Exploring the Role of mRNA Methylation in Insect Biology and Resistance
Simple Summary
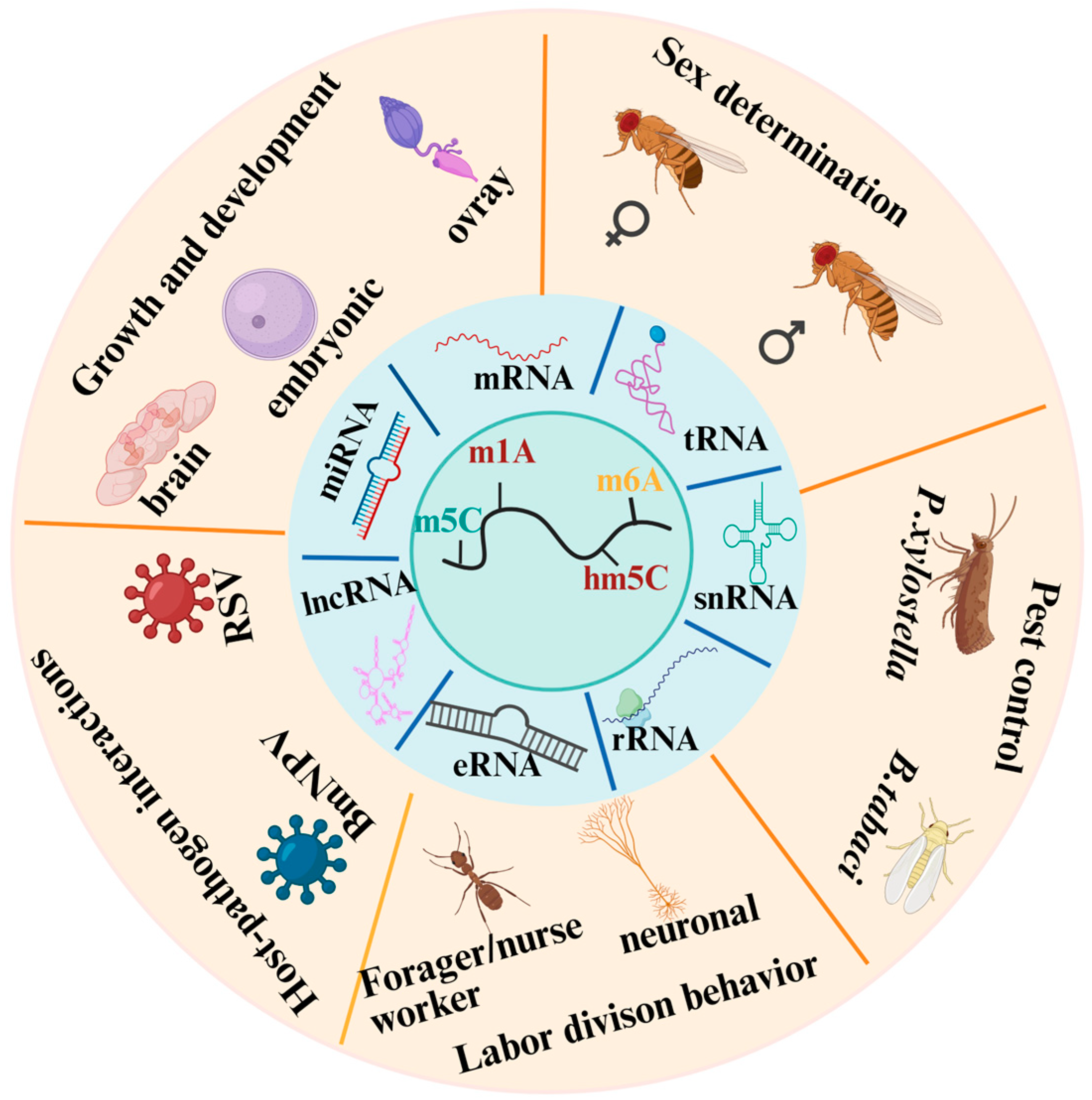
Abstract
1. Introduction
2. m6A Methylation in Insects
3. m5C Methylation in Insects
4. Other Types of RNA Methylation in Insects
5. Discussion and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| m6A | N6-methyladenosine |
| m5C | 5-methylcytosine |
| m1A | N1-methyladenosine |
| H. armigera | Helicoverpa armigera |
| B. mori | Bombyx mori |
| D. melanogaster | Drosophila melanogaster |
| T. molitor | Tenebrio molitor |
| JH | Juvenile hormone |
| P. xylostella | Plutella xylostella |
| L. striatellus | Laodelphax striatellus |
| S. frugiperda | Spodoptera frugiperda |
| B. tabaci | Bemisia tabaci |
| Dop1 | Dopamine receptor 1 |
| DAT | Dopamine transporter |
| NSUN | NOL1/NOP2/Sun domain |
| DNMT2 | DNA methyltransferase 2 |
| lncRNA | Non-coding RNAs |
| YPS | Ypsilon schachtel |
| YBX1 | Y box binding protein 1 |
| GSC | Germ line stem cell |
| PUS | Pseudouridine |
References
- Sendinc, E.; Shi, Y. RNA m6A methylation across the transcriptome. Mol. Cell 2023, 83, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.K.; Gao, C.C.; Yang, Y.G. Emerging Roles of RNA Methylation in Development. Accounts Chem. Res. 2023, 56, 3417–3427. [Google Scholar] [CrossRef]
- Chen, Y.H.; Jiang, T.; Yasen, A.; Fan, B.Y.; Zhu, J.; Wang, M.X.; Shen, X.J. RNA N6-methyladenosine of DHAPAT and PAP involves in regulation of diapause of Bombyx mori via the lipid metabolism pathway. Bull. Entomol. Res. 2023, 113, 665–675. [Google Scholar] [CrossRef]
- Liu, S.; Tian, H.; Xu, Y.; Wang, H. Juvenile hormone regulates silk gene expression by m6A RNA methylation. Cell. Mol. Life Sci. 2023, 80, 331. [Google Scholar] [CrossRef]
- Liu, J.; Huang, T.; Chen, W.; Ding, C.; Zhao, T.; Zhao, X.; Cai, B.; Zhang, Y.; Li, S.; Zhang, L.; et al. Developmental mRNA m5C landscape and regulatory innovations of massive m5C modification of maternal mRNAs in animals. Nat. Commun. 2022, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Hayashi, G.; Ueda, H.; Ota, S.; Nagae, G.; Aburatani, H.; Okamoto, A. Base-resolution analysis of 5-hydroxymethylcytidine by selective oxidation and reverse transcription arrest. Org. Biomol. Chem. 2021, 19, 6478–6486. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Kariko, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Oerum, S.; Degut, C.; Barraud, P.; Tisne, C. m1A Post-Transcriptional Modification in tRNAs. Biomolecules 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Podicheti, R.; Rusch, D.B.; Tracey, W.D. Transcriptome-wide analysis of pseudouridylation in Drosophila melanogaster. G3-Genes Genom. Genet. 2023, 13, jkac333. [Google Scholar] [CrossRef]
- Bataglia, L.; Simoes, Z.; Nunes, F. Active genic machinery for epigenetic RNA modifications in bees. Insect. Mol. Biol. 2021, 30, 566–579. [Google Scholar] [CrossRef]
