Larvicidal Activity of Extracts from the Artemisia arborescens L. Plant and Hyrtios erectus Sponge Against the Culex pipiens Mosquito (Diptera: Culicidae) and Toxicological Assessment on Danio rerio Zebrafish Embryos as Non-Target Organism
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Materials
2.2. Collection of a Marine Sponge
2.3. Preparation of Crude Extract
2.3.1. Fractionation of Crude Extract
2.3.2. Preparation of Stock Solution
2.4. Maintenance of Mosquitoes’ Colony
2.5. Mosquito Larvicidal Bioassay
2.6. Evalution of Biotoxicity Against Non-Target Organisms
2.7. Statistical Analysis
3. Results
3.1. Mosquito Larvicidal Activity
3.2. Biotoxicity Against Non-Target Organisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siam, M.H.; Owaresat, J.; Khan, A. Mosquito Control Management Using Phytochemicals: A Review. Int. J. Mosq. Res. 2022, 9, 10–17. [Google Scholar] [CrossRef]
- Saleem, M.A.; Lobanova, I. Mosquito-Borne Diseases. In Dengue Virus Disease: From Origin to Outbreak; Qureshi, A.I., Saeed, O., Eds.; Academic Press: New York, NY, USA, 2020; pp. 57–83. ISBN 9780128182703. [Google Scholar]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Marm Kilpatrick, A. “Bird Biting” Mosquitoes and Human Disease: A Review of the Role of Culex pipiens Complex Mosquitoes in Epidemiology. Infect. Genet. and Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef]
- Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Peña, R.; Montalvo, T.; Bueno-Marí, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the Current and Future Distribution of the West Nile Virus Mosquito Vector Culex pipiens in Spain. Environ. Res. 2020, 188, 109837. [Google Scholar] [CrossRef] [PubMed]
- Brugman, V.A.; Hernández-Triana, L.M.; Medlock, J.M.; Fooks, A.R.; Carpenter, S.; Johnson, N. The Role of Culex pipiens L. (Diptera: Culicidae) in Virus Transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef]
- Turell, M.J. Members of the Culex pipiens Complex as Vectors of Viruses1. J. Am. Mosq. Control Assoc. 2012, 28, 123–126. [Google Scholar] [CrossRef]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile Virus Transmission Potential by Local Culex pipiens Mosquitoes in North-Western Europe. One Health 2015, 1, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kassem, Y.E.; El-Baghdady, K.Z.; Saleh, N.E.M.; Wahba, M.M.I. Biological Control of Culex pipiens Mosquito by Local Bacterial Isolates. Afr. J. Biol. Sci. 2018, 14, 21–40. [Google Scholar] [CrossRef]
- Ferraguti, M.; Heesterbeek, H.; Martínez-de la Puente, J.; Jiménez-Clavero, M.Á.; Vázquez, A.; Ruiz, S.; Llorente, F.; Roiz, D.; Vernooij, H.; Soriguer, R.; et al. The Role of Different Culex Mosquito Species in the Transmission of West Nile Virus and Avian Malaria Parasites in Mediterranean Areas. Transbound. Emerg. Dis. 2021, 68, 920–930. [Google Scholar] [CrossRef]
- Marcolin, L.; Zardini, A.; Longo, E.; Caputo, B.; Poletti, P.; Di Marco, M. Mapping the Habitat Suitability of Culex Pipiens in Europe Using Ensemble Bioclimatic Modelling. bioRxiv 2025. [Google Scholar] [CrossRef]
- Wani, J.A.; Wali, A.F.; Majid, S.; Rasool, S.; Rehman, M.U.; Rashid, S.M.; Ali, S.; Farooq, S.; Rasool, S.; Ahmad, A.; et al. Bio-Pesticides: Application and Possible Mechanism of Action. In Bioremediation and Biotechnology; Bhat, R.A., Hakeem, K.R., Dervash, M.A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 2, pp. 97–119. ISBN 9783030403331. [Google Scholar]
- Meier, C.J.; Rouhier, M.F.; Hillyer, J.F. Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides. Insects 2022, 13, 1093. [Google Scholar] [CrossRef]
- Gajger, I.T.; Dar, S.A. Plant Allelochemicals as Sources of Insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Parra, J.A.; Cortés, T.R.; Ramirez-Lepe, M. Mosquito-Borne Diseases, Pesticides Used for Mosquito Control, and Development of Resistance to Insecticides. In Insecticides Resistance; Trdan, S., Ed.; IntechOpen: London, UK, 2016; Volume 2, pp. 111–134. [Google Scholar]
