1. Introduction
The increasing burden of diseases transmitted by mosquitoes, including dengue, Zika, Rift Valley fever, and West Nile viruses, underscores the urgent need for the development of effective and sustainable strategies in disease vector control. In the absence of effective vaccines or treatments for most of these pathogens, vector control programs have heavily depended on the use of chemical insecticides to suppress the population of mosquitoes. However, this dependence on chemicals has raised concerns, as prolonged exposure led to insecticide resistance in the targeted mosquito population and unintended harm to the environments [
1]. Therefore, there is growing interest in innovative biological control tools that provide eco-friendly and effective alternatives. These include microbial toxins (e.g.,
Bacillus thuringiensis subsp.
israelensis), genetically modified mosquitoes, and endosymbiotic bacteria (e.g.,
Wolbachia) that show efficacy in the lowering of mosquito populations and disease transmission [
2,
3,
4,
5,
6,
7]. As research advances, these biological control agents offer a promising path toward integrated and sustainable mosquito control strategies that reduce reliance on chemical insecticides and reduce the risk of insecticide resistance.
Bacillus velezensis (
Bv) is a bacterium that produces a toxin [
8]. This Gram-positive spore-forming bacterium is characterized by its ability to produce a variety of bioactive metabolites with potent antimicrobial and insecticidal properties. Recent studies have shown that
Bv has significant larvicidal activity against a variety of mosquito species, making it a strong candidate for mosquito management [
9,
10]. The bacterium produces a complex array of secondary metabolites, including lipopeptides, polyketides, and hydrolases, which effectively disrupt larval development and significantly reduce mosquito population density [
11]. In addition,
Bv is characterized by its environmental friendliness, as its highly selective mode of action minimizes unintended ecological consequences and it has low toxicity to non-target organisms, making it a sustainable and safe alternative for mosquito control [
12]. Due to its high efficacy and ecological safety,
Bv represents a transformative tool for integrated vector management strategies, with the potential to improve current mosquito control efforts and limit the spread of mosquito-borne diseases.
Despite the promising mosquitocidal potential of
Bv, its effects on the mosquito life history traits remain poorly understood. While it is known that
Bv can reduce mosquito populations by directly killing larvae, less attention has been paid to potential sublethal effects on surviving individuals. Sublethal exposures may result in significant but less obvious consequences, such as altered developmental duration, reduced adult emergence, and impaired reproductive success, all of which can affect mosquito population dynamics and vectorial capacity [
13,
14]. Understanding these sublethal effects is critical, as they could complement larvicidal efficacy by further suppressing mosquito populations and altering disease transmission potential. Therefore, this study aimed to investigate the lethal and sublethal effects of
Bv strain WHk23 isolated from Wadi Hanifah against
Cx. quinquefasciatus, the main vector of arboviral and parasitic diseases. Specifically, mosquito mortality at different concentrations was evaluated to assess larvicidal efficacy while examining key life history traits, including development time, adult emergence, survival, and reproductive capacity, to fully understand its potential as a biocontrol agent.
2. Materials and Methods
2.1. Mosquito Rearing
The Cx. quinquefasciatus colony used in this experiment was reared and maintained under standard insectary conditions at 27 ± 1 °C, 60 ± 10% relative humidity, and a 12:12 h light–dark photoperiod. Eggs were hatched in plastic trays containing 1.5 L of dechlorinated water. Larvae were reared at a density of 300 individuals per tray and fed 0.2 g (approximately 0.67 mg/larva) of finely ground TetraMin fish food (Tetra Holding Inc., Blacksburg, VA, USA) every two days, except during the 24 h exposure period to the test toxin. Each pupa was individually transferred to a 30 mL plastic cup with dechlorinated water and maintained until adult emergence. Newly emerged adults were transferred to insect rearing cages (30 cm3, BioQuip Products, Rancho Dominguez, CA, USA) and provided with 10% sucrose solution via dental cotton rolls. Female mosquitoes were blood-fed once using defibrinated chicken blood delivered through the Hemotek membrane feeding system (Hemotek Ltd., Blackburn, UK) for egg production.
