Shape as a Key to Taxonomy: Morphometric Analysis of Tetropium Species (Coleoptera: Cerambycidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Morphometrics Analyses
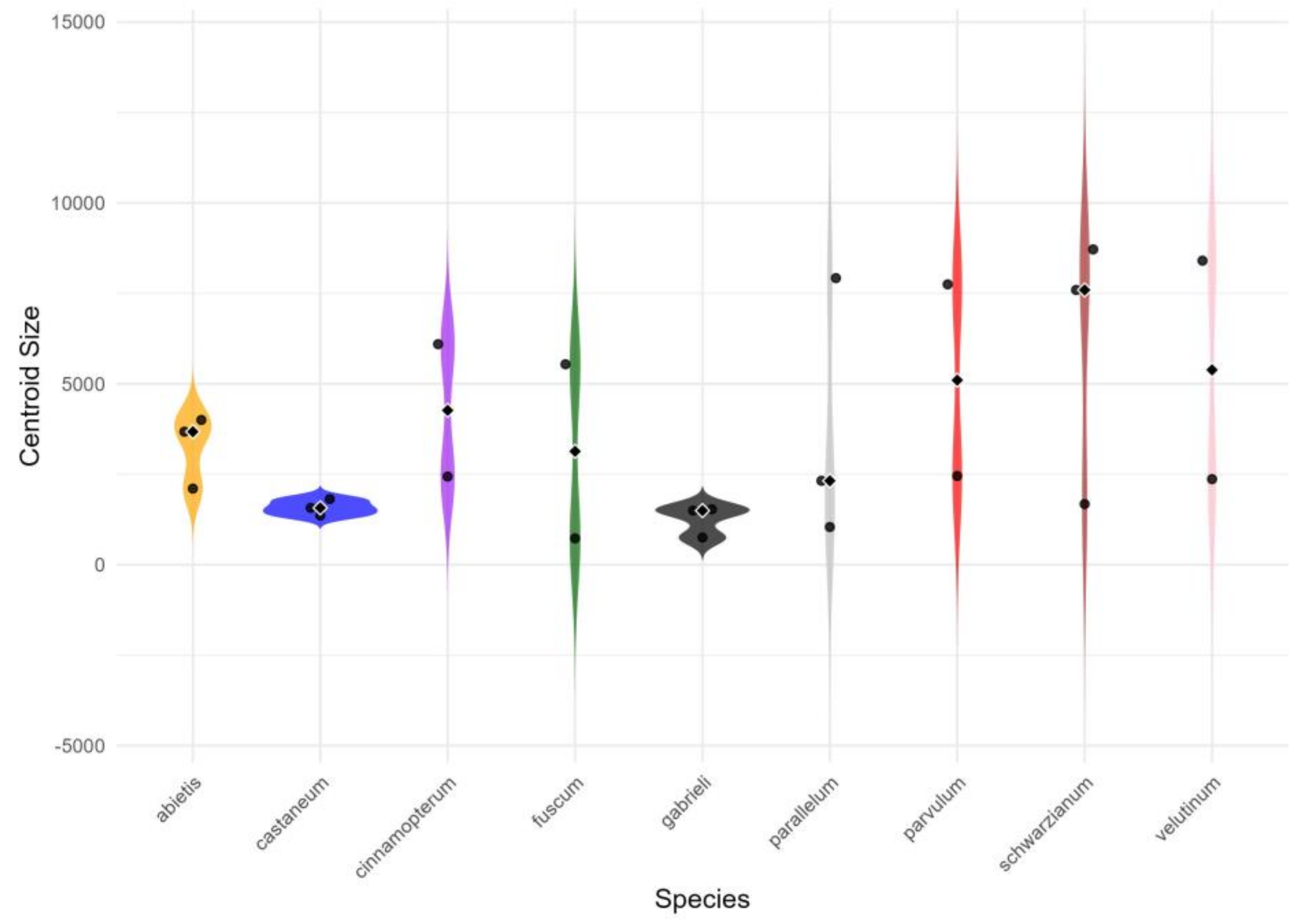
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, G.; Hurley, J.E. First North American record of the Palearctic species Tetropium fuscum (Fabricius)(Coleoptera: Cerambycidae). Coleopt. Bull. 2000, 54, 540. [Google Scholar]
- Rossa, R.; Goczał, J. Global diversity and distribution of longhorn beetles (Coleoptera: Cerambycidae). Eur. Zool. J. 2021, 88, 289–302. [Google Scholar]
- Lee, S.; Lee, S. Molecular identification of immature Cerambycinae (Coleoptera: Cerambycidae): Revisiting the significance of immature morphologies. Zool. J. Linn. Soc. 2024, 202, zlad182. [Google Scholar]
- Wu, Y.; Trepanowski, N.F.; Molongoski, J.J.; Reagel, P.F.; Lingafelter, S.W.; Nadel, H.; Myers, S.W.; Ray, A.M. Identification of wood-boring beetles (Cerambycidae and Buprestidae) intercepted in trade-associated solid wood packaging material using DNA barcoding and morphology. Sci. Rep. 2017, 7, 40316. [Google Scholar] [CrossRef]
- Zamoroka, A.; Trócoli Gracia, S.; Shparyk, V.Y.; Semaniuk, D. Polyphyly of the genus Stenurella (Coleoptera, Cerambycidae): Consensus of morphological and molecular data. Biosyst. Divers. 2022, 30, 119–136. [Google Scholar]
- Karpiński, L.; Gorring, P.; Cognato, A.I. DNA vs. morphology in delineating species boundaries of endemic Mongolian Eodorcadion taxa (Coleoptera: Cerambycidae). Diversity 2023, 15, 662. [Google Scholar] [CrossRef]
- Song, N.; Wang, M.; Zhai, Q.; Zhang, H. Insights into the phylogeny of longhorn beetles from phylogenomic data. Zool. J. Linn. Soc. 2025, 203, zlae174. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procustes methods for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J. Ecological character displacement in Plethodon: Biomechanical differences found from a geometric morphometric study. Proc. Natl. Acad. Sci. USA 2000, 97, 4106–4111. [Google Scholar] [CrossRef]
- Alibert, P.; Moureau, B.; Dommergues, J.L.; David, B.J.Z.S. Differentiation at a microgeographical scale within two species of ground beetle, Carabus auronitens and C. nemoralis (Coleoptera, Carabidae): A geometrical morphometric approach. Zool. Scr. 2001, 30, 299–311. [Google Scholar]
- Benítez, H.A.; Lemic, D.; Bazok, R.; Gallardo-Araya, C.M.; Mikac, K.M. Evolutionary directional asymmetry and shape variation in Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae): An example using hind wings. Biol. J. Linn. Soc. 2014, 111, 110–118. [Google Scholar] [CrossRef]
- Mikac, K.M.; Lemic, D.; Bažok, R.; Benítez, H.A. Wing shape changes: A morphological view of the Diabrotica virgifera virgifera European invasion. Biol. Invasions 2016, 18, 3401–3407. [Google Scholar]
