Simple Summary
The parasitic mite Varroa destructor is the most damaging biotic stressor of honey bees (Apis mellifera) worldwide. Breeding bees for resistance against V. destructor is a sustainable long-term approach to managing the mite. This study analyzed several mechanisms of resistance of honey bees that were bred for low (resistant) and high (susceptible) Varroa population growth (LVG and HVG, respectively). After three generations of selection based on mite fall, LVG bees had significantly higher immunity detected as stronger behavioral (hygienic and grooming behaviors), cellular (haemocyte concentration), and humoral (hymenoptaecin 2 and defensin 2 antimicrobial peptide gene expression and lower DWV levels) immunity compared to HVG bees. These results indicate that selecting bees for LVG indirectly selects for bees with multiple resistance mechanisms against V. destructor. Thus, it appears that the LVG trait is associated with multiple genes.
Abstract
Honey bees (Apis mellifera) bred for resistance to the parasitic mite, Varroa destructor, were examined for potential Varroa resistance mechanisms following bidirectional selection for low (resistant) or high (susceptible) Varroa population growth (LVG and HVG, respectively) based on mite fall in colonies at two different time points. Hygienic and grooming behavior rates in LVG colonies were significantly higher than those in HVG colonies for two out of three generations of selection, indicating that behavioral resistance to the mite increased. For the third generation, grooming start time was significantly shorter, and grooming intensity more frequent in LVG bees than in HVG bees. Cellular immunity was increased as well, based on significantly higher haemocyte concentrations in non-parasitized and Varroa-parasitized LVG bees. Humoral immunity was increased with Varroa-parasitized LVG bees, which had significantly higher expression of the antimicrobial peptide gene, hymenoptaecin 2. In addition, antiviral resistance may be involved as there were significantly lower levels of deformed wing virus (DWV) in Varroa-parasitized LVG bees. While selection for LVG and HVG bees was solely based on Varroa population growth, it appears that behavioral, cellular, and humoral mechanisms were all selected along with this resistance. Thus, LVG resistance appears to be a multi-gene trait, involving multiple resistance mechanisms.
1. Introduction
Varroa destructor is a parasitic mite of the Western honey bee (Apis mellifera L.) that has been linked to colony losses worldwide, mainly in countries in the Northern hemisphere [1]. Varroa feeds upon the fat body tissue and haemolymph of honey bees [2,3], suppressing their immune system and shortening their lifespan [4,5,6]. A major factor in the detrimental effects of Varroa is its role as a vector of bee viruses, including deformed wing virus (DWV) [7], which reduces the longevity of adult bees and causes a deformed body and wings in developing bees [8,9]. Hence, both V. destructor and DWV are linked to honey bee colony losses.
One control strategy is breeding honey bees for Varroa resistance [10]. For example, De la Mora et al. [11,12] carried out three generations of bidirectional selection for low (resistant) and high (susceptible) Varroa population growth (LVG and HVG, respectively) based on mite fall in colonies at two different time points. The LVG genotype demonstrated lower Varroa population growth and levels, as well as lower colony DWV levels and winter colony mortality than the HVG genotype. However, the mechanisms involved in low Varroa population growth were not reported. One possible mechanism is grooming behavior, in which bees bite and dislodge mites from their bodies [13]. Variation between bee genotypes has been demonstrated for the number of visibly damaged Varroa among the fallen mites, presumably due to grooming behavior [14,15,16]. This trait has been used for selecting honey bee stocks, such as the ‘mite-biter’ [17]. Another possible resistance mechanism is hygienic behavior, in which bees identify and remove not only diseased or dead broods from their cells, but also Varroa-infested broods [10,18,19]. Honey bee stocks have been selected for this trait, such as the Varroa-sensitive hygiene stock that was selected for high detection and removal of mite-infested broods [20,21].
There are other mechanisms possible for Varroa resistance in addition to behavioral responses. One is an enhanced cellular immunity, such as increased haemocytes, and another is higher humoral immunity, such as increased defense compounds [22]. Haemocytes are immune cells that engulf pathogens present in the bees’ haemolymph [23]. They also promote wound healing and haemolymph clotting after the V. destructor attack [24] and produce anti-viral compounds [25,26]. Humoral defense compounds include antimicrobial peptides (AMPs) that neutralize pathogenic microorganisms, including viruses [1,27]. An example of antiviral immunity is the trait called ‘Suppressed in ovo virus infection (SOV)’ that is found in resilient colonies to DWV infections [28]. The primary mechanism of insect antiviral defense is RNA interference (RNAi), and high virus levels in honey bees can suppress key RNAi components [29].