- Thongni, A.; Ariina, M.S.; Susngi, W.E. Botanical Pesticides- An Alternative for Insect Pest Management. Just Agric. 2023, 3, 49–58. [Google Scholar]
- Gharsan, F.N. A Review of the Bioactivity of Plant Products Against Aedes aegypti (Diptera: Culicidae). J. Entomol. Sci. 2019, 54, 256–274. [Google Scholar] [CrossRef]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides and Innovative Nano-based Application Strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef]
- Joseph, B.; Sujatha, S.; Jeevitha, M.V. Screening of Pesticidal Activities of Some Marine Sponge Extracts against Chosen Pests. J. Biopest. 2010, 3, 495–498. [Google Scholar] [CrossRef]
- Mathivanan, A.; Ravikumar, S.; Selvakumar, G. Bioprospecting of Sponge and Its Symbionts: New Tool for Mosquitocidal & Insecticidal Metabolites. Biocatal. Agric. Biotechnol. 2019, 19, 101158. [Google Scholar] [CrossRef]
- Varijakzhan, D.; Loh, J.Y.; Yap, W.S.; Yusoff, K.; Seboussi, R.; Lim, S.H.E.; Lai, K.S.; Chong, C.M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef]
- Anteneh, Y.S.; Yang, Q.; Brown, M.H.; Franco, C.M.M. Factors Affecting the Isolation and Diversity of Marine Sponge-Associated Bacteria. Appl. Microbiol. Biotechnol. 2022, 106, 1729–1744. [Google Scholar] [CrossRef]
- Reegan, A.D.; Kinsalin, A.V.; Paulraj, M.G.; Ignacimuthu, S. Larvicidal, Ovicidal, and Repellent Activities of Marine Sponge Cliona celata (Grant) Extracts against Culex quinquefasciatus Say and Aedes aegypti L. (Diptera: Culicidae). ISRN Entomol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- El-Gamal, A.A.; Al-Massarani, S.M.; Shaala, L.A.; Alahdald, A.M.; Al-Said, M.S.; Ashour, A.E.; Kumar, A.; Abdel-Kader, M.S.; Abdel-Mageed, W.M.; Youssef, D.T.A. Cytotoxic Compounds from the Saudi Red Sea Sponge Xestospongia testudinaria. Mar. Drugs 2016, 14, 82. [Google Scholar] [CrossRef]
- Otsuka, H. Purification by Solvent Extraction Using Partition Coefficient. In Natural Products Isolation; Sarker, S.D., Latif, Z., Gary, A.I., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 269–370. [Google Scholar]
- Ahmed, A.M.; Hussein, H.I.; El-Kersh, T.A.; Al-Sheikh, Y.A.; Ayaad, T.H.; El-Sadawy, H.A.; Al-Mekhlafi, F.A.; Ibrahim, M.S.; Al-Tamimi, J.; Nasr, F.A. Larvicidal Activities of Indigenous Bacillus thuringiensis Isolates and Nematode Symbiotic Bacterial Toxins against the Mosquito Vector, Culex pipiens (Diptera: Culicidae). J. Arthropod-Borne Dis. 2017, 11, 260–277. [Google Scholar]
- El-Sadawy, H.A.; El Namaky, A.H.; Hafez, E.E.; Baiome, B.A.; Ahmed, A.M.; Ashry, H.M.; Ayaad, T.H. Silver Nanoparticles Enhance the Larvicidal Toxicity of Photorhabdus and Xenorhabdus Bacterial Toxins: An Approach to Control the Filarial Vector, Culex pipiens. Trop. Biomed. 2018, 35, 392–407. [Google Scholar] [PubMed]
- Abutaha, N.; Mashaly, A.M.A.; Al-Mekhlafi, F.A.; Farooq, M.; Al-shami, M.; Wadaan, M.A. Larvicidal Activity of Endophytic Fungal Extract of Cochliobolus spicifer (Pleosporales: Pleosporaceae) on Aedes caspius and Culex pipiens (Diptera: Culicidae). Appl. Entomol. Zool. 2015, 50, 405–414. [Google Scholar] [CrossRef]
- Ahmed, A.M.; El-Kersh, T.A.; Hussein, H.I.; Ayaad, T.H.; El-Sadawy, H.A.; Ibrahim, M.S.; Amoudi, M.A.; Aseery, G.M. Larvicidal Activities of Local Bacillus thuringiensis Isolates and Toxins from Nematode Bacterial Symbionts against the Rift Valley Fever Vector, Aedes caspius (Diptera: Culicidae). J. Afr. Zool. 2021, 56, 65–75. [Google Scholar] [CrossRef]
- WHO. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; WHO: Geneva, Switzerland, 2005; Available online: https://iris.who.int/bitstream/handle/10665/69101/WHO_CDS_WHOPES_GCDPP_2005.13.pdf?sequence=1 (accessed on 24 January 2025).
