Environmental Heterogeneity and Altitudinal Gradients Drive Darkling Beetle Diversity in an Alluvial Fan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Sites and Selection
2.3. Collection of Darkling Beetles
2.4. Investigation of Environmental Factors
2.5. Data Analysis
2.5.1. Analysis of the Diversity of Darkling Beetle Communities
2.5.2. Non-Metric Multidimensional Scaling Analysis
2.5.3. Beta Diversity Analysis
2.5.4. Analysis of the Relationship Between the Diversity of Darkling Beetle Communities and Environmental Factors
3. Results
3.1. Composition of Darkling Beetle Communities
3.2. Diversity of Darkling Beetle Communities
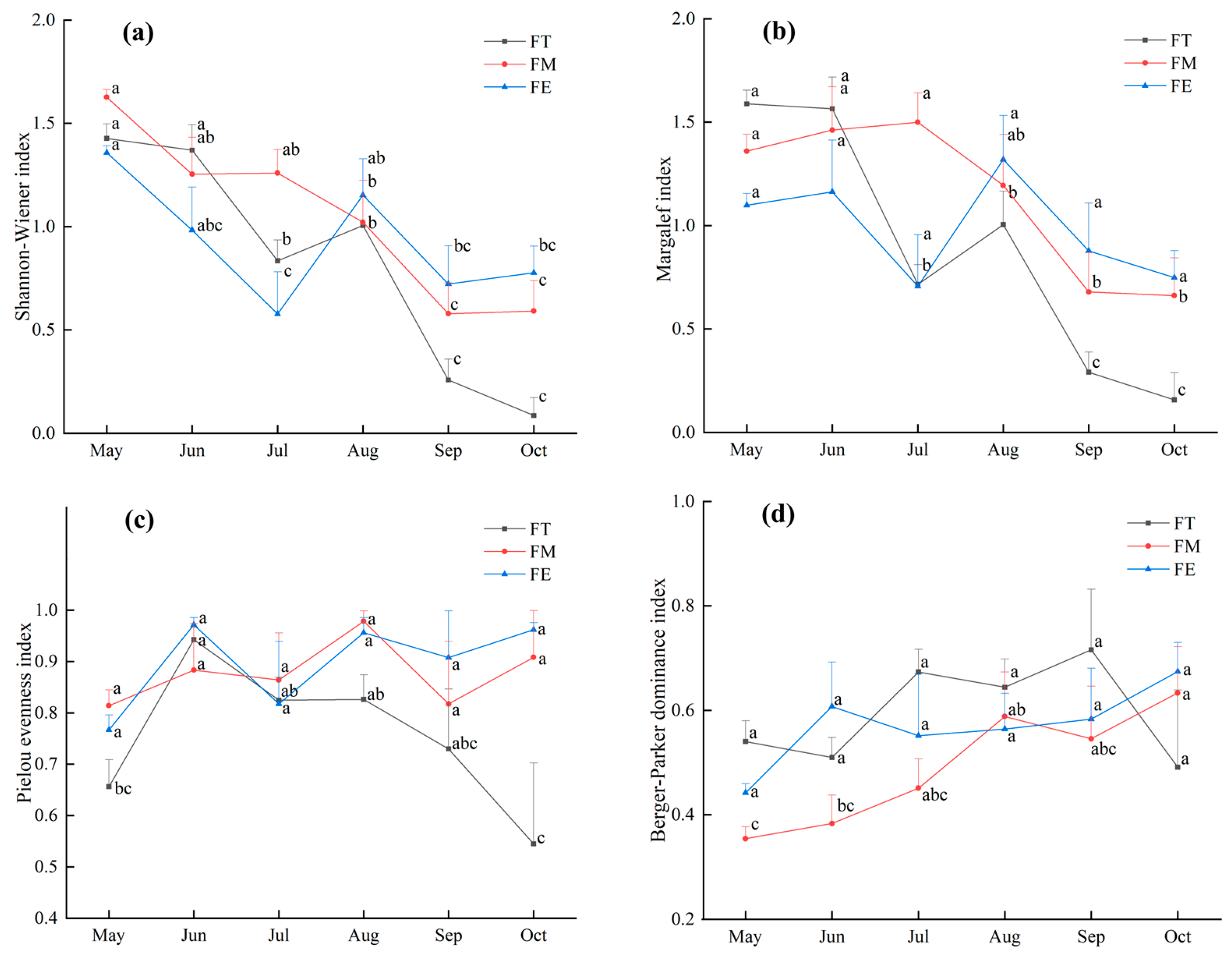
3.2.1. Temporal Dynamics of the Diversity of Darkling Beetle Communities in Different Sample Plots
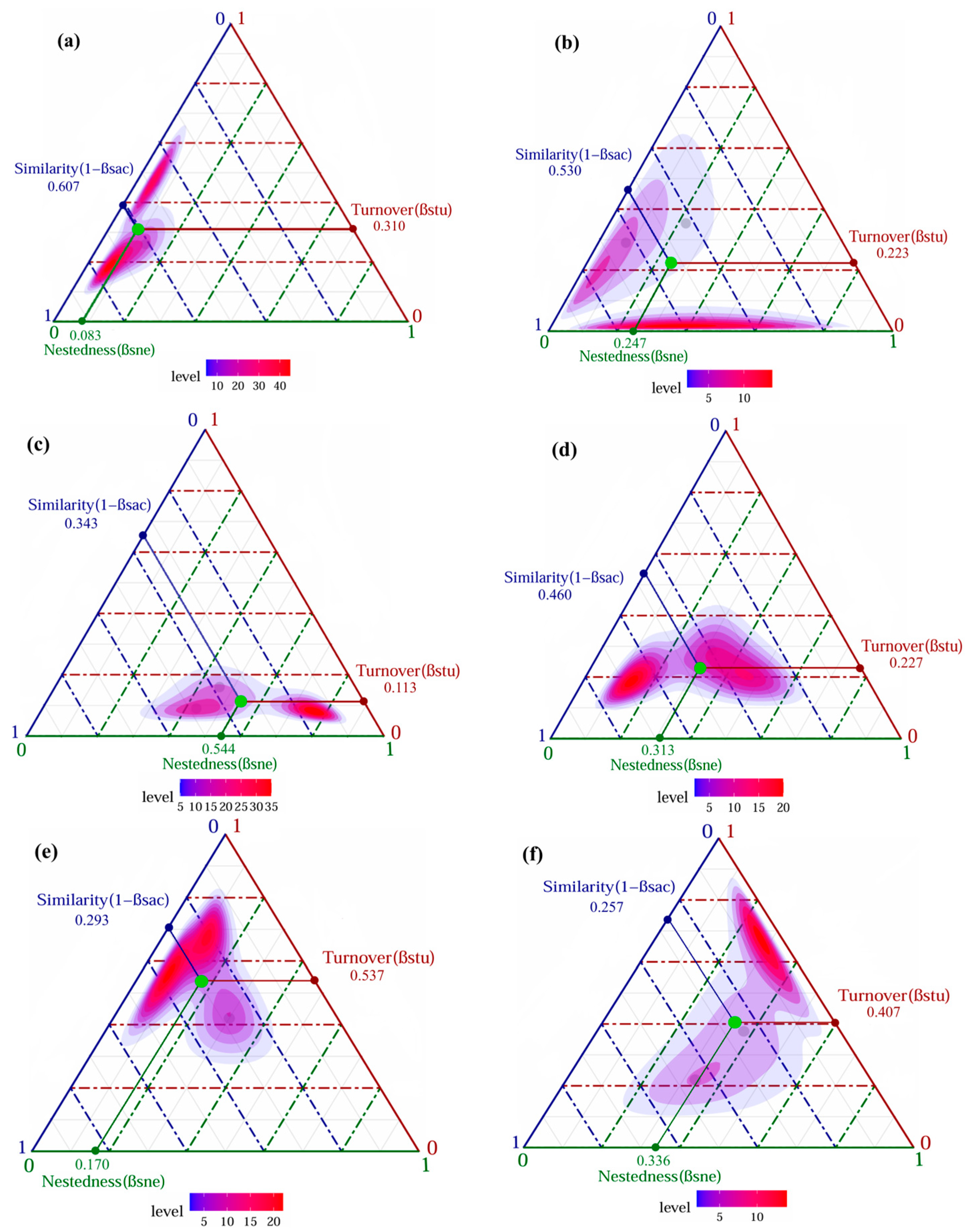
3.2.2. Beta Diversity and Its Components of Darkling Beetle Communities
3.3. The Relationship Between the Distribution of Darkling Beetle Species and Environmental Factors
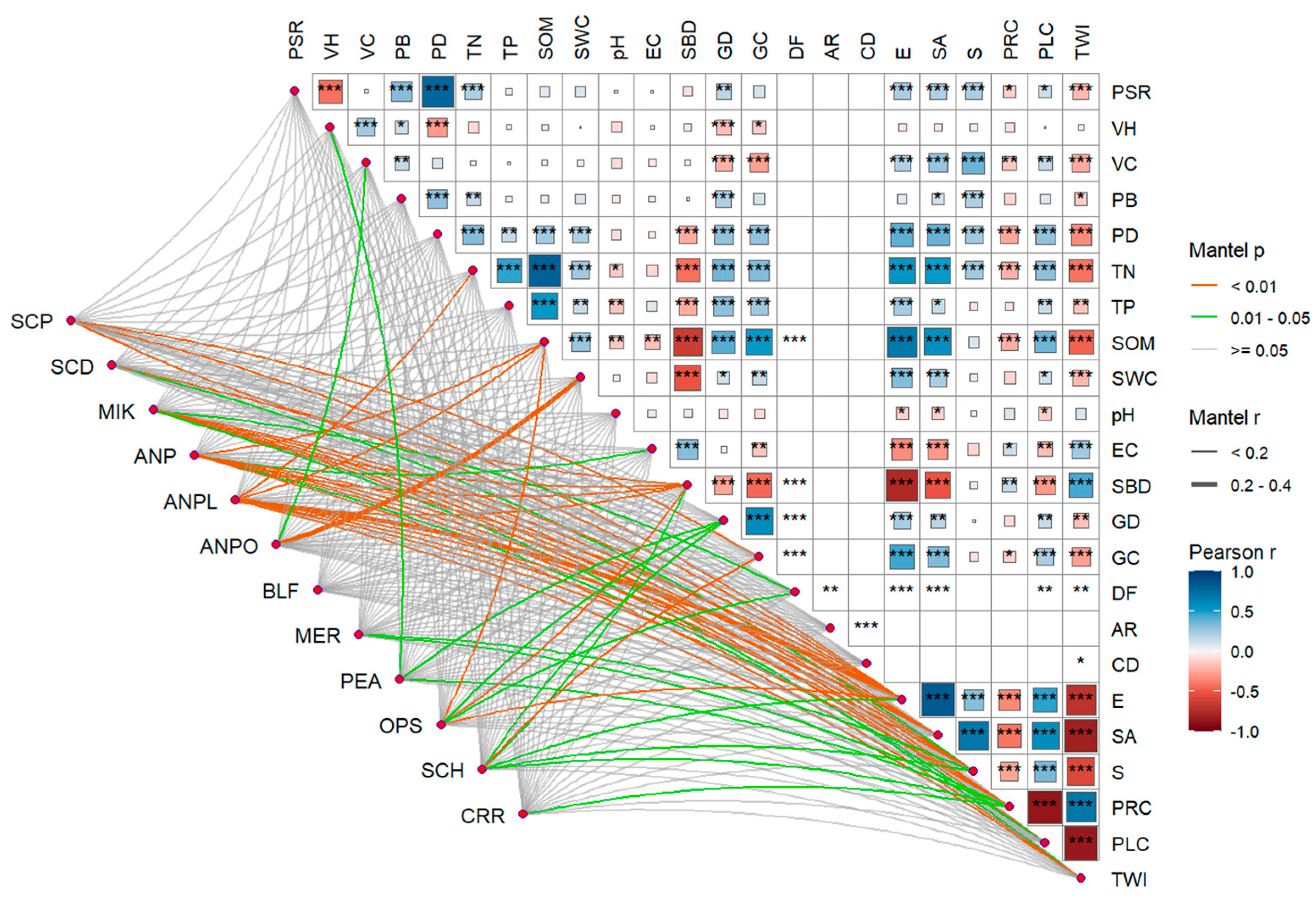
3.3.1. The Correlation Between Species Abundance and Environmental Factors
3.3.2. Influencing Factors of the Distribution of Darkling Beetle Species in Different Sample Plots
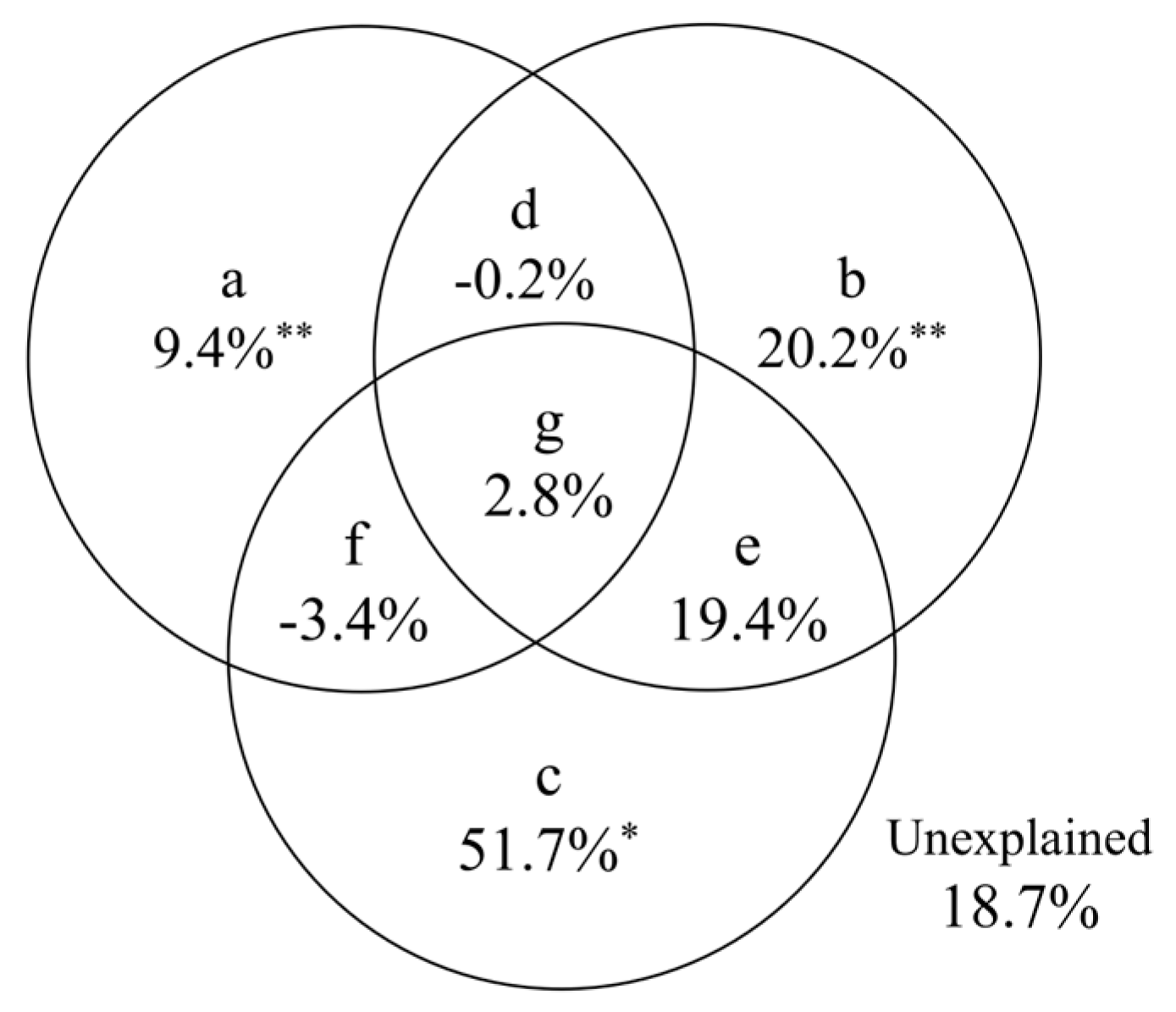
3.3.3. Influencing Factors of the Distribution of Darkling Beetle Species in Fan Landscapes
4. Discussion
4.1. Dominant Species of Darkling Beetle Communities in Alluvial Fan Landforms
4.2. Diversity Changes of Darkling Beetle Communities in Alluvial Fan Landforms
4.3. Analysis of the Causes for the Distribution of Darkling Beetle Species in Alluvial Fan Landforms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, G.; Yu, Y. Tenebrionidae Insects in the Deserts and Semi–Deserts of China; Hebei University Press: Baoding, China, 1999; pp. 19–23. (In Chinese) [Google Scholar]
- Zhang, D.; Chen, X.; He, D. Species Diversity of Darkling Beetles in Desert Landscape and Their Value as Bioindicators. Appl. Entomol. 2012, 49, 229–235. (In Chinese) [Google Scholar]