- Zhang, M.; Ruan, Y.; Bai, M.; Chen, X.; Li, L.; Yang, X.; Meng, Z.; Liu, Y.; Du, X. Geometric Morphometric Analysis of Genus Chaetocnema (Coleoptera: Chrysomelidae: Alticini) with Insights on Its Subgenera Classification and Morphological Diversity. Diversity 2023, 15, 918. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Geiser, M.; Yang, Y. Morphology and geometric morphometrics unveil a new genus of Cantharidae (Coleoptera, Elateroidea) from mid-Cretaceous Burmese amber, with a preliminary investigation on the phylogenetic position. Invertebr. Syst. 2022, 36, 608–621. [Google Scholar] [CrossRef]
- Sumruayphol, S.; Chaiphongpachara, T. Geometric morphometrics as a tool for three species identification of the firefly (Coleoptera: Lampyridae) in Thailand: Geometric Morphometrics as a Tool for Three Species Identification of the Firefly. Biodiversitas J. Biol. Divers. 2019, 20. [Google Scholar]
- Zhang, M.; Ruan, Y.; Wan, X.; Tong, Y.; Yang, X.; Bai, M. Geometric morphometric analysis of the pronotum and elytron in stag beetles: Insight into its diversity and evolution. ZooKeys 2019, 833, 21. [Google Scholar]
- Okutaner, A.Y.; Sarikaya, A.D.; Sarikaya, Z.; Zdikmen, H. A geometric morphometric evaluation on three populations of endemic species, Dorcadion micans (Cerambycidae, Coleoptera) in Ankara Province from Turkey with a new subspecies description. Zootaxa 2021, 5004, 167–180. [Google Scholar] [PubMed]
- Ospina-Garcés, S.M.; Hernández-Cardenas, J.A.; Toledo-Hernández, V.H.; Corona-López, A.M.; Flores-Palacios, A. Head shape variation in cerambycid saproxylic beetles as a function of host plant selection. Arthropod Struct. Dev. 2018, 47, 2–11. [Google Scholar] [CrossRef]
- Rossa, R.; Goczał, J.; Tofilski, A. Hind wing morphology facilitates discrimination between two sibling species: Leiopus nebulosus and L. linnei (Coleoptera: Cerambycidae). Zootaxa 2017, 4227, 266–278. [Google Scholar] [CrossRef]
- Rossa, R.; Goczał, J.; Pawliczek, B.; Ohbayashi, N. Hind wing variation in Leptura annularis complex among European and Asiatic populations (Coleoptera, Cerambycidae). ZooKeys 2017, 724, 31. [Google Scholar] [CrossRef]
- Okutaner, A.Y.; Sariyaka, A.D. Sexual Dimorphism of the Pronotum in Dorcadion micans J. Thomson, 1867 (Coleoptera: Cerambycidae) Using Geometric Morphometrics. J. Anatol. Environ. Anim. Sci. 2021, 6, 88–91. [Google Scholar]
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 9–12. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Adams, D.C.; Otárola-Castillo, E. Geomorph: An R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 2011, 3, 180–185. [Google Scholar]
- Gojković, N.; Ludoški, J.; Milankov, V. The encounter of distinct Morimus asper (Coleoptera: Cerambycidae) phylogeographic lineages on the Balkan Peninsula: Conservation implications. J. Insect Conserv. 2022, 26, 773–792. [Google Scholar]