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Abutaha, N.; Al-Mekhlafi, F.A.; Farooq, M. Target and Nontarget Toxicity of Cassia fistula Fruit Extract Against Culex pipiens (Diptera: Culicidae), Lung Cells (BEAS-2B) and Zebrafish (Danio rerio) Embryos. J. Med. Entomol. 2020, 57, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Moungthipmalai, T.; Puwanard, C.; Aungtikun, J.; Sittichok, S.; Soonwera, M. Ovicidal Toxicity of Plant Essential Oils and Their Major Constituents against Two Mosquito Vectors and Their Non-Target Aquatic Predators. Sci. Rep. 2023, 13, 2119. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, 3rd ed.; Cambridge University Press: London, UK, 1947. [Google Scholar]
- Litchfield, J.T.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 92, 99–113. [Google Scholar] [CrossRef]
- Pavela, R. Essential Oils for the Development of Eco-Friendly Mosquito Larvicides: A Review. Ind. Crops. Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Falaiye, O.E.; Ojo, O.B.; Adeoti, A.; Amoo, Z.A.; Olaleye, M.T. Effect of Extraction Technique, Solvent Polarity, and Plant Matrix on the Antioxidant Properties of Chrysophyllum albidum G. Don (African Star Apple). Bull. Natl. Res. Cent. 2022, 46, 40. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Chebbac, K.; Abchir, O.; Chalkha, M.; El Moussaoui, A.; El kasmi-Alaoui, M.; Lafraxo, S.; Chtita, S.; Alanazi, M.M.; Alanazi, A.S.; Hefnawy, M.; et al. Larvicidal Properties of Essential Oils of Three Artemisia species against the Chemically Insecticide-Resistant Nile Fever Vector Culex pipiens (L.) (Diptera: Culicidae): In Vitro and in Silico Studies. Open Chem 2024, 22, 20240108. [Google Scholar] [CrossRef]