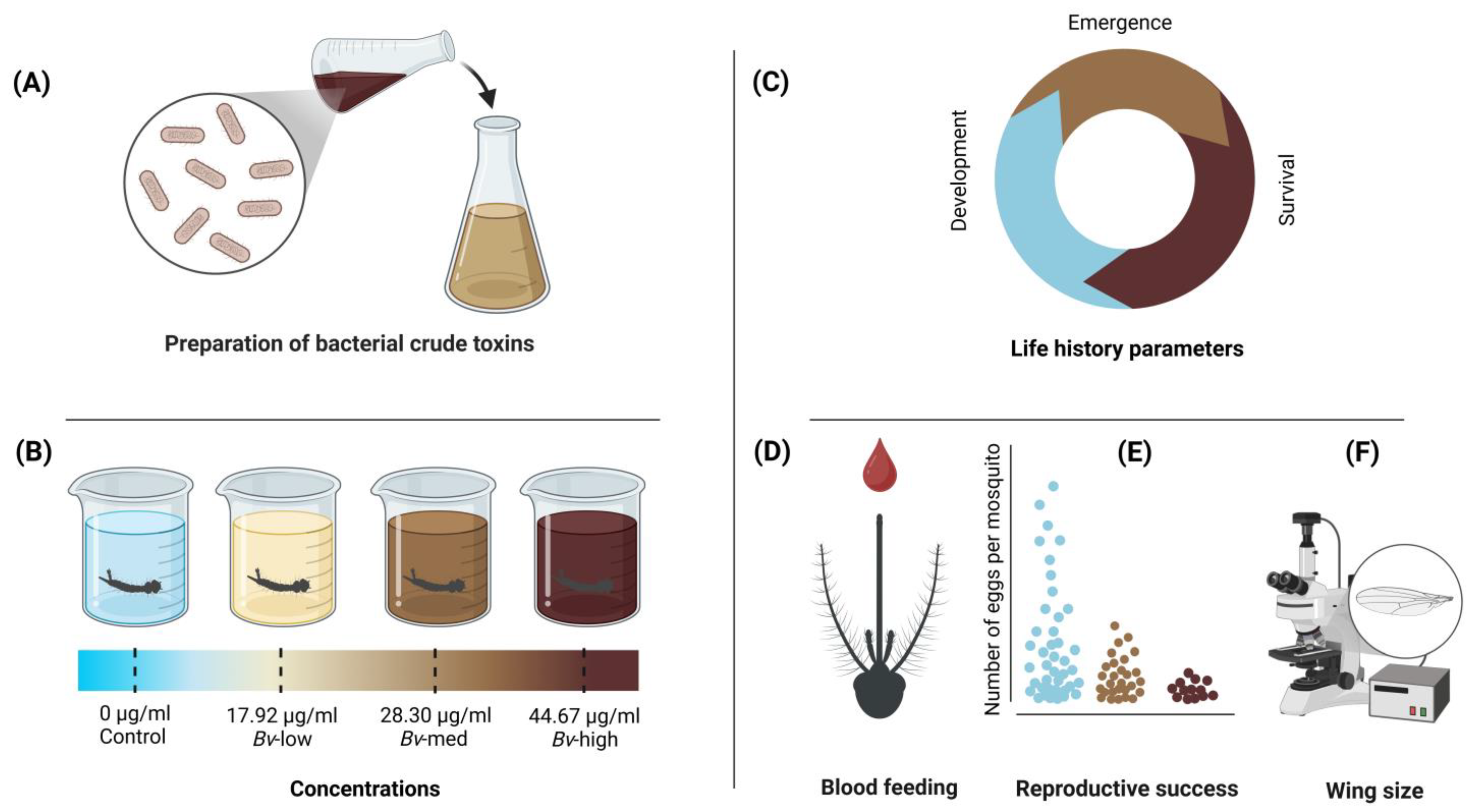
2.2. Preparation of Bacterial Crude Toxins
The strain of
Bv WHk23 was isolated from a soil sample collected in Wadi Hanifah, Riyadh, as described previously [
9]. The bacteria were inoculated into 300 mL of NB2 in a sterile 1 L flask and incubated at 30 °C for two nights with shaking at 200 rpm. The culture was then centrifuged at 13,000×
g for 10 min at 4 °C to obtain the supernatant. The resulting supernatant was acidified to pH 2 with 6N HCl and stored at 4 °C overnight to facilitate the precipitation of the crude toxin. The precipitate was collected by centrifugation at 9000×
g at 4 °C for 30 min. The resulting precipitate was resuspended in dH
2O, and the pH was adjusted to 7 with 1N NaOH to ensure solubilization. The solubilized original toxin was then lyophilized, weighed, and stored at 4 °C until being used for experiments.