While the LVG genotype clearly showed resistance to Varroa compared to the HVG genotype with three rounds of selection for low rates of Varroa population growth [12], the mechanisms were not examined. The current study examined LVG and HVG bees for several potential mechanisms of Varroa resistance, including behavioral resistance (grooming and hygienic behavior), cellular immunity (haemocyte concentrations), humoral immunity (expression of the AMP genes, defensin 2 (AmDef-2), and hymenoptaecin 2 (AmHym-2)). Additionally, Varroa parasitized bees of the two genotypes were assessed for DWV levels to determine if antiviral resistance was involved for bees individually parasitized by the mite. Therefore, this study provides a better understanding of whether selection for LVG involves one or more mite-resistance mechanisms.
2. Materials and Methods
2.1. Location and Genotype Selection
Experiments were conducted at the Honey Bee Research Center (HBRC), University of Guelph, Guelph, ON, Canada (43.5448° N, 80.2482° W). Honey bees underwent three generations of selection for either low (resistant) or high (susceptible) Varroa population growth based on mite fall in colonies at two different time points to obtain the LVG and HVG genotypes, respectively [11,12]. Each generation was one year and Varroa treatments were applied in colonies in the Fall of each year using amitraz (Apivar, Veto-Pharma, Saint-Benoit-du-Sault, 36310 Chaillac, France) following the manufacturer’s instructions.
2.2. Hygienic and Grooming Behaviors at the Colony Level
Seventeen randomly selected colonies per genotype were tested on each generation in late May to assess hygienic behavior by freeze-sacrificing capped worker brood with liquid nitrogen as per Spivak and Reuter [30]. Briefly, a metal cylinder (4.10 cm internal diameter) was placed on four sections of comb containing ~140 capped cells (~35 capped brood cells/section) from each of the tested colonies. Then, 250 mL of liquid nitrogen was poured into its interior to kill the brood. The number of cells uncapped and cleaned by the bees was counted 24 h after freezing the brood to calculate the percentages of uncapped and cleaned cells.
Twenty randomly selected colonies per genotype were assessed for each generation in late August for grooming behavior by calculating the percentage of damaged mites with body dents and/or mutilated legs out of the total number of mites collected from sticky boards [17,31]. The mites were transferred with a fine paint brush from a colony sticky board into a Petri dish. To observe damage to mites, they were positioned with their ventral side facing up under a stereoscopic microscope (Olympus SD-ILK, Optical Co., Tokyo, Japan). The number of mites with mutilated legs and/or dents on the idiosoma were counted. The rate of mutilated mites was determined by dividing the number of damaged mites over the total number of mites analyzed.
2.3. Grooming Behavior at the Individual Level
Three randomly selected colonies per genotype of the third generation were sampled for adult bees in late August to assess self-grooming behavior in the laboratory [32]. A total of 1534 bees were assessed. Briefly, bees from brood-chamber frames were shaken into a 5 L container, then 500 mL of them was scooped into an open-mouth 1 L container with a 100 micron honey filter cloth (Dancing Bee, Port Hope, ON, Canada) as a lid. Each bee taken from the container was individually placed in a Petri dish (100 mm × 15 mm; Fisher Scientific, Mississauga, ON, Canada) that had a perforated plastic lid, and was allowed to acclimate for 2 min. Then, approx. 20 mg of wheat flour (Robin Hood, Markham, ON, Canada) was applied onto the thorax of the bee using a fine paint brush (6 mm × 11 mm; DeSerres, Oakville, ON, Canada) as an irritant to stimulate grooming instances. Wheat flour is a proxy of V. destructor for grooming behavior assays [32]. The time (s) taken by each bee to start performing the first grooming instance was measured for up to 3 min using a stopwatch with a resolution of 1/100 s and an accuracy of 0.001% (Catalog number 06-662-56. Fisherbrand, Mississauga, ON, Canada). This was performed as a blind test to the observer. The intensity of grooming (light or intense) was also recorded as per Guzman-Novoa et al. [15]. Bees were classified as ‘light’ groomers if they used no more than two legs and if slow motions to remove the irritant were observed, or as ‘intense’ groomers if they used three or more legs and if vigorous shaking and wiping motions to remove the irritant were observed. Bees showing intermediate expressions of grooming instances were discarded from the analysis.
2.4. Source of V. destructor
To obtain female Varroa, frames covered with adult bees from highly infested colonies that were unrelated to the experimental bees were shaken inside a plastic bag, and then the bees were anesthetized with CO2. The bees were transferred to a plastic chamber (30 × 18 × 4 cm) that was divided into upper and lower areas by a 3 mm metal mesh that served to support the anesthetized bees. The chamber was placed on an orbital shaker (Eberbach, Van Buren, MI, USA) set at 400 rpm for 10 min to dislodge mites attached to the bees [33]. Fallen mites were recovered from the lower area of the chamber and placed in a plastic Petri dish lined with a moist paper towel using a fine paint brush. The bees used for this procedure were returned to their original hive after recovering from anesthesia.