- Sofi, M.A.; Nanda, A.; Sofi, M.A.; Maduraiveeran, R.; Nazir, S.; Siddiqui, N.; Nadeem, A.; Shah, Z.A.; Rehman, M.U. Larvicidal Activity of Artemisia absinthium Extracts with Special Reference to Inhibition of Detoxifying Enzymes in Larvae of Aedes aegypti L. J. King Saud Univ. Sci. 2022, 34, 102248. [Google Scholar] [CrossRef]
- Alami, A.; Ez zoubi, Y.; Fadil, M.; Annemer, S.; Bassouya, M.; Moustaid, W.; Farah, A. Exploring Ternary Essential Oil Mixtures of Moroccan Artemisia species for Larvicidal Effectiveness Against Culex pipiens Mosquitoes: A Mixture Design Approach. J Parasitol. Res. 2025, 2025, 2379638. [Google Scholar] [CrossRef]
- Reegan, A.D.; Kinsalin, A.V.; Paulraj, M.G.; Ignacimuthu, S. Larvicidal, Ovicidal and Repellent Activities of Marine Sponge Cliona celata (Grant) Extracts against Anopheles stephensi Liston (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2015, 8, 29–34. [Google Scholar] [CrossRef]
- Alkenani, N.A.; Basabreen, M.A.; Shaala, L.A.; Alshaeri, M.A.; Mahyoub, J.A.; Ullah, I.; Algamdi, K.M.; Youssef, D.T. Larvicidal Effects of Carbon Nanotubes Loaded with Selected Marine Sponges Extracts. Arch. Pharm. Pract. 2021, 12, 100–104. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Sudarshan, S.; Sivasubramani, K.; Nandini, M.S.; Narenkumar, J.; Ramachandran, V.; Almutairi, B.O.; Arunkumar, P.; Rajasekar, A.; Jayalakshmi, S. Larvicidal and Anti-Termite Activities of Microbial Biosurfactant Produced by Enterobacter cloacae SJ2 Isolated from Marine Sponge Clathria sp. Sci. Rep. 2023, 13, 15153. [Google Scholar] [CrossRef]