2.3. Toxicity Bioassay
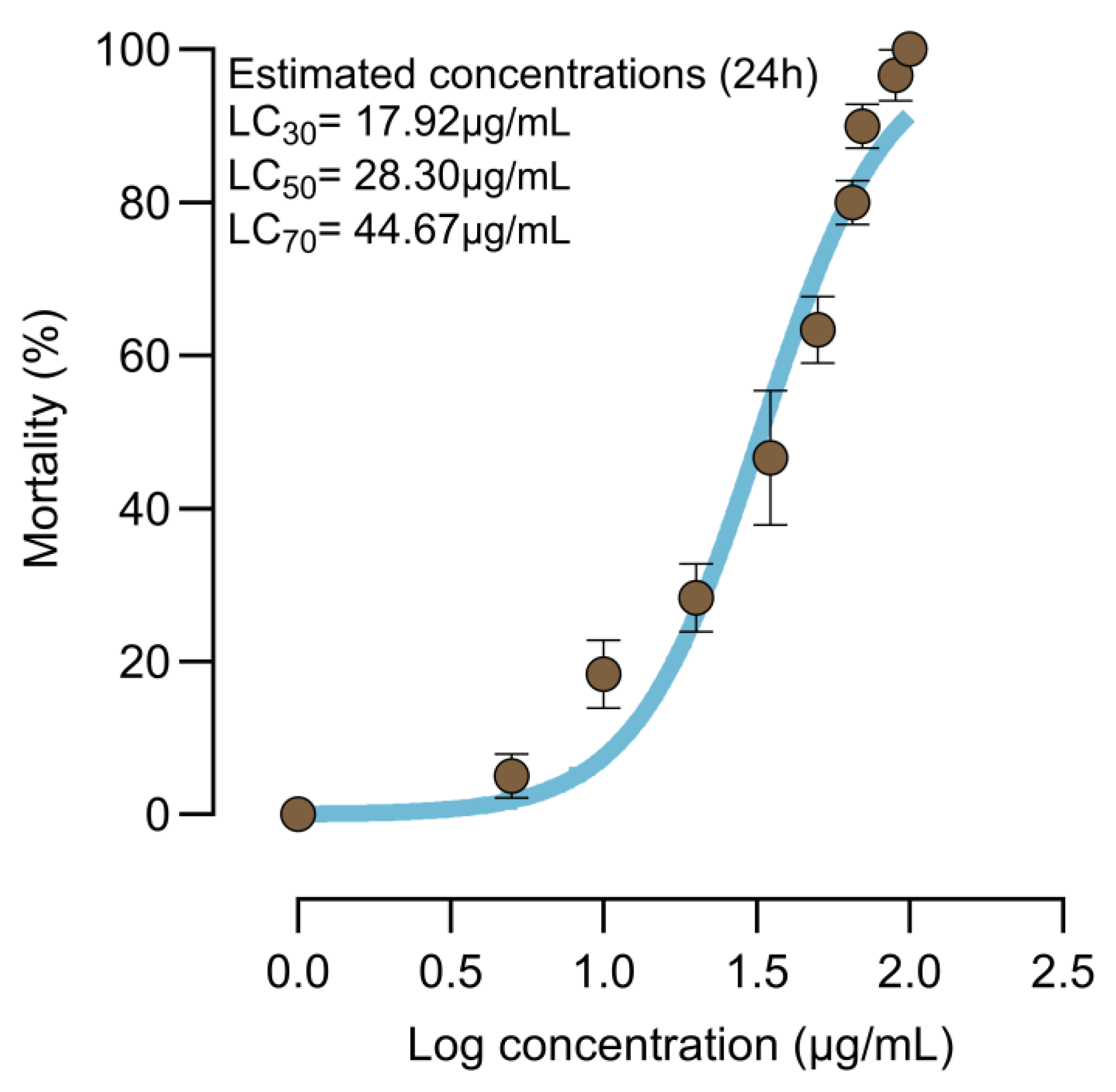
A 1 mg/mL stock solution of Bv crude toxin was prepared with dechlorinated water and then serially diluted to test the susceptibility of larvae of Cx. quinquefasciatus to Bv. Several concentrations of the crude toxin were tested (5, 10, 20, 35, 50, 65, 70, 90, and 100 µg/mL). Twenty third instar larvae were placed in plastic cups containing 200 mL of dechlorinated water with the desired toxin concentration. Each concentration was replicated three times and incubated at a controlled temperature of 27 °C. Larval mortality was recorded after 24 h of exposure. If the mortality of the control group exceeded 20%, the results were considered invalid, and the experiment was repeated. Based on the concentration–response data from the bioassay, the lethal concentrations (LC30, LC50, and LC70) for subsequent experiments were determined using probit analysis.