- Bartholomew, A.; Hafezi, S.A.; Karimi, S. Effects of Habitat Complexity on the Abundance, Species Richness and Size of Darkling Beetles (Tenebrionidae) in Artificial Vegetation. Arid. Environ. 2016, 129, 35–41. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, J. Prediction of the Spatial Patterns of Species Richness Based on the Plant–Topography Relationship: An Application of GAMs Approach. Acta Ecol. Sin. 2007, 27, 953–962. (In Chinese) [Google Scholar]
- Ma, Y.; Ma, C.; Ouyang, F.; Hang, J.; Shi, Y. Response of Spatial Heterogeneity of Surface Beetles to Environmental Changes in the Loess Hilly Region. Northwest A F Univ. Nat. Sci. Ed. 2021, 49, 68–75. (In Chinese) [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Yang, Y.; Yang, G. Communities Biodiversity of Ground–Dwelling Beetle and Its Relations with Environmental Factors in Helan Mountains, Northwestern China. Arid. Land. Resour. Environ. 2020, 34, 154–161. (In Chinese) [Google Scholar] [CrossRef]
- Yang, G.; Jia, L.; Zhang, J.; Yu, Y. Distribution of Darkling Beetles and Its Relationships with Topography in Helan Mountain, Ningxia. Environ. Entomol. 2016, 38, 77–86. (In Chinese) [Google Scholar]
- Liu, J.; Zhao, W.; Li, F.; Fang, J. Effects of Microtopography Variation on the Distribution of Ground Darkling Beetles in a Sandy Desert Ecosystem. Arid Zone Res. 2017, 36, 388–1394. (In Chinese) [Google Scholar] [CrossRef]
- Ford, H.; Evans, B.; Van Klink, R.; Skov, M.W.; Garbutt, A. The Importance of Canopy Complexity in Shaping Seasonal Spider and Beetle Assemblages in Saltmarsh Habitats. Ecol. Entomol. 2017, 42, 145–155. [Google Scholar] [CrossRef]
- Bartholomew, A.; El Moghrabi, J. Seasonal Preference of Darkling Beetles (Tenebrionidae) for Shrub Vegetation Due to High Temperatures, Not Predation or Food Availability. Arid. Environ. 2018, 156, 34–40. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liu, C.; Liu, Q. Influences of Shrub Vegetation on Distribution and Diversity of a Ground Beetle Community in a Gobi Desert Ecosystem. Biodivers. Conserv. 2012, 21, 2601–2619. [Google Scholar] [CrossRef]
