Lepidoptera pests on different cabbage vegetables. J. Plant Prot. Res. 2006, 46, 181–190. [Google Scholar]
- Cartea, M.E.; Padilla, G.; Vilar, M.; Velasco, P. Incidence of the major Brassica pests in Northwestern Spain. J. Econ. Entomol. 2009, 102, 767–773. [Google Scholar] [CrossRef]
- Cartea, M.E.; Soengas, P.; Sotelo, T.; Abilleira, R.; Velasco, P. Determining the host-plant resistance mechanisms for Mamestra brassicae (Lepidoptera: Noctuidae) pest in cabbage. Ann. Appl. Biol. 2014, 164, 270–285. [Google Scholar] [CrossRef][Green Version]
- Nikam, P.K.; Pawar, C.V. Life tables and intrinsic rate of increase of Bracon hebetor Say (Hym., Braconidae) population on Corcyra cephalonica Staint. (Lep., Pyralidae), a key parasitoid of Helicoverpa armigera Hbn. (Lep., Noctuidae). J. Appl. Entomol. 1993, 115, 210–213. [Google Scholar] [CrossRef]
- Borzoui, E.; Naseri, B.; Mohammadzadeh-Bidarani, M. Adaptation of Habrobracon hebetor (Hymenoptera: Braconidae) to rearing on Ephestia kuehniella (Lepidoptera: Pyralidae) and Helicoverpa armigera (Lepidoptera: Noctuidae). J. Insect Sci. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Kfir, R. Parasitoids of Plutella xylostella (Lep.: Plutellidae) in South Africa: An annotated list. BioControl 1997, 42, 517–523. [Google Scholar] [CrossRef]
- Madyarov, S.R.; Mirzaeva, G.S.; Otarbaev, D.O.; Khamidi, K.S.; Kamilova, S.I.; Akhmerov, R.N.; Khamraev, A.S. Mulberry silkworm, Bombyx mori L., as a host for neurotoxic Braconidae I. Insect-toxic properties of Bracon venom gland extract and its fractions. Int. J. Ind. Entomol. Biomater. 2003, 7, 235–239. [Google Scholar]
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A promising model organism in life science. J. Insect Sci. 2017, 17, 97. [Google Scholar] [CrossRef]
- Abdelli, N.; Peng, L.; Keping, C. Silkworm, Bombyx mori, as an alternative model organism in toxicological research. Environ. Sci. Pollut. Res. 2018, 25, 35048–35054. [Google Scholar] [CrossRef]
- Allonsius, C.N.; Van Beeck, W.; De Boeck, I.; Wittouck, S.; Lebeer, S. The microbiome of the invertebrate model host Galleria mellonella is dominated by Enterococcus. Anim. Microbiome 2019, 1, 7. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Chernyak, E.I.; Kryukova, N.; Tyurin, M.; Krivopalov, A.; Yaroslavtseva, O.; Senderskiy, I.; Polenogova, O.; Zhirakovskaia, E.; Glupov, V.V.; et al. Parasitoid venom alters the lipid composition and development of microorganisms on the wax moth cuticle. Ent. Exp. Appl. 2022, 170, 852–868. [Google Scholar] [CrossRef]
- Duan, R.; Xu, H.; Gao, S.; Gao, Z.; Wang, N. Effects of different hosts on bacterial communities of parasitic wasp Nasonia vitripennis. Front. Microbiol. 2020, 11, 1435. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.X.; Zhuang, Y.H.; Wu, Y.X.; Huang, T.W.; Song, Z.R.; Du, Y.Z.; Zhu, Y.X. Wolbachia infection alters the microbiota of the invasive leaf-miner Liriomyza huidobrensis (Diptera: Agromyzidae). Microorganisms 2025, 13, 302. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.V.; de Roode, J.C.; Gerardo, N.M. Diet–microbiome–disease: Investigating diet’s influence on infectious disease resistance through alteration of the gut microbiome. PLoS Pathog. 2019, 15, e1007891. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, A.; Lievens, B. Microbe-mediated alterations in floral nectar: Consequences for insect parasitoids. Curr. Opin. Insect Sci. 2023, 60, 101116. [Google Scholar] [CrossRef]
- Gruntenko, N.Е.; Ilinsky, Y.Y.; Adonyeva, N.V.; Burdina, E.V.; Bykov, R.A.; Menshanov, P.N.; Rauschenbach, I.Y. Various Wolbachia genotypes differently influence host Drosophila dopamine metabolism and survival under heat stress conditions. BMC Evol. Biol. 2017, 17, 15–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).