- Truong, L.; Harper, S.L.; Tanguay, R.L. Evaluation of Embryotoxicity Using the Zebrafish Model. Methods Mol. Biol. 2011, 691, 271–279. [Google Scholar] [CrossRef]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, C.D.; Jayawardena, U.A. Toxicity Assessment of Herbal Medicine Using Zebrafish Embryos: A Systematic Review. J. Evid. Based Complementary Altern. Med. 2019, 2019, 7272808. [Google Scholar] [CrossRef] [PubMed]
- Caballero, M.V.; Candiracci, M. Zebrafish as Screening Model for Detecting Toxicity and Drugs Efficacy. J. Unexplored Med. Data 2018, 3, 4. [Google Scholar] [CrossRef]
- Carnovali, M.; Ciavatta, M.L.; Mollo, E.; Roussis, V.; Banfi, G.; Carbone, M.; Mariotti, M. Aerophobin-1 from the Marine Sponge Aplysina aerophoba Modulates Osteogenesis in Zebrafish Larvae. Mar. Drugs. 2022, 20, 135. [Google Scholar] [CrossRef]
- Hanif, N.; Ardianti, R.; Ahmadi, P.; Setiawan, A.; Mohamad, K.; de Voogd, N.J.; Murni, A.; Tanaka, J. Ichthyotoxic Principles against Zebrafish Embryos from the Indonesian Marine Sponge Neopetrosia chaliniformis. J. Appl. Pharm. Sci. 2018, 8, 044–048. [Google Scholar] [CrossRef]

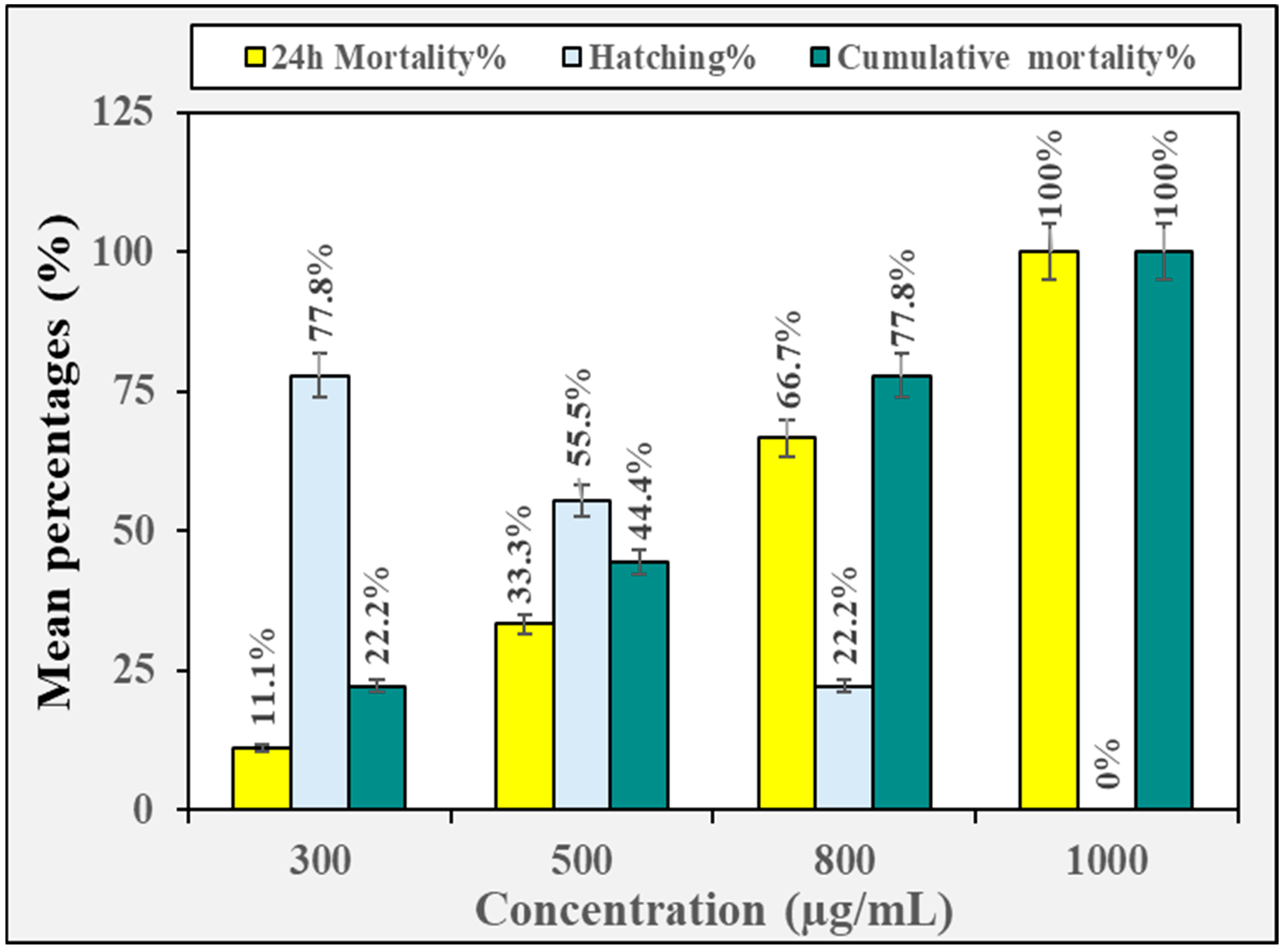
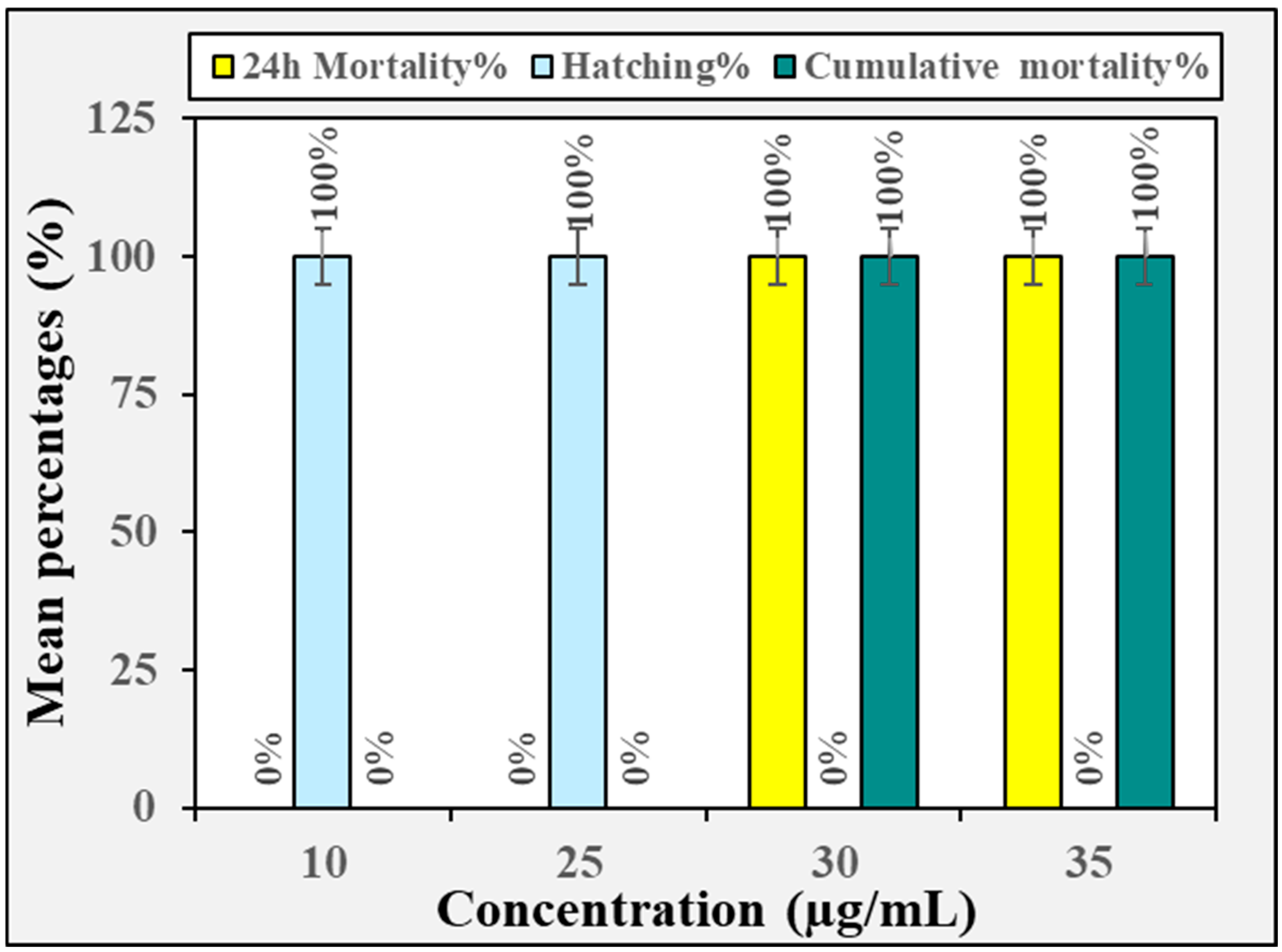

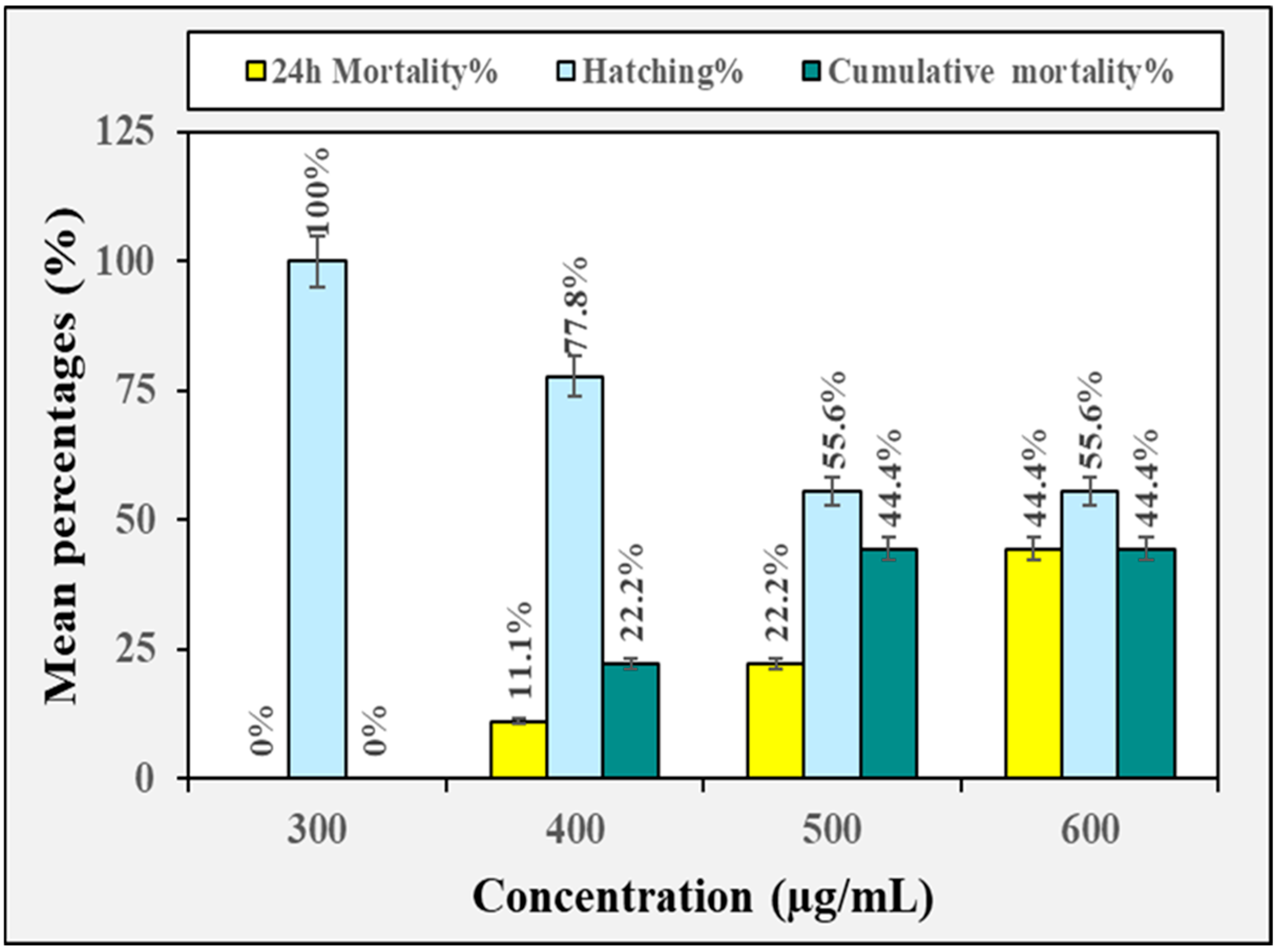
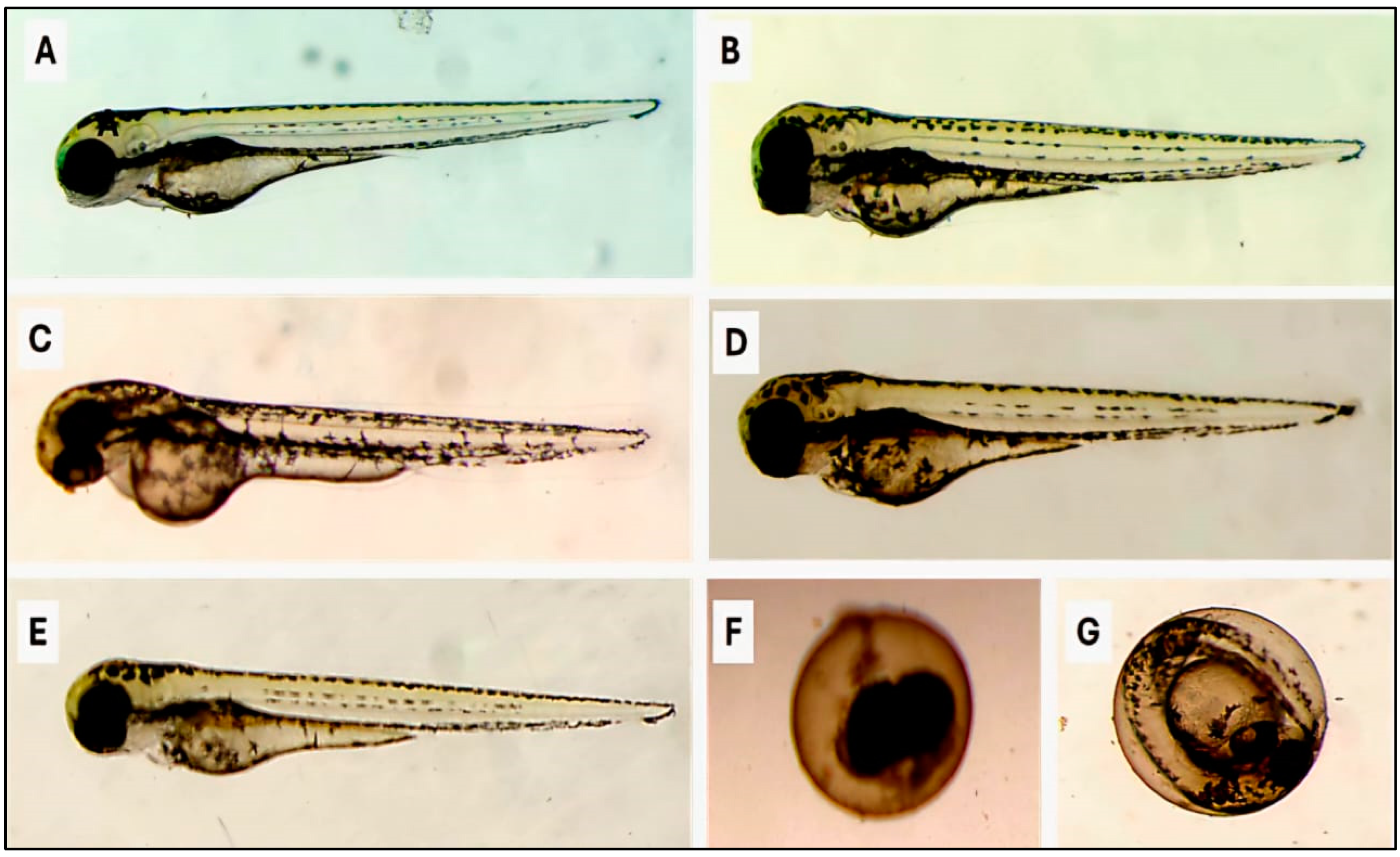
| Extract/Fraction | Concentration (µg/mL) | Mean Mortality% ± SE | |
|---|---|---|---|
| Exposure Period | |||
| 24 h | 48 h | ||
| Crude methanolic extract | 1200 | 10 ± 3.16 | 14 ± 4.00 |
| 1500 | 40 ± 6.32 | 52 ± 8.00 | |
| 1700 | 70 ± 4.47 | 76 ± 5.10 | |
| 2000 | 86 ± 5.10 | 94 ± 2.48 | |
| n-Hexane fraction | 200 | 8 ± 4.90 | 16 ± 4.00 |
| 300 | 32 ± 8.00 | 50 ± 8.60 | |
| 400 | 64 ± 7.48 | 84 ± 8.12 | |
| 500 | 86 ± 6.78 | 96 ± 4.00 | |
| Dichloromethane fraction | 1200 | 10 ± 0.0 | 14 ± 3.16 |