2.5. Cellular Immunity
Brood nest frames of three colonies of each of the LVG and HVG genotypes of the third generation of selection were shaken into a 5 L container, and 50 bees were individually transferred to five Benton wooden cages (75 × 25 × 16 mm) with 10 bees per cage. The bees in the cages were treated as follows: (1) HVG bees without Varroa; (2) HVG bees with Varroa, (3) LVG bees without Varroa; (4) LVG bees with Varroa. The experiment was replicated three times using a different source colony per replicate. For Varroa exposure, bees were artificially parasitized with one mite per individual by placing a mite with a fine paintbrush onto each bee through the cage screen, and the cages were maintained at 32–35 °C and 60% RH for eight days [4]. The bees were fed queen candy (65% sucrose syrup mixed with powdered sucrose as needed to obtain a soft mass) and watered twice a day by placing a clean 2 × 2 cm sponge piece (Scotch Brite, Two Harbors, MN, USA) with dsH2O on the cage screen. Samples of 10 bees of each genotype that had or had not been exposed to V. destructor were collected at eight days post-exposure to the mite. Each bee was collected directly from the cage and held manually with the index and thumb fingers to expose the dorsal part of the insect’s abdomen. Then, each bee was pierced between the third and fourth tergite with a #7 entomological pin (Bioquip, Rancho Dominguez, CA, USA), and a sample of 4 µL of haemolymph was collected with a 10 µL micropipette (Labnet International, Edison, NJ, USA).
The concentration of haemocytes/µL of haemolymph was measured as per Koleoglu et al. [5]. Briefly, the haemolymph sample was evenly spread over a microscope slide previously marked with two 1 × 1 cm squares, each divided into four 0.5 × 0.5 cm squares using a fine point permanent marker (Sharpie, Atlanta, GA, USA). The smears were allowed to airdry for 15 min and then fixed with 10 µL of 95% methanol. The smears were dyed with the Hema 3 Stat Pack kit (Fisher Scientific, Fair Lawn, NJ, USA) following the manufacturer’s instructions. After staining the smears, haemocytes were counted at 400× magnification under an optic microscope (Leica Microsystems, Wetzlar, Germany) equipped with a 10 × 10 mm ocular reticule having a 100 cells grid (2.5 μm2 each). Eight counts were performed with two areas selected in each 0.5 × 0.5 cm square, one near the center and the other near the corner of the 1 × 1 cm square. Haemocytes were counted in a zig-zag pattern starting at the top left-hand corner of each square. To calculate the number of haemocytes per μL of haemolymph in each sample, the following equation was used:
No. haemocytes/µL = ((Ʃ haemocytes/8) × 1322)/4
The adjustment factor of 1322 was calculated from Murphy and Davidson [34], which corresponds to the number of microscope reticles. This procedure was repeated three times.
2.6. Humoral Immunity
Samples of 10 bees harvested from broodnest frames that were treated in cages as per the cellular immunity measurements were collected eight days after being infested and stored at −80 °C. Detection and quantification of gene expression was performed as per Morfin et al. [35], with modifications for RNA extraction. Briefly, bees were macerated with a pestle and mortar in 5000 µL of One Step RNA Reagent (BioBasic, Markham, ON, Canada), following instructions from the manufacturer. The macerate was transferred to a new 1.5 mL centrifuge tube and incubated for 5 min at 20–22 °C. Then, 300 µL of chloroform was added, and the tube was vortexed (Thermo Fisher, Waltham, MA, USA) at 7000 rpm for 15 s. After incubation at 20–22° for 2–3 min, the tube was centrifuged (VWR, Mississauga, ON, Canada) at 12,000× g for 15 min at 4 °C. The aqueous phase was transferred to a new 1.5 mL microcentrifuge tube, and 500 µL of 99% isopropanol was added. The tube was incubated at 20–22 °C for 10 min and centrifuged at 12,000× g for 10 min at 4 °C. The supernatant was discarded, and the pellet was washed by adding 1000 µL of 70% ethanol, mixing by inversion and followed by centrifugation at 7500× g for 5 min at 4 °C. This was repeated two additional times. The pellet was airdried at room temperature. Finally, the RNA pellet was dissolved in 30 µL of Invitrogen UltraPure H2O (Fisher Scientific, Waltham, MA, USA), and RNA quality was assessed using a NanoDrop Lite (Thermo Fisher, Waltham, MA, USA). The RNA samples were stored at −80 °C.