2.4. Larval Development Time and Adult Emergence
Three hundred first instar larvae (<24 h old) were placed into each experimental tray (experimental unit) containing 1.5 L of dechlorinated water with 0.2 g of larval food (TetrMin fish food). The experimental trays were assigned to one of four treatments—control,
Bv-low (LC
30),
Bv-medium (LC
50), and
Bv-high (LC
70) concentrations of
Bv—and replicated three times (
Figure 1).
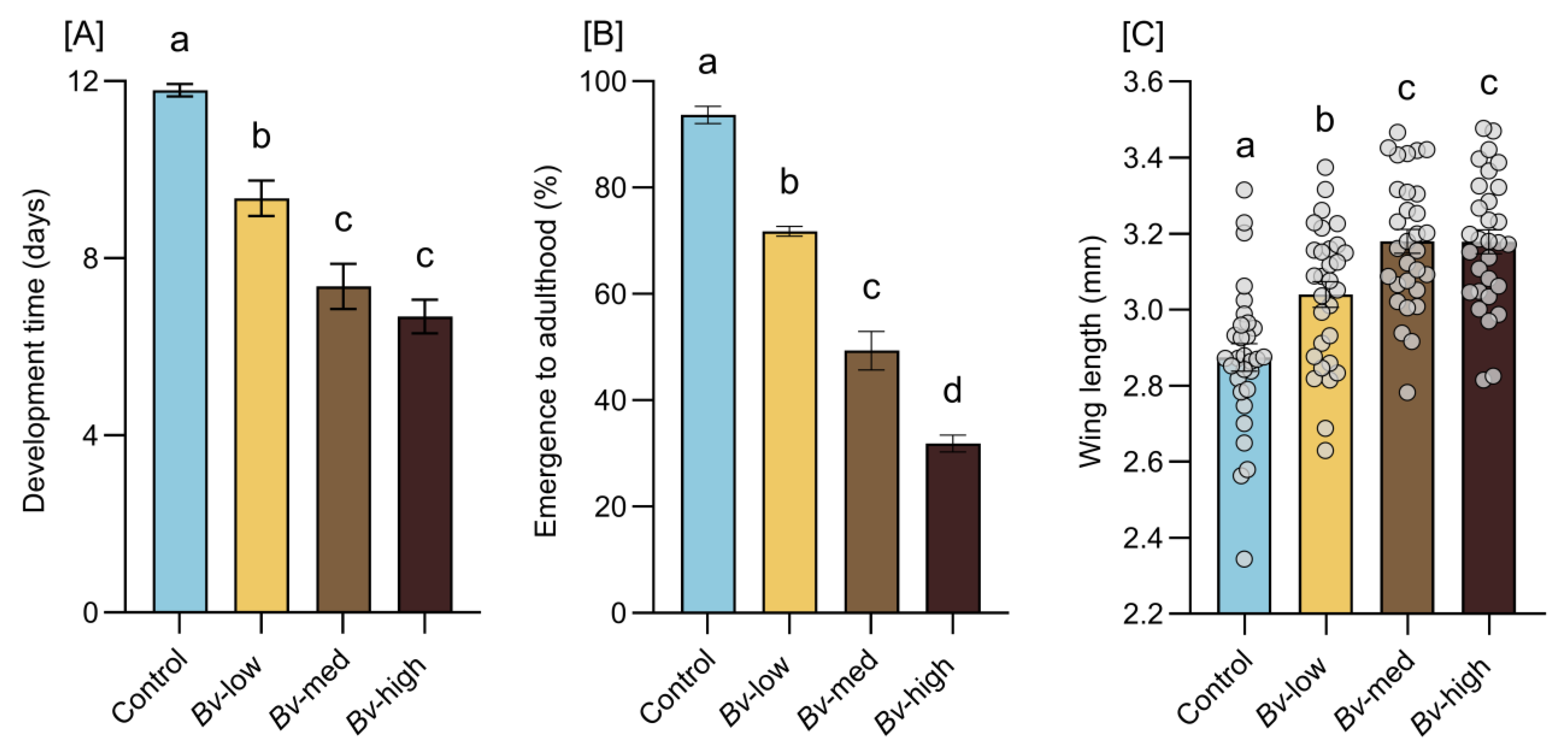
These concentrations were applied to Bv treatments when the majority reached the third instar stage, while control groups received no Bv exposure. During the 24 h exposure period, no food was provided for the larvae. After this period, surviving larvae from each treatment were transferred to new trays containing dechlorinated water without toxins. New pupae were collected daily and placed individually in 30 mL plastic cups for adult emergence. After emerging, adults were fed a 10% sucrose solution. For each experimental tray, larval development time and adult emergence (total number of adults emerged divided by the initial number of larvae introduced, expressed as a percentage) were monitored daily. All experimental trays were kept under controlled conditions with a 12 h light–dark photoperiod, a temperature of 27 °C, and a relative humidity of 60 ± 10%.