- Jiao, Y.; Palli, S.R. N6-adenosine (m6A) mRNA methylation is required for Tribolium castaneum development and reproduction. Insect Biochem. Mol. 2023, 159, 103985. [Google Scholar] [CrossRef] [PubMed]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Jia, S.Z.; Tian, H.; Li, Y.H.; Hu, K.W.; Tao, J.G.; Lu, Y.C.; Xu, Y.S.; Wang, H.B. Evolution of m6A-related genes in insects and the function of METTL3 in silkworm embryonic development. Insect Mol. Biol. 2023, 32, 316–327. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, Y.; Li, Y.; Wang, X.; Qi, S.; Wang, Y.; Zhao, L.; Wang, K.; Peng, W.; Luo, G.Z.; et al. RNA m6A Modification Functions in Larval Development and Caste Differentiation in Honeybee (Apis mellifera). Cell Rep. 2021, 34, 108580. [Google Scholar] [CrossRef]
- Castro-Vargas, C.; Linares-Lopez, C.; Lopez-Torres, A.; Wrobel, K.; Torres-Guzman, J.C.; Hernandez, G.A.; Wrobel, K.; Lanz-Mendoza, H.; Contreras-Garduno, J. Methylation on RNA: A Potential Mechanism Related to Immune Priming within But Not across Generations. Front. Microbiol. 2017, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Piou, C.; Marescot, L. Spatiotemporal risk forecasting to improve locust management. Curr. Opin. Insect Sci. 2023, 56, 101024. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Romeis, J. Managing the Invasive Fall Armyworm through Biotech Crops: A Chinese Perspective. Trends Biotechnol. 2021, 39, 105–107. [Google Scholar] [CrossRef]
- Timani, K.; Bastarache, P.; Morin, P.J. Leveraging RNA Interference to Impact Insecticide Resistance in the Colorado Potato Beetle, Leptinotarsa decemlineata. Insects 2023, 14, 418. [Google Scholar] [CrossRef]
- Quan, Y.; Wu, K. Managing Practical Resistance of Lepidopteran Pests to Bt Cotton in China. Insects 2023, 14, 179. [Google Scholar] [CrossRef]
- Saavedra-Rodriguez, K.; Campbell, C.L.; Lozano, S.; Penilla-Navarro, P.; Lopez-Solis, A.; Solis-Santoyo, F.; Rodriguez, A.D.; Perera, R.; Black, I.W. Permethrin resistance in Aedes aegypti: Genomic variants that confer knockdown resistance, recovery, and death. PLoS Genet. 2021, 17, e1009606. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, Y.; Zheng, Q.; Zhang, J.; Li, H.; Wu, W. Epigenetic targeting of autophagy for cancer: DNA and RNA methylation. Front. Oncol. 2023, 13, 1290330. [Google Scholar] [CrossRef] [PubMed]
- Scholler, E.; Weichmann, F.; Treiber, T.; Ringle, S.; Treiber, N.; Flatley, A.; Feederle, R.; Bruckmann, A.; Meister, G. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA 2018, 24, 499–512. [Google Scholar] [CrossRef]
- Perlegos, A.E.; Shields, E.J.; Shen, H.; Liu, K.F.; Bonini, N.M. Mettl3-dependent m6A modification attenuates the brain stress response in Drosophila. Nat. Commun. 2022, 13, 5387. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Asgari, S. ALKBH8 as a potential N6-methyladenosine (m6A) eraser in insects. Insect Mol. Biol. 2023, 32, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Hase, H.; Tanikawa, S.; Okawa, K.; Chen, L.; Yamaguchi, T.; Nakai, M.; Kitae, K.; Ago, Y.; Nakagawa, S.; et al. ALKBH8 contributes to neurological function through oxidative stress regulation. Proc. Natl. Acad. Sci. Nexus 2024, 3, 115. [Google Scholar] [CrossRef]