- Allema, B.; Hemerik, L.; Rossing, W.A.H.; Groot, J.C.J.; van Lenteren, J.C.; van der Werf, W. Dispersal of a Carabid Beetle in Farmland Is Driven by Habitat-Specific Motility and Preference at Habitat Interfaces. Entomol. Exp. Appl. 2019, 167, 741–754. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Wang, M.; Jia, L. Niche and Interspecific Association of Darkling Beetles in a Desert Grassland of Alluvial Fans in Helan Mountain, Northwestern China. Acta Entomol. Sin. 2021, 64, 840–850. (In Chinese) [Google Scholar] [CrossRef]
- Padial, A.A.; Ceschin, F.; Declerck, S.A.J.; Meester, L.D.; Bonecker, C.C.; Lansac-Tôha, F.A.; Rodrigues, L.; Rodrigues, L.C.; Train, S.; Velho, L.F.M.; et al. Dispersal Ability Determines the Role of Environmental, Spatial and Temporal Drivers of Metacommunity Structure. PLoS ONE 2014, 9, e111227. [Google Scholar] [CrossRef] [PubMed]
- De Los Santos, A.; Gómez-González, L.A.; Alonso, C.; Arbelo, C.D.; De Nicolás, J.P. Adaptive Trends of Darkling Beetles (Col. Tenebrionidae) on Environmental Gradients on the Island of Tenerife (Canary Islands). J. Arid. Environ. 2000, 45, 85–98. [Google Scholar] [CrossRef]
- Krasnov, B.; Ward, D.; Shenbrot, G. Body Size and Leg Length Variation in Several Species of Darkling Beetles (Coleoptera: Tenebrionidae) Along a Rainfall and Altitudinal Gradient in the Negev Desert (Israel). J. Arid. Environ. 1996, 34, 477–489. [Google Scholar] [CrossRef]
- Hendrickx, F.; Maelfait, J.-P.; Desender, K.; Aviron, S.; Bailey, D.; Diekotter, T.; Lens, L.; Liira, J.; Schweiger, O.; Speelmans, M.; et al. Pervasive Effects of Dispersal Limitation on Within- and Among-Community Species Richness in Agricultural Landscapes. Glob. Ecol. Biogeogr. 2009, 18, 607–616. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milne, B.T.; Wiens, J.A. Diffusion in Fractcal Landscapes: Simulations and Experimental Studies of Tenebrionid Beetle Movements. Ecology 1992, 73, 1968–1983. [Google Scholar] [CrossRef]
- Mo, D.; Zhu, Z.; Wan, L. The Alluvial Fans along the Eastern Foot of Helan Mountain. Acta Sci. Nat. Univ. Pekin. 1999, 35, 816–823. (In Chinese) [Google Scholar] [CrossRef]