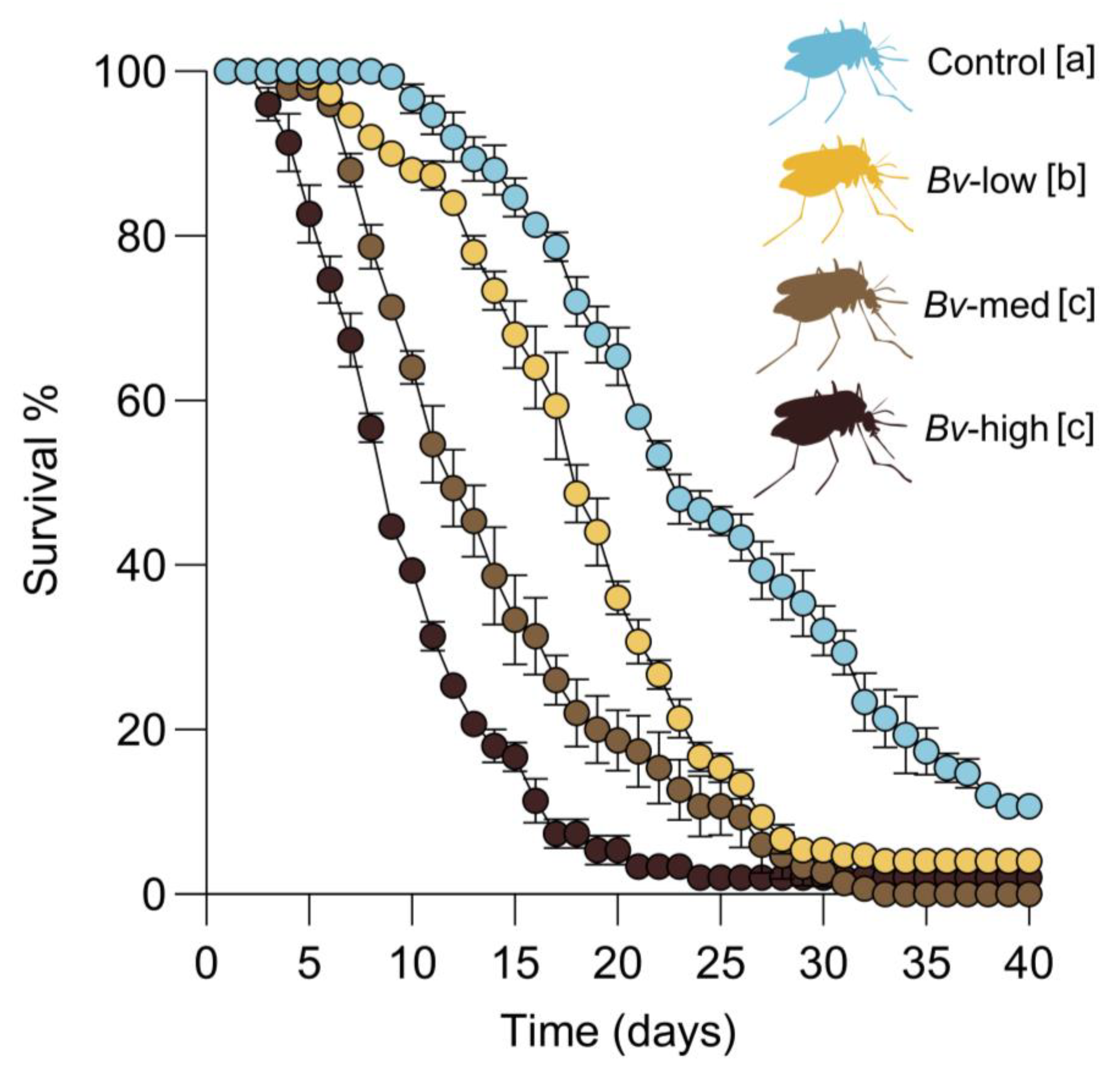
2.5. Female Survival, Reproductive Fitness, and Offspring Viability
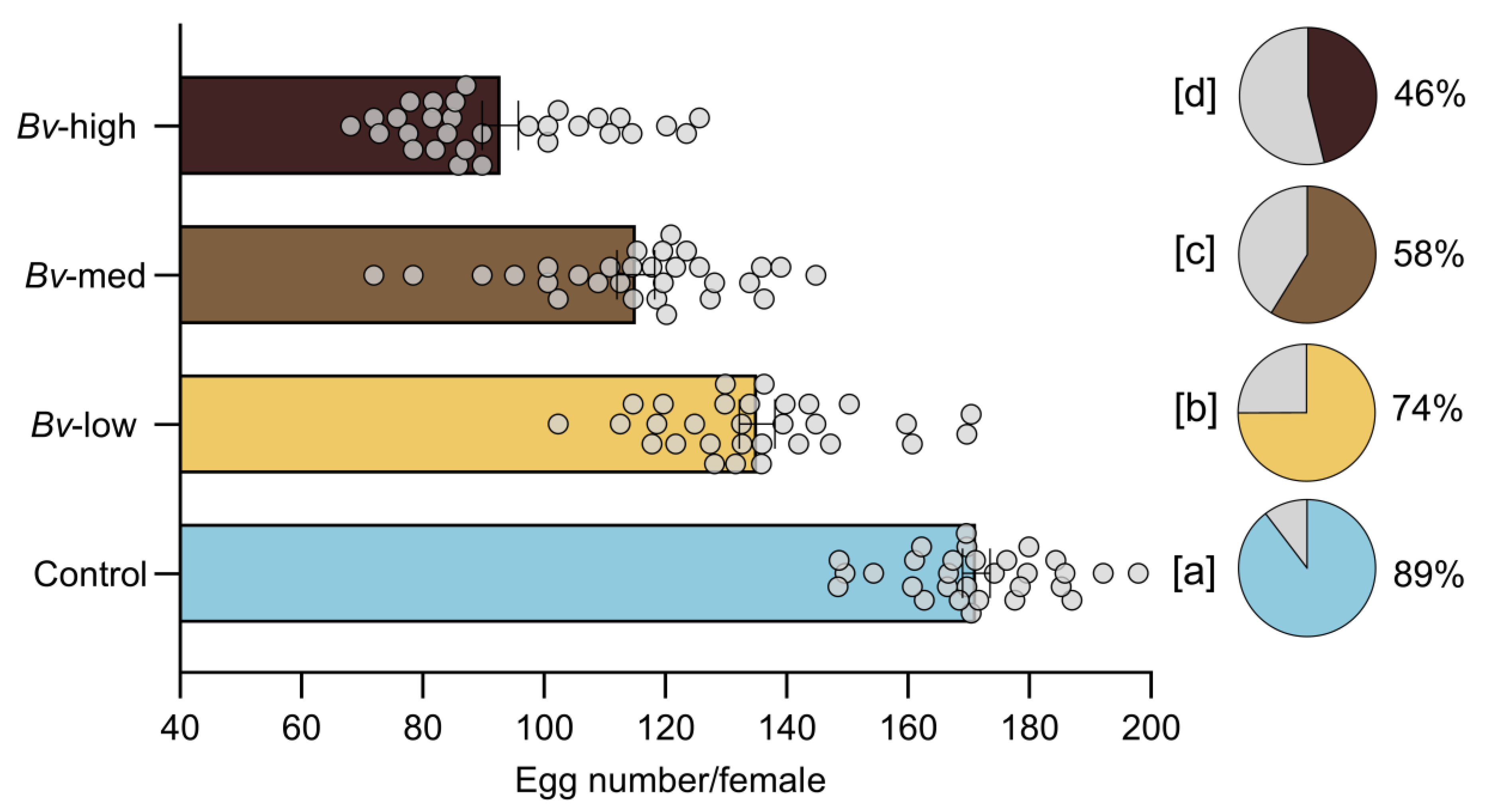
Female survival was assessed by placing newly emerged female mosquitoes into 16 oz paper-cup cages. Access to 10% sucrose solution was provided by a cotton ball placed on the top of the cages and changed every three days. Female survival was monitored daily. The female cages were kept at 27 °C until the end of the survival experiment. To assess the effect of Bv on mosquito reproduction (fecundity), emerged adults from each treatment group were first maintained in mesh cages with both males and females together to allow for natural mating. Subsequently, a group of five- to eight-day-old female mosquitoes were deprived of sucrose overnight, and then offered a single blood meal consisting of defibrinated chicken blood for 1 h using a Hemotek membrane blood feeder preheated to 37 °C. After feeding, mosquitoes were immobilized by cold