| 1500 | 28 ± 7.35 | 34 ± 8.12 | |
| 1700 | 40 ± 8.37 | 52 ± 10.7 | |
| 2000 | 78 ± 5.83 | 80 ± 10.5 | |
| Ethyl acetate fraction | 1800 | 4 ± 2.45 | 6 ± 2.45 |
| 2000 | 20 ± 5.48 | 20 ± 5.48 | |
| 2300 | 36 ± 8.72 | 44 ± 5.10 | |
| 2500 | 62 ± 12.0 | 70 ± 8.94 | |
| Exposure Period | Extract/Fraction | LC50 (µg/mL) (LCL-UCL) | LC90 (µg/mL) (LCL-UCL) | Slope ± SE | Chi-Square |
|---|---|---|---|---|---|
| 24 h | Crude methanolic extract | 1564.44 (1517.63–1611.56) | 2046.76 (1951.60–2183.55) | 10.981 ± 1.0 | 1.4786 |
| n-Hexane fraction | 346.74 (328.86–365.45) | 554.26 (509.29–621.47) | 6.291 ± 0.567 | 0.927 | |
| Dichloromethane fraction | 1722.37 (1663.77–1791.66) | 2377.80 (2212.36–2644.81) | 8.634 ± 0.925 | 1.612 | |
| Ethyl acetate fraction | 2392.88 (2328.44–2478.21) | 3006.58 (2832.64–3292.91) | 12.926 ± 1.46 | 3.173 | |
| 48 h | Crude methanolic extract | 1491.22 (1442.63–1537.73) | 1957.59 (1866.92–2089.13) | 10.844 ± 1.04 | 1.169 |
| n-Hexane fraction | 289.78 (274.33–304.49) | 454.78 (426.62–492.57) | 6.548 ± 0.507 | 0.928 | |
| Dichloromethane fraction | 1643.06 (1585.26–1706.68) | 2312.53 (2153.37–2568.49) | 8.634 ± 0.925 | 1.612 | |
| Ethyl acetate fraction | 2318.78 (2264.67–2384.08) | 2862.38 (2726.98–3071.54) | 14.012 ± 1.44 | 1.074 |
| Extract/Fraction | Concentration (µg/mL) | Mean Mortality% ± SE | |
|---|---|---|---|
| Exposure Period | |||
| 24 h | 48 h | ||
| Crude methanolic extract | 200 | 20 ± 3.16 | 44 ± 3.16 |
| 300 | 56 ± 5.10 | 66 ± 5.10 | |
| 400 | 82 ± 6.63 | 86 ± 5.10 | |
| 500 | 92 ± 5.83 | 98 ± 4.00 | |
| n-Hexane fraction | 60 | 20 ± 3.16 | 26 ± 6.78 |
| 70 | 64 ± 5.10 | 72 ± 9.17 | |
| 80 | 78 ± 3.74 | 84 ± 9.27 | |