- Shen, A.; Zhao, N.; Shi, Y.; Mi, W.; She, J.; Zhang, F.; Guo, R.; Wu, T.; Li, Z.; Li, J.; et al. Soil Ecological Stoichiometry in Varied Micro-Topographies of an Alluvial Fan at Eastern Helan Mountains, Northwest China. J. Arid. Land. 2024, 16, 1648–1663. [Google Scholar] [CrossRef]
- Chu, G.; Wang, M.; Zhang, S. Spatial Point Patters of Anabasis Aphylla Populations in the Proluvial Fan of South Junggar Basin. Sci. Silvae Sin. 2014, 50, 8–14. (In Chinese) [Google Scholar]
- Ding, J.; Zhang, P.; Zhang, H.; Li, Z.; Feng, Y. Spatial Heterogeneity of Ephedra Przewalskii Populations in the Stony Desert in the Middle of Southern Foot of Tianshan Mountains, China. Chin. J. Appl. Ecol. 2020, 31, 3997–4003. (In Chinese) [Google Scholar] [CrossRef]
- Parker, K.C. Effects of Complex Geomorphic History on Soil and Vegetation Patterns on Arid Alluvial Fans. J. Arid. Environ. 1995, 30, 19–39. [Google Scholar] [CrossRef]
- Flores, D.G.; Suvires, G.M.; Ocaña, R. Actividad Geomorfológica y Colonización Vegetal En Depósitos de Abanicos Aluviales Del Desierto Del Monte Central de Argentina. Cuad. Investig. Geográfica Geogr. Res. Lett. 2017, 43, 293–308. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Wang, J.; Xu, M.; Ali, A.; Xu, Y.; Lamb, D.; Duan, L.-C.; Yan, K.-H.; Yang, S.-T. Effects of the Ephemeral Stream on Plant Species Diversity and Distribution in an Alluvial Fan of Arid Desert Region: An Application of a Low Altitude UAV. PLoS ONE 2019, 14, e0212057. [Google Scholar] [CrossRef]
- Fu, S.; Lu, B.; Ye, Z. Effects of Rock Fragments on Runoff and Soil Erosion. J. Soil. Water Conserv. 2010, 24, 15–18+34. (In Chinese) [Google Scholar] [CrossRef]
- Pietrasiak, N.; Drenovsky, R.E.; Santiago, L.S.; Graham, R.C. Biogeomorphology of a Mojave Desert Landscape—Configurations and Feedbacks of Abiotic and Biotic Land Surfaces during Landform Evolution. Geomorphology 2014, 206, 23–36. [Google Scholar] [CrossRef]
- Lv, W.; Qiu, Y.; Xie, Z.; Wang, X.; Wang, Y. Effects of Mulch Gravels with Different Sizes on the Process of Water Infiltration. Res. Soil. Water Conserv. 2021, 28, 46–51. (In Chinese) [Google Scholar] [CrossRef]