treatment (2–3 min), and partially fed and unfed individuals were discarded. Fully engorged females from each treatment group were placed individually in new cup cages with mesh screens and provided with 10% sucrose solution via cotton balls. Each cage contained a 100 mL plastic oviposition cup filled with dechlorinated water. The total number of eggs laid by each female mosquito was recorded as a measure of fecundity. Fertility was assessed approximately 72 h after oviposition by counting the number of neonates hatched from each egg raft.
2.6. Female Body-Size Determination
To assess the effects of
Bv on mosquito body size, groups of female mosquitoes were collected from each treatment and body size was determined by measuring wing length as a proxy for body size [
15]. One wing from each female was selected, mounted on a microscope slide, and the distance from the axillary margin to the apical notch without considering wing fringe was measured in millimeters. Measurements were made using a calibrated dissecting microscope equipped with an ocular micrometer.
2.7. Data Analysis
Concentration–response data were analyzed using probit analysis, corrected for control mortality, when necessary, to estimate LC30, LC50, and LC70 values and their 95% confidence intervals. Data on the effects of exposure on mosquito life history, including development time, adult emergence, fecundity, fertility, and wing length, were analyzed using separate ANOVA tests followed by multiple comparisons using Tukey post hoc tests. Adult survival data of females were analyzed using a Cox proportional hazards regression analysis of survival data. Statistical analyses were performed using GraphPad Prism 10 (GraphPad Software, San Diego, CA, USA), with a p value of <0.05 considered statistically significant.
4. Discussion
Biological control agents represent a promising alternative to conventional chemical insecticides for mosquito control.
Bacillus velezensis has emerged as a potential biocontrol agent due to its ability to produce a wide range of secondary metabolites and bioactive compounds with insecticidal properties. Although it is not as extensively studied or widely used as
Bti, recent research indicates that
Bv may hold significant promise in targeting mosquito larvae and reducing vector populations [
9,
10].