- Tian, S.; Wu, N.; Zhang, L.; Wang, X. RNA N6 -methyladenosine modification suppresses replication of rice black streaked dwarf virus and is associated with virus persistence in its insect vector. Mol. Plant Pathol. 2021, 22, 1070–1081. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, N.; Zhong, J.; Chen, C.; Liu, W.; Ren, Y.; Wang, X.; Jin, H. N6-methyladenosine modification of the mRNA for a key gene in purine nucleotide metabolism regulates virus proliferation in an insect vector. Cell Rep. 2024, 43, 113821. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Xu, X.; Lin, S.; Wu, J.; Lin, G.; Bai, J.; Song, Q.; You, M.; Xie, M. Novel miR-108 and miR-234 target juvenile hormone esterase to regulate the response of Plutella xylostella to Cry1Ac protoxin. Ecotoxicol. Environ. Safe 2023, 254, 114761. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, Y.; Zhang, X.; Guo, L.; Zhu, L.; Sun, D.; Sun, K.; Xu, X.; Yang, X.; Xie, W.; et al. RNA m6A Methylation Suppresses Insect Juvenile Hormone Degradation to Minimize Fitness Costs in Response to A Pathogenic Attack. Adv. Sci. 2024, 11, e2307650. [Google Scholar] [CrossRef]
- Wang, R.; Che, W.; Wang, J.; Qu, C.; Luo, C. Cross-resistance and biochemical mechanism of resistance to cyantraniliprole in a near-isogenic line of whitefly Bemisia tabaci Mediterranean (Q biotype). Pestic. Biochem. Physiol. 2020, 167, 104590. [Google Scholar] [CrossRef]
- Yang, X.; Wei, X.; Yang, J.; Du, T.; Yin, C.; Fu, B.; Huang, M.; Liang, J.; Gong, P.; Liu, S.; et al. Epitranscriptomic regulation of insecticide resistance. Sci. Adv. 2021, 7, eabe5903. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jiang, Q.; Yang, Y.; Feyereisen, R.; Wu, Y. Pyrethroid metabolism by eleven Helicoverpa armigera P450s from the CYP6B and CYP9A subfamilies. Insect Biochem. Mol. 2021, 135, 103597. [Google Scholar] [CrossRef]
- Nauen, R.; Vontas, J.; Kaussmann, M.; Wolfel, K. Pymetrozine is hydroxylated by CYP6CM1, a cytochrome P450 conferring neonicotinoid resistance in Bemisia tabaci. Pestic. Manag. Sci. 2013, 69, 457–461. [Google Scholar] [CrossRef]
- Wang, R.; Che, W.; Wang, J.; Luo, C. Monitoring insecticide resistance and diagnostics of resistance mechanisms in Bemisia tabaci Mediterranean (Q biotype) in China. Pesti. Biochem. Physiol. 2020, 163, 117–122. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Q.; Lu, W.; Yuan, J.; Yang, Y.; Oakeshott, J.; Wu, Y. Divergent amplifications of CYP9A cytochrome P450 genes provide two noctuid pests with differential protection against xenobiotics. Proc. Natl. Acad. Sci. USA 2023, 120, e2308685120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guan, Z.; Sun, L.; Fan, X.; Wang, D.; Yu, X.; Lyu, L.; Qi, G. N6-methyladenosine modification of RNA controls dopamine synthesis to influence labour division in ants. Mol. Ecol. 2024, 33, e17322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Pan, J.; Gong, C.; Hu, X. Identification and Characterization of BmNPV m6A Sites and Their Possible Roles During Viral Infection. Front. Immunol. 2022, 13, 869313. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, K.; Liu, M.; Yang, S.; Xu, W.; Feng, T.; Jie, M.; Liu, Z.; Sheng, X.; Chen, H.; et al. Phase separation of BuGZ regulates gut regeneration and aging through interaction with m6A regulators. Nat. Commun. 2023, 14, 6700. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, C.; Xu, L.; Yuan, Y.; Zhao, J.; Zhao, W.; Chen, Y.; Qiu, J.; Meng, M.; Zheng, Y.; et al. N6-Methyladenosine Reader Protein YT521-B Homology Domain-Containing 2 Suppresses Liver Steatosis by Regulation of mRNA Stability of Lipogenic Genes. Hepatology 2021, 73, 91–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Kan, D.; Yang, Y.; Shen, J.; Han, C.; Liu, X.; Yang, J. m6A-mediated nonhomologous end joining (NHEJ) pathway regulates senescence in Brachionus plicatilis (Rotifera). Arch. Gerontol. Geriatr. 2023, 111, 104994. [Google Scholar] [CrossRef]
- Haussmann, I.U.; Bodi, Z.; Sanchez-Moran, E.; Mongan, N.P.; Archer, N.; Fray, R.G.; Soller, M. m6A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 2016, 540, 301–304. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; He, J.; Zhang, Y.; Xi, H.; Liu, M.; Ma, J.; Wu, L. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 12626. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, Y.; Liu, R.; Yao, L.; Yu, X.Q.; Wang, X. Transcriptome-wide analysis of mRNA N6 -methyladenosine modification in the embryonic development of Spodoptera frugiperda. Insect Sci. 2023, 30, 1229–1244. [Google Scholar] [CrossRef]
- Sami, J.D.; Spitale, R.C.; Cleary, M.D. mRNAs encoding neurodevelopmental regulators have equal N6-methyladenosine stoichiometry in Drosophila neuroblasts and neurons. Neural Dev. 2022, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, P.; Huang, Z.H.; Yang, J.Y.; Wang, J.; Xie, X.Z.; Wang, Y.H.; Li, C.C.; Xu, J.P. RNA methyltransferase BmMettl3 and BmMettl14 in silkworm (Bombyx mori) and the regulation of silkworm embryonic development. Arch. Insect Biochem. 2023, 113, e22005. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhou, M.; Chen, W.; Geng, W.; Lu, L.; Shen, G.; Lin, P.; Xia, Q.; Zhao, P.; Li, Z. N6-methyladenosine modification of host Hsc70 attenuates nucleopolyhedrovirus infection in the lepidopteran model insect Bombyx mori. Int. J. Biol. Macromol. 2025, 298, 139869. [Google Scholar] [CrossRef] [PubMed]
- Van Ekert, E.; Heylen, K.; Rouge, P.; Powell, C.A.; Shatters, R.J.; Smagghe, G.; Borovsky, D. Aedes aegypti juvenile hormone acid methyl transferase, the ultimate enzyme in the biosynthetic pathway of juvenile hormone III, exhibits substrate control. J. Insect Physiol. 2014, 64, 62–73. [Google Scholar] [CrossRef]
- Jalloh, B.; Lancaster, C.L.; Rounds, J.C.; Brown, B.E.; Leung, S.W.; Banerjee, A.; Morton, D.J.; Bienkowski, R.S.; Fasken, M.B.; Kremsky, I.J.; et al. The Drosophila Nab2 RNA binding protein inhibits m6A methylation and male-specific splicing of Sex lethal transcript in female neuronal tissue. eLife 2023, 12, e64904. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Ren, H.; Ma, L.; Guo, J.; Mao, D.; Lu, Z.; Lu, L.; Yan, D. Role of Hakai in m6A modification pathway in Drosophila. Nat. Commun. 2021, 12, 2159. [Google Scholar] [CrossRef]
- Bawankar, P.; Lence, T.; Paolantoni, C.; Haussmann, I.U.; Kazlauskiene, M.; Jacob, D.; Heidelberger, J.B.; Richter, F.M.; Nallasivan, M.P.; Morin, V.; et al. Hakai is required for stabilization of core components of the m6A mRNA methylation machinery. Nat. Commun. 2021, 12, 3778. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, K.; Lin, G.; Liu, P.; Yan, Y.; Ye, T.; Yao, G.; Barr, M.P.; Liang, D.; Wang, Y.; et al. Ajuba inhibits hepatocellular carcinoma cell growth via targeting of beta-catenin and YAP signaling and is regulated by E3 ligase Hakai through neddylation. J. Exp. Clin. Cancer Res. 2018, 37, 165. [Google Scholar] [CrossRef] [PubMed]