- Hassanzadeh Bashtian, M.; Karimi, A.; Sepehr, A.; Lakzian, A.; Caballero, E.R. Spatial Relationship of Landform Surface Features and Biocrusts, and Their Effect on Soil Microbial Biomass on an Alluvial Fan. Earth Surf. Process. Landf. 2024, 49, 1348–1360. [Google Scholar] [CrossRef]
- Ríos-Casanova, L.; Valiente-Banuet, A.; Rico-Gray, V. Ant Diversity and Its Relationship with Vegetation and Soil Factors in an Alluvial Fan of the Tehuacán Valley, Mexico. Acta Oecologica 2006, 29, 316–323. [Google Scholar] [CrossRef]
- Treonis, A.M.; Sutton, K.A.; Unangst, S.K.; Wren, J.E.; Dragan, E.S.; McQueen, J.P. Soil Organic Matter Determines the Distribution and Abundance of Nematodes on Alluvial Fans in Death Valley, California. Ecosphere 2019, 10, e02659. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Y.; Chen, D.; Zhang, H. Spatial and Temporal Effects on the Value of Ecosystem Services in Arid and Semi-Arid Mountain Areas—A Case Study from Helan Mountain in Ningxia, China. Front. Ecol. Evol. 2023, 10. [Google Scholar] [CrossRef]
- Cheli, G.H.; Bosco, T.; Flores, G.E. The Role of Nyctelia circumundata (Coleoptera: Tenebrionidae) on Litter Fragmentation Processes and Soil Fertility in Northeastern Arid Patagonia. Geoderma 2022, 415, 115770. [Google Scholar] [CrossRef]
- Cheli, G.H.; Bosco, T.; Flores, G.E. The Role of Nyctelia dorsata Fairmaire, 1905 (Coleoptera: Tenebrionidae) on Litter Fragmentation Processes and Soil Biogeochemical Cycles in Arid Patagonia. Ann. Zool. 2022, 72, 129–134. [Google Scholar] [CrossRef]
- de Vega, C.; Arista, M.; Ortiz, P.L.; Herrera, C.M.; Talavera, S. Endozoochory by Beetles: A Novel Seed Dispersal Mechanism. Ann. Bot. 2011, 107, 629–637. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Su, J.; Wen, Y. Spatially Differentiated Characteristics of Vegetation Carbon Sequestration Function in Helan Mountain Area and Its Driving Factors. Acta Ecol. Sin. 2023, 43, 10250–10262. (In Chinese) [Google Scholar] [CrossRef]