- Smith-Pardo, A.H.; Irons, L.; Morse, G.E. Landmark-and Semi-Landmark-Based Geometric Morphometrics as a Tool for the Identification of Genera of Seed Beetles (Coleoptera: Chrysomelidae: Bruchinae). Coleopt. Bull. 2024, 78, 553–566. [Google Scholar]
- Dujardin, J.-P. Morphometrics applied to medical entomology. Infect. Genet. Evol. 2008, 8, 875–890. [Google Scholar]
- Jaramillo-O, N.; Dujardin, J.P.; Calle-Londoño, D.; Fonseca-González, I. Geometric morphometrics for the taxonomy of 11 species of A nopheles (N yssorhynchus) mosquitoes. Med. Vet. Entomol. 2015, 29, 26–36. [Google Scholar]
- Lemic, D.; Mikac, K.M.; Kozina, A.; Benitez, H.A.; McLean, C.M.; Bažok, R. Monitoring techniques of the western corn rootworm are the precursor to effective IPM strategies. Pest Manag. Sci. 2016, 72, 405–417. [Google Scholar]



| Species | Origin | Specimen ID |
|---|---|---|
| T. abietis | Native | 146896-Tabietis |
| 146898-Tabietis | ||
| 146899-Tabietis | ||
| T. castaneum | Exotic | 003199-Tcastaneum |
| 013872-Tcastaneum | ||
| 198017-Tcastaneum | ||
| T. cinnamopterum | Native | 010876-Tcinnamopterum |
| 010877-Tcinnamopterum | ||
| T. fuscum | Exotic | 010880-Tfuscum |
| 016942-Tfuscum | ||
| T. gabrieli | Exotic | 0001CC-Tgabrieli |
| 0001CZ-Tgabrieli | ||
| 0002CC-Tgabrieli | ||
| 040811-Tgabrieli-WK | ||
| 309258-Tgabrieli- BIO | ||
| T. parallelum | Native | 002INA-Tparallelum |
| 146942-Tparallelum | ||
| 146943-Tparallelum | ||
| T. parvulum | Native | 146955-Tparvulum |
| 146956-Tparvulum | ||
| T. schwarzianum | Native | 146966-Tschwarzianum |
| 146973-Tschwarzianum | ||
| 146974-Tschwarzianum | ||
| T. velutinum | Native | 146979-Tvelutinum |
| 146980-Tvelutinum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith-Pardo, A.H.; Lingafelter, S.W.; Laroze, D.; Piñeiro-Gonzalez, A.; Benítez, H.A. Shape as a Key to Taxonomy: Morphometric Analysis of Tetropium Species (Coleoptera: Cerambycidae). Insects 2025, 16, 386. https://doi.org/10.3390/insects16040386
Smith-Pardo AH, Lingafelter SW, Laroze D, Piñeiro-Gonzalez A, Benítez HA. Shape as a Key to Taxonomy: Morphometric Analysis of Tetropium Species (Coleoptera: Cerambycidae). Insects. 2025; 16(4):386. https://doi.org/10.3390/insects16040386
Chicago/Turabian StyleSmith-Pardo, Allan H., Steven W. Lingafelter, David Laroze, Alejandro Piñeiro-Gonzalez, and Hugo A. Benítez. 2025. "Shape as a Key to Taxonomy: Morphometric Analysis of Tetropium Species (Coleoptera: Cerambycidae)" Insects 16, no. 4: 386. https://doi.org/10.3390/insects16040386
APA StyleSmith-Pardo, A. H., Lingafelter, S. W., Laroze, D., Piñeiro-Gonzalez, A., & Benítez, H. A. (2025). Shape as a Key to Taxonomy: Morphometric Analysis of Tetropium Species (Coleoptera: Cerambycidae). Insects, 16(4), 386. https://doi.org/10.3390/insects16040386