- Perlegos, A.E.; Quan, X.; Donnelly, K.M.; Shen, H.; Shields, E.J.; Elashal, H.; Fange, L.K.; Bonini, N.M. dTrmt10A impacts Hsp70 chaperone m6A levels and the stress response in the Drosophila brain. Sci. Rep. 2023, 13, 22999. [Google Scholar] [CrossRef]
- Knuckles, P.; Lence, T.; Haussmann, I.U.; Jacob, D.; Kreim, N.; Carl, S.H.; Masiello, I.; Hares, T.; Villasenor, R.; Hess, D.; et al. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 2018, 32, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, F.; Chen, J.; Liu, J.; Lin, Y.B.; Li, L.; Chen, C.B.; Xu, Q. N6-methyladenosine modification of CENPK mRNA by ZC3H13 promotes cervical cancer stemness and chemoresistance. Mil. Med. Res. 2022, 9, 19. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yang, W.L.; Zhao, Y.L.; Yang, Y.G. Dynamic transcriptomic m5C and its regulatory role in RNA processing. Wiley Interdiscip. Rev. RNA 2021, 12, e1639. [Google Scholar] [CrossRef]
- Bohnsack, K.E.; Hobartner, C.; Bohnsack, M.T. Eukaryotic 5-methylcytosine (m5C) RNA Methyltransferases: Mechanisms, Cellular Functions, and Links to Disease. Genes 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.B.; Shelby, E.A.; McKinney, E.C.; Simmons, A.M.; Moore, A.J.; Moore, P.J. An association between Dnmt1 and Wnt in the production of oocytes in the whitefly Bemisia tabaci. Insect Mol. Biol. 2024, 33, 467–480. [Google Scholar] [CrossRef]
- Abbas, Z.; Rehman, M.U.; Tayara, H.; Zou, Q.; Chong, K.T. XGBoost framework with feature selection for the prediction of RNA N5-methylcytosine sites. Mol. Ther. 2023, 31, 2543–2551. [Google Scholar] [CrossRef]
- Mansfield, J.H.; Wilhelm, J.E.; Hazelrigg, T. Ypsilon Schachtel, a Drosophila Y-box protein, acts antagonistically to Orb in the oskar mRNA localization and translation pathway. Development 2002, 129, 197–209. [Google Scholar] [CrossRef]
- Thieringer, H.A.; Singh, K.; Trivedi, H.; Inouye, M. Identification and developmental characterization of a novel Y-box protein from Drosophila melanogaster. Nucleic Acids Res. 1997, 25, 4764–4770. [Google Scholar] [CrossRef]
- Zou, F.; Tu, R.; Duan, B.; Yang, Z.; Ping, Z.; Song, X.; Chen, S.; Price, A.; Li, H.; Scott, A.; et al. Drosophila YBX1 homolog YPS promotes ovarian germ line stem cell development by preferentially recognizing 5-methylcytosine RNAs. Proc. Natl. Acad. Sci. USA 2020, 117, 3603–3609. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Qi, S.; Jiang, N.; Li, Q.; Zhao, L.; Wu, L.; Huang, S.; Wang, M. Exploring RNA methylation as a promising biomarker for assessing sublethal effects of fipronil on honeybees (Apis mellifera L.). Ecotoxicol. Environ. Safe 2023, 262, 115152. [Google Scholar] [CrossRef]
- Degut, C.; Roovers, M.; Barraud, P.; Brachet, F.; Feller, A.; Larue, V.; Al, R.A.; Caillet, J.; Droogmans, L.; Tisne, C. Structural characterization of B. subtilis m1A22 tRNA methyltransferase TrmK: Insights into tRNA recognition. Nucleic Acids Res. 2019, 47, 4736–4750. [Google Scholar] [CrossRef]