- Ren, G.; Ba, Y. Fauna of Soil Darkling Beetles in China Vol. 2 Tenty Riforms (Coleoptera: Tenebrionidae); Science Press: Beijing, China, 2010; pp. 26–187. (In Chinese) [Google Scholar]
- Ren, G.; Yang, X. Fauna of Soil Darkling Beetles in China Vol. 1 Opatriformes (Coleoptera: Tenebrionidae); Science Press: Beijing, China, 2006; pp. 36–201. (In Chinese) [Google Scholar]
- Bao, S. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000; pp. 25–400. (In Chinese) [Google Scholar]
- Qian, G.; Dong, Z.; Luo, W.; Feng, Y.; Wu, B.; Yang, W. Gravel Morphometric Analysis Based on Digital Images of Different Gobi Surfaces in Northwestern China. J. Desert Res. 2014, 34, 625–633. (In Chinese) [Google Scholar]
- Yang, Y.; Yang, G.; Wang, J. Effects of Topographic Factors on the Distribution Pattern of Carabid Species Diversity in the Helan Mountains, Northwestern China. Acta Entomol. Sin. 2017, 60, 1060–1073. (In Chinese) [Google Scholar] [CrossRef]
- Ge, F. Principle and Methods of Inesct Ecology; Higher Education Press: Beijing, China, 2008; pp. 249–251. (In Chinese) [Google Scholar]
- Begon, M.; Townsend, C.; Harper, J. Ecology: From Individuals to Ecosystems, 4th ed.; Wiley—Blackwell: Hoboken, NJ, USA, 2016; pp. 447–455. [Google Scholar]
- Baselga, A. Partitioning the Turnover and Nestedness Components of Beta Diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Yang, G.; Yu, Y.; Wang, X. Tendbrionidae Fauna and Ecological Distribution in Ningxia Helan Mountain Nature Reserve. J. Ningxia Univ. Nat. Sci. Ed. 2011, 32, 67–72. (In Chinese) [Google Scholar]
- Maeno, K.; Nakamura, S.; Babah, M. Nocturnal and Sheltering Behaviours of the Desert Darkling Beetle, Pimelia senegalensis (Coleoptera: Tenebrionidae), in the Sahara Desert. Afr. Entomol. 2014, 22, 499–504. [Google Scholar] [CrossRef]
- Harrison, S.; Ross, S.; Lawton, J. Beta Diversity on Geographic Gradients in Britain. J. Anim. Ecol. 1992, 61, 151–158. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Stoll, S.; Jähnig, S.C.; Haase, P. Contrasting Metacommunity Structure and Beta Diversity in an Aquatic-Floodplain System. Oikos 2016, 125, 686–697. [Google Scholar] [CrossRef]