| 100 | 94 ± 6.00 | 98 ± 2.00 | |
| Chloroform fraction | 50 | 22 ± 7.35 | 30 ± 6.32 |
| 70 | 68 ± 10.20 | 68 ± 5.83 | |
| 90 | 80 ± 8.94 | 82 ± 4.90 | |
| 100 | 96 ± 2.45 | 96 ± 4.00 | |
| n-Butanol fraction | 60 | 32 ± 4.90 | 36 ± 6.00 |
| 80 | 68 ± 4.90 | 72 ± 3.74 | |
| 120 | 80 ± 4.47 | 86 ± 5.10 | |
| 150 | 96 ± 2.45 | 98 ± 2.00 | |
| Exposure Period | Extract/Fraction | LC50 (µg/mL) (LCL-UCL) | LC90 (µg/mL) (LCL-UCL) | Slope ± SE | Chi-Square |
|---|---|---|---|---|---|
| 24 h | Crude methanolic extract | 280.74 (254.72–304.62) | 470.44 (421.28–555.59) | 5.717 ± 0.743 | 0.049 |
| n-Hexane fraction | 68.39 (64.995–71.37) | 89.65 (84.28–98.99) | 10.903 ± 1.551 | 3.885 | |
| Chloroform fraction | 63.03 (58.00–67.38) | 94.80 (87.18–107.28) | 7.231 ± 0.939 | 3.482 | |
| n-Butanol fraction | 71.23 (62.03–78.78) | 132.8 (117.3–161.1) | 4.738 ± 0.680 | 3.418 | |
| 48 h | Crude methanolic extract | 225.98 (190.8–252.8) | 426.01 (377.88–513.68) | 4.654 ± 0.708 | 1.956 |
| n-Hexane fraction | 65.54 (61.65–68.62) | 86.79 (81.61–96.07) | 10.509 ± 1.60 | 1.571 | |
| Chloroform fraction | 60.58 (55.55–64.78) | 90.93 (84.34–101.04) | 7.265 ± 0.893 | 2.368 | |
| n-Butanol fraction | 67.49 (58.85–74.43) | 118.37 (105.43–142.02) | 5.252 ± 0.785 | 2.810 |
| Extract/Fraction | LC50 (µg/mL) (UCL-LCL) * | Biosafety Index |
|---|---|---|
| Crude methanolic extract | 511 (462.54–566.34) | 1.82 |
| n-Hexane fraction | not determined | not determined |
| Chloroform fraction | not determined | not determined |