- Adami, R.; Bottai, D. S-adenosylmethionine tRNA modification: Unexpected/unsuspected implications of former/new players. Int. J. Biol. Sci. 2020, 16, 3018–3027. [Google Scholar] [CrossRef]
- Safra, M.; Sas-Chen, A.; Nir, R.; Winkler, R.; Nachshon, A.; Bar-Yaacov, D.; Erlacher, M.; Rossmanith, W.; Stern-Ginossar, N.; Schwartz, S. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 2017, 551, 251–255. [Google Scholar] [CrossRef]
- Liu, F.; Clark, W.; Luo, G.; Wang, X.; Fu, Y.; Wei, J.; Wang, X.; Hao, Z.; Dai, Q.; Zheng, G.; et al. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell 2016, 167, 816–828.e16. [Google Scholar] [CrossRef] [PubMed]
- Simonova, A.; Romanska, V.; Benoni, B.; Skubnik, K.; Smerdova, L.; Prochazkova, M.; Spustova, K.; Moravcik, O.; Gahurova, L.; Paces, J.; et al. Honeybee Iflaviruses Pack Specific tRNA Fragments from Host Cells in Their Virions. Chembiochem 2022, 23, e202200281. [Google Scholar] [CrossRef]
- Aviles-Pagan, E.E.; Orr-Weaver, T.L. Activating embryonic development in Drosophila. Semin. Cell Dev. Biol. 2018, 84, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Franco, G.; Taillebourg, E.; Delfino, E.; Homolka, D.; Gueguen, N.; Brasset, E.; Pandey, R.R.; Pillai, R.S.; Fauvarque, M.O. The catalytic-dead Pcif1 regulates gene expression and fertility in Drosophila. RNA 2023, 29, 609–619. [Google Scholar] [CrossRef]
- Angelova, M.T.; Dimitrova, D.G.; Da, S.B.; Marchand, V.; Jacquier, C.; Achour, C.; Brazane, M.; Goyenvalle, C.; Bourguignon-Igel, V.; Shehzada, S.; et al. tRNA 2′-O-methylation by a duo of TRM7/FTSJ1 proteins modulates small RNA silencing in Drosophila. Nucleic Acids Res. 2020, 48, 2050–2072. [Google Scholar] [CrossRef]
- Dohnalkova, M.; Krasnykov, K.; Mendel, M.; Li, L.; Panasenko, O.; Fleury-Olela, F.; Vagbo, C.B.; Homolka, D.; Pillai, R.S. Essential roles of RNA cap-proximal ribose methylation in mammalian embryonic development and fertility. Cell Rep. 2023, 42, 112786. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chai, P.; Jia, R.; Fan, X. Novel insight into the regulatory roles of diverse RNA modifications: Re-defining the bridge between transcription and translation. Mol. Cancer 2020, 19, 78. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wang, C.; Wang, X. TET (Ten-eleven translocation) family proteins: Structure, biological functions and applications. Signal Transduct. Target. Ther. 2023, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Delatte, B.; Wang, F.; Ngoc, L.V.; Collignon, E.; Bonvin, E.; Deplus, R.; Calonne, E.; Hassabi, B.; Putmans, P.; Awe, S.; et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 2016, 351, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, H.; Mao, F.; Wang, H.; Wang, S.; Chen, Z.; Zhang, Y.; Xu, Z.; Xing, J.; Cui, Z.; et al. N4-acetyldeoxycytosine DNA modification marks euchromatin regions in Arabidopsis thaliana. Genome Biol. 2022, 23, 5. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Qiao, J.; Zheng, C.; Zheng, W.; Zhang, H. METTL3/METTL14-mediated RNA m6A modification is involved in male reproductive development in Bactrocera dorsalis. In Insect Science; Wiley: Hoboken, NJ, USA, 2025. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Reinberg, D.; Desplan, C. A molecular toolkit for superorganisms. Trends Genet. 2021, 37, 846–859. [Google Scholar] [CrossRef]