- Imeni, S.; Sadough, H.; Bahrami, S.; Mehrabian, A.; Nosrati, K. Geomorphological Controls on Vegetation Changes: A Case Study of Alluvial Fans in Southwest of Miami City, Northeastern Iran. Arab. J. Geosci. 2021, 14, 349. [Google Scholar] [CrossRef]
- Sepehr, A.; Hosseini, A.; Naseri, K.; Gholamhosseinian, A. Biological Soil Crusts Impress Vegetation Patches and Fertile Islands over an Arid Pediment, Iran. J. Ecol. Environ. 2022, 46, 31–40. [Google Scholar] [CrossRef]
- Soberón, J.M. Niche and Area of Distribution Modeling: A Population Ecology Perspective. Ecography 2010, 33, 159–167. [Google Scholar] [CrossRef]
- Yuan, P.; Wang, M.; Wang, Y.; Yang, G. Interspecific Association and Community Stability of Ground–Dwelling Beetle in a Desert Grassland of Alluvial Fan in Helan Mountain. Chin. J. Ecol. 2022, 41, 741–749. (In Chinese) [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Strona, G.; Pace, L.; Biondi, M. Elevational Patterns of Generic Diversity in the Tenebrionid Beetles (Coleoptera Tenebrionidae) of Latium (Central Italy). Diversity 2020, 12, 47. [Google Scholar] [CrossRef]
- Parmenter, R.R.; Macmahon, J.A. Factors Limiting Populations of Arid-Land Darkling Beetles (Coleoptera: Tenebrionidae): Predation by Rodents. Environ. Entomol. 1988, 17, 280–286. [Google Scholar] [CrossRef]
- Ayal, Y.; Merkl, O. Spatial and Temporal Distribution of Tenebrionid Species (Coleoptera) in the Negev Highlands, Israel. J. Arid. Environ. 1994, 27, 347–361. [Google Scholar] [CrossRef]



| Month | Precipitation (mm) | Maximum Temperature (°C) | Mean Temperature (°C) | Minimum Temperature (°C) |
|---|---|---|---|---|
| April | 9 | 17.3 | 9.8 | 2.3 |
| May | 18 | 23.6 | 16.4 | 9.1 |
| June | 42 | 27.5 | 20.8 | 14.1 |
| July | 67 | 29.1 | 22.8 | 16.5 |
| August | 51 | 27.2 | 21.1 | 15.1 |
| September | 25 | 22.5 | 15.9 | 9.3 |
| October | 13 | 16.0 | 9.0 | 2.0 |
| Species | Abbreviation | FT | FM | FE | Percentage (%) | Levins’ Index | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency * | Proportion | Frequency | Proportion | Frequency | Proportion | |||||