The third instar larval toxicity bioassay revealed that the LC
50 of
Bv against
Cx. quinquefasciatus was estimated to be 28.30 µg/mL after 24 h of exposure, supporting previous findings on the biocontrol potential of this strain. Consistent with this result, the same strain has been reported to exhibit significant larvicidal activity, with LC
50 values of 25.41 and 33.97 µg/mL against
Anopheles stephensi and
Cx. quinquefasciatus, respectively [
9]. A study by Kannan Revathi et al. [
16] demonstrated that the culture supernatant of
B. subtilis exhibited significant larvicidal activity against
Aedes aegypti, with LC
50 values of 1.73 µg/mL. Similarly,
B. amyloliquefaciens culture supernatant showed species-specific toxicity, with LC
50 values of 26.4 µg/mL for
An. stephensi, 22.2 µg/mL for
Cx. quinquefasciatus, and 20.5 µg/mL for
Ae. aegypti [
17]. In comparison,
Bv strains B64a and B15 exhibited larvicidal activity against
Ae. aegypti, with crude lipopeptide extracts reaching LC
50 values of 110 µg/mL and 120 µg/mL, respectively, where the authors suggested that the larvicidal effect was mainly due to metabolites produced by these bacteria [
12]. Furthermore, silver nanoparticles synthesized using
Bv demonstrated strong toxicity against third instar
Ae. aegypti larvae, with an LC
50 of 0.76 µg/mL [
10]. These findings highlight the potential of
Bv as an effective larvicidal agent, although its efficacy varies depending on the strain, mosquito species, and extraction or formulation method. This underscores the need for careful strain selection and optimization to enhance biocontrol efficacy against mosquito vectors.
Several studies have shown that sublethal exposure to
Bacillus spp. and chemical insecticides during larval stages can result in a carry-over effect on the survival and fitness of adult mosquitoes. For instance,
Ae. aegypti larvae exposed to
Bti developed into adults with reduced survival rates [
18]. Similarly, a study examined the effects of sublethal permethrin exposure on various fitness parameters of
Cx. quinquefasciatus, where the exposure resulted in a significant reduction in the survival of adult females. These detrimental effects are suggested to result from physiological stress and energy costs associated with detoxification processes, ultimately compromising the overall fitness and survival of adult mosquitoes [
19]. In another study, larval exposure to pyriproxyfen resulted in reduced survival of
Ae. aegypti adults [
20]. In the current study, the sublethal exposure to
Bv significantly shortened the survival of
Cx quinquefasciatus females, with the effect being more pronounced at higher exposure concentrations. This indicates a potential dose-dependent physiological cost associated with larval exposure to
Bv, which may impact female survival. Further studies are needed to elucidate the exact mechanism responsible for the reduction in female survival following the larval exposure. Collectively, these findings underscore the importance of considering sublethal effects in mosquito control programs. Beyond direct larvicidal activity, biocontrol agents such as
Bv may also contribute to population suppression through a reduction in adult mosquito survival, which could have implications for vector control efficacy and disease transmission dynamics.
The current study demonstrated that exposure to
Bv led to a dose-dependent larval mortality, reducing intraspecific competition for food and space. This reduction in larval competition contributed to a shortened development time and the emergence of larger adults [
21]. These effects are consistent with previous findings where exposure to chemical agents such as malathion resulted in larval mortality that promoted faster development and larger adult body size [
22,
23]. Under high densities, competition prolongs development and often results in smaller adults, whereas reduced competition promotes faster development and yields larger individuals with the potential for increased reproductive output. However, no fitness advantage was observed in terms of reproductive success in the emergence of larger females following
Bv exposure. Contrary to the common assumption that larger mosquitoes exhibit higher fecundity, this study revealed that exposure to
Bv negatively affected both fecundity and fertility, leading to fewer eggs laid and lower hatching rates. These results align with earlier observations in
An. coluzzii, where larval exposure to increasing concentrations of
Bti produced larger adults but was accompanied by a decline in egg production [
24]. Similar trends have been reported in other studies, where larval exposure to
Bti resulted in reductions in fecundity of
Ae. albopictus [
18]. Although this study did not directly investigate the underlying mechanisms of sublethal effects, these findings suggest that larval exposure to
Bv may influence not only mosquito development and size but also induce physiological effects that impair reproductive capacity. These results underscore the complex trade-offs associated with sublethal exposure to insecticides, where increased body size does not necessarily translate into enhanced fecundity [
15,
25]. Understanding such effects is critical for evaluating the broader implications of
Bv as a biocontrol agent and for optimizing its integration into mosquito management programs.