- Tortoriello, G.; de Celis, J.F.; Furia, M. Linking pseudouridine synthases to growth, development and cell competition. FEBS J. 2010, 277, 3249–3263. [Google Scholar] [CrossRef]
- Vicidomini, R.; Di Giovanni, A.; Petrizzo, A.; Iannucci, L.F.; Benvenuto, G.; Nagel, A.C.; Preiss, A.; Furia, M. Loss of Drosophila pseudouridine synthase triggers apoptosis-induced proliferation and promotes cell-nonautonomous EMT. Cell Death Dis. 2015, 6, e1705. [Google Scholar] [CrossRef]
- Song, W.; Ressl, S.; Tracey, W.D. Loss of Pseudouridine Synthases in the RluA Family Causes Hypersensitive Nociception in Drosophila. G3-Genes Genom. Genet. 2020, 10, 4425–4438. [Google Scholar] [CrossRef]
- Lence, T.; Akhtar, J.; Bayer, M.; Schmid, K.; Spindler, L.; Ho, C.H.; Kreim, N.; Andrade-Navarro, M.A.; Poeck, B.; Helm, M.; et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 2016, 540, 242–247. [Google Scholar] [CrossRef]
- Guo, J.; Tang, H.W.; Li, J.; Perrimon, N.; Yan, D. Xio is a component of the Drosophila sex determination pathway and RNA N6-methyladenosine methyltransferase complex. Proc. Natl. Acad. Sci. USA 2018, 115, 3674–3679. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, X.Y.; He, Z.; Shen, C.; Li, Y.L.; Qin, L.; Zhao, C.Q.; Luo, G.H.; Fang, J.C.; Ji, R. The dynamics of N6-methyladenine RNA modification in resistant and susceptible rice varieties responding to rice stem borer damage. Insect Sci. 2025, 32, 530–550. [Google Scholar] [CrossRef] [PubMed]
- Honeybee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006, 443, 931–949. [Google Scholar] [CrossRef]
- Muthu, L.B.C.; Murugan, M.; Pavithran, S.; Naveena, K. Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: Role of transcriptional and post-transcriptional events. Front. Mol. Biosci. 2023, 10, 1257859. [Google Scholar]
- Wang, Y.; Liu, B.; Zhao, G.; Lee, Y.; Buzdin, A.; Mu, X.; Zhao, J.; Chen, H.; Li, X. Spatial transcriptomics: Technologies, applications and experimental considerations. Genomics 2023, 115, 110671. [Google Scholar] [CrossRef]
- Liu, L.; Chen, A.; Li, Y.; Mulder, J.; Heyn, H.; Xu, X. Spatiotemporal omics for biology and medicine. Cell 2024, 187, 4488–4519. [Google Scholar] [CrossRef]
- Li, B.; Zheng, L.; Yang, J.; Qu, L. Targeting oncogene-induced cellular plasticity for tumor therapy. Adv. Biotechnol. 2024, 2, 24. [Google Scholar] [CrossRef]

| Factor/Enzyme | Species | Pathways | Function | Up/Down |
|---|---|---|---|---|
| PxMETTL3, PxMETTL14, and YTHDF2 [30,42] | P. xylostella | CCR4/NOT complex and HRSP12-RNase P/MRP complex | Regulation of PxJHE expression | Down |
| LsIMPDH [27] | L. striatellus | RSV replication | GTP synthesis | Up |
| SfrMETTL3 and SfrMETTL14 [43] | S. frugiperda | / | Embryonic development | / |
| CYP4C64, WTAP, and KIAA1429 [31] | B. tabaci | / | Insecticide resistance | Up |
| DmMETTL3 [44] | D. melanogaster | Wnt | Embryonic development | Down |