| Pimeliinae | Scytosoma pygmaeum | SCP | 6 | 8.11 | 4 | 1.79 | 5 | 4.57 | 5.03 | 2.14 |
| Scytosoma dissilimarginis | SCD | 4 | 7.71 | 4 | 2.39 | 5 | 6.32 | 5.57 | 2.44 | |
| Microdera kraatzi | MIK | 6 | 50.30 | 5 | 32.26 | 6 | 23.52 | 36.66 | 2.53 | |
| Anatolica pandaroides | ANP | 5 | 2.74 | 5 | 17.20 | 5 | 24.33 | 13.71 | 2.28 | |
| Anatolica planata | ANPL | 2 | 3.14 | 6 | 18.40 | 5 | 30.65 | 16.09 | 2.22 | |
| Anatolica polita | ANPO | 2 | 3.65 | 4 | 7.65 | 6 | 6.85 | 5.88 | 2.85 | |
| Tenebrioninae | Blaps femoralis | BLF | 6 | 4.87 | 6 | 15.05 | 4 | 0.81 | 7.01 | 1.78 |
| Melanesthes rugipennis | MER | 0 | 0.00 | 2 | 0.96 | 0 | 0.00 | 0.31 | 1.00 | |
| Penthicus alashanicus | PEA | 6 | 1.22 | 6 | 1.91 | 2 | 0.27 | 1.17 | 2.23 | |
| Opatyum subaratum | OPS | 2 | 5.68 | 2 | 0.72 | 1 | 0.27 | 2.49 | 1.29 | |
| Scleropatrum horridum | SCH | 4 | 12.07 | 5 | 1.31 | 5 | 2.42 | 5.77 | 1.50 | |
| Diaperinae | Crypticus rufipes | CRR | 2 | 0.51 | 1 | 0.36 | 0 | 0.00 | 0.31 | 1.88 |
| Total | 986 | 837 | 744 | 100.00 | - | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Wang, Y.; Yang, W.; Zhu, Y.; Zhang, S.; Liang, Y.; Yang, G. Environmental Heterogeneity and Altitudinal Gradients Drive Darkling Beetle Diversity in an Alluvial Fan. Insects 2025, 16, 388. https://doi.org/10.3390/insects16040388
Zhao M, Wang Y, Yang W, Zhu Y, Zhang S, Liang Y, Yang G. Environmental Heterogeneity and Altitudinal Gradients Drive Darkling Beetle Diversity in an Alluvial Fan. Insects. 2025; 16(4):388. https://doi.org/10.3390/insects16040388
Chicago/Turabian StyleZhao, Min, Yuan Wang, Wenbin Yang, Yachao Zhu, Shuyu Zhang, Yongliang Liang, and Guijun Yang. 2025. "Environmental Heterogeneity and Altitudinal Gradients Drive Darkling Beetle Diversity in an Alluvial Fan" Insects 16, no. 4: 388. https://doi.org/10.3390/insects16040388
APA StyleZhao, M., Wang, Y., Yang, W., Zhu, Y., Zhang, S., Liang, Y., & Yang, G. (2025). Environmental Heterogeneity and Altitudinal Gradients Drive Darkling Beetle Diversity in an Alluvial Fan. Insects, 16(4), 388. https://doi.org/10.3390/insects16040388