| n-Butanol fraction | 616.47 (551.40–854.78) | 8.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqurashi, S.A.; Ahmed, A.M.; El Gamal, A.A.; Al-Massarani, S.M.; Basudan, O.A.; Youssef, D.T.A.; Shaala, L.A.; Khan, M.F. Larvicidal Activity of Extracts from the Artemisia arborescens L. Plant and Hyrtios erectus Sponge Against the Culex pipiens Mosquito (Diptera: Culicidae) and Toxicological Assessment on Danio rerio Zebrafish Embryos as Non-Target Organism. Insects 2025, 16, 448. https://doi.org/10.3390/insects16050448
Alqurashi SA, Ahmed AM, El Gamal AA, Al-Massarani SM, Basudan OA, Youssef DTA, Shaala LA, Khan MF. Larvicidal Activity of Extracts from the Artemisia arborescens L. Plant and Hyrtios erectus Sponge Against the Culex pipiens Mosquito (Diptera: Culicidae) and Toxicological Assessment on Danio rerio Zebrafish Embryos as Non-Target Organism. Insects. 2025; 16(5):448. https://doi.org/10.3390/insects16050448
Chicago/Turabian StyleAlqurashi, Sadeem A., Ashraf M. Ahmed, Ali A. El Gamal, Shaza M. Al-Massarani, Omer A. Basudan, Diaa T. A. Youssef, Lamiaa A. Shaala, and Muhammad Farooq Khan. 2025. "Larvicidal Activity of Extracts from the Artemisia arborescens L. Plant and Hyrtios erectus Sponge Against the Culex pipiens Mosquito (Diptera: Culicidae) and Toxicological Assessment on Danio rerio Zebrafish Embryos as Non-Target Organism" Insects 16, no. 5: 448. https://doi.org/10.3390/insects16050448
APA StyleAlqurashi, S. A., Ahmed, A. M., El Gamal, A. A., Al-Massarani, S. M., Basudan, O. A., Youssef, D. T. A., Shaala, L. A., & Khan, M. F. (2025). Larvicidal Activity of Extracts from the Artemisia arborescens L. Plant and Hyrtios erectus Sponge Against the Culex pipiens Mosquito (Diptera: Culicidae) and Toxicological Assessment on Danio rerio Zebrafish Embryos as Non-Target Organism. Insects, 16(5), 448. https://doi.org/10.3390/insects16050448