| BmMETTL3 and BmMETTL14 [13,45] | B. mori | Wnt and Toll/Imd | Embryonic development | Up |
| BmYTHDC [13] | B. mori | / | Embryonic development | / |
| BmMETTL3 [46] | B. mori | HSC70 | Attenuates nucleopolyhedrovirus infection | Up |
| Juvenile hormone analog and BmMETTL3 [4] | B. mori | Biosynthetic pathway of JH III [47] | Embryonic development | Up |
| N2b2 [48] | D. melanogaster | / | Male-specific lethal regulation | Down |
| Hakai (E3 ubiquitin ligase) [49,50] | D. melanogaster | RACK1 Slit-Robo [51] | Defects in morphological traits | Up |
| ALKBH8 | D. melanogaster /Aedes aegypti | / | mRNA regulation | Down |
| dTrmt10A [52] | D. melanogaster | Neuronal signaling Heat stress pathway | Stress response | Up |
| Zinc finger CCCH domain-containing protein 13/Flacc [Fl(2)d-associated complex component] [53] | D. melanogaster | Wnt/β-catenin signaling p53 pathway [54] | mRNA regulation | Up |
| Types of Modification | Different Insects | Function |
|---|---|---|
| m6A | B. dorsalis | Regulates male reproductive system development [76] |
| m6A | S. invicta | Regulates dopamine synthesis [36] |
| m6A | B. mori | Regulates Hsc70 expression to suppress BmNPV infection [46] |
| m6A | L. striatellus | Regulates GTP levels to inhibit viral replication [27] |
| m6A | H. armigera | Regulates expression of P450 genes, potentially involved in pesticide resistance [30,31,32] |
| m6A | P. xylostella | Improving Bt resistance and growth–defense balance [29] |
| m6A | B. plicatilis | Reduces fecundity and lifespan; involved in DNA repair [24,40] |
| m5C | T. molitor | Enhances immune priming by regulating translation of immune proteins [1] |
| m5C | A. mellifera | Associated with RNA metabolism and immune regulation [2] |
| m5C | B. mori | Regulates maternal mRNA stability during early embryogenesis [59,60] |
| m5C | D. melanogaster | Regulates germ line stem cell development via YPS-m5C RNA interactions [60,61] |
| m5C | B. tabaci | Knockdown increases ftz-f1 expression, which disrupts hormone levels and choriogenesis [62] |
| m1A | A. mellifera | Influences selective packaging of tRNA fragments into virions by iflaviruses [67] |
| m1A | D. melanogaster | Regulates maternal-to-zygotic transition, including mRNA storage, translational activation, and developmental timing during early embryogenesis [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lin, L.; Huang, B.; Liu, H.; Li, H.; Wu, W. Exploring the Role of mRNA Methylation in Insect Biology and Resistance. Insects 2025, 16, 463. https://doi.org/10.3390/insects16050463
Zhang J, Lin L, Huang B, Liu H, Li H, Wu W. Exploring the Role of mRNA Methylation in Insect Biology and Resistance. Insects. 2025; 16(5):463. https://doi.org/10.3390/insects16050463
Chicago/Turabian StyleZhang, Jiayang, Luobin Lin, Botian Huang, Huoxi Liu, Huaqin Li, and Wenmei Wu. 2025. "Exploring the Role of mRNA Methylation in Insect Biology and Resistance" Insects 16, no. 5: 463. https://doi.org/10.3390/insects16050463
APA StyleZhang, J., Lin, L., Huang, B., Liu, H., Li, H., & Wu, W. (2025). Exploring the Role of mRNA Methylation in Insect Biology and Resistance. Insects, 16(5), 463. https://doi.org/10.3390/insects16050463