cDNA was prepared by using 2000 ng of RNA and the Fermentas Revert Aid H Minus First Strand cDNA Synthesis Kit (Fisher Scientific, Mississauga, ON, Canada), following the manufacturer’s instructions. Quantitative real-time PCR (RT-qPCR) was used to analyze the expression of the immune-related genes, defensin 2 (AmDef-2) and hymenoptaecin 2 (AmHym-2) [36]. For AmDef-2, a 20 µL reaction contained 2 µL cDNA, 10 µL PowerUp SYBRgreen (Supermix 2×) (Applied Biosystems, Foster City, CA, USA), 0.8 µL each of the 400 nM forward and reverse primers (Table S1) [37], and 6.4 µL of nuclease free H2O. For AmHym-2, a 20 µL reaction contained the same reagents and volumes, except for 0.6 µL each of the 400 nM forward and reverse primers (Table S1) [37] and 6.8 µL of nuclease-free H2O. The expression of those genes was measured relative to the expression of the constitutive gene 40S ribosomal protein S5 (AmRPS5) [36] with each reaction containing 2 µL of cDNA, 10 µL of Supermix 2×, 1.4 µL each of the 700 nM forward and reverse primers (Table S1) [37], and 5.2 µL of nuclease-free H2O. The negative control included 2 µL of nuclease free H2O, and the positive control included 2 µL of the diluted synthetic 300 bp gBlock of the target gene (Integrated DNA Technologies, Coralville, IA, USA) (Table S2) [35]. RT-qPCR was performed with a QuantStudio3 thermocycler (Real-Time PCR Systems, Fisher Scientific, Pittsburgh, PA, USA) with one cycle at 50 °C for 2 min, one cycle at 95 °C for 10 min, and 40 cycles at 95 °C for 10 s and 60 °C for 60 s. The expression level of the AmRPS5 reference gene in each sample was used to normalize the expression level of the target gene using the 2−ΔΔ (Livak) method [38] with the HVG genotype as calibrator. The QuantStudio3 real-time PCR detection system was used to calculate the normalized expression level.
2.7. DWV Infection Levels
Samples of 18–22 bees treated the same as per the cellular immunity measurements were collected at eight days post-treatment and stored at −80 °C. Detection and quantification of DWV was performed as per Morfin et al. [39], with modifications for RNA extraction as described above. cDNA was prepared with 2000 ng of RNA for each sample using the Fermentas Revert Aid H Minus First Strand cDNA Synthesis Kit (Fisher Scientific, Burlington, ON, Canada), following the manufacturer’s instructions. cDNA was amplified by RT-qPCR with a QuantStudio3 thermocycler (Real-Time PCR Systems, Fisher, Pittsburgh, PA, USA). Each qPCR reaction of the helicase gene of DWV type A consisted of 20 µL containing 2 µL of cDNA, 0.4 µL of both 200 nM forward and reverse primers (Table S1) [40], 10 µL PowerUpTM Sybergreen (2×) (Applied Biosystems, Foster City, CA, USA), and 7.2 µL nuclease-free H2O. The negative control was 2 µL of nuclease-free H2O instead of cDNA. The positive control included 2 µL of a 300 bp gBlock (Integrated DNA Technologies, Coralville, IA, USA) that included the sequences of the forward primer, amplicon, and reverse primer (Table S2) [39]. PCR conditions consisted of one cycle at 48 °C for 15 min, one cycle at 95 °C for 10 min, 40 cycles at 95 °C for 15 s and 60 °C for 60 s, followed by one cycle at 68 °C for 7 min. Calibration curves to convert Ct values to DWV genome copies (gc) were carried out using 300 bp gBlocks (Integrated DNA Technologies, Coralville, IA, USA) (Table S2) diluted in dsH2O to 10 ng/μL that was then used to make 10-fold serial dilutions from 109 to 102. Using a plot of Ct values versus DWV copy number (log10), a linear equation was used to calculate the DWV gc [41,42]. Three technical repetitions were performed for each qRT-PCR. Randomly selected amplicons of DWV PCR product were sequenced at the University of Guelph Laboratory Services to confirm identity.
2.8. Statistical Analyses
Data were analyzed for normality with the Shapiro–Wilk test. When not normally distributed, data were transformed. Data on DWV copies, haemocyte counts, gene expression, and time to start grooming were log-transformed. Data on percentage hygienic behavior were arcsine-square root transformed. The transformed data were subjected to analyses of variance and Fisher-protected LSD tests to compare means when significant differences were found. Data on percentages or proportions, including damaged mites, and grooming intensity between genotypes, were analyzed with contingency tables and corrected χ2 tests. Statistical analyses were performed with the R 4.1.1. software [43].
3. Results
3.1. Hygienic and Grooming Behaviors at the Colony Level
Hygienic behavior estimated from the percentage of frozen brood removed by bees of the LVG colonies was significantly higher than that of the HVG colonies in the first and second generations, but not in the third generation (F1,97 = 15.25, p < 0.001) (Figure 1a). There were no significant effects of genotype x generation interaction (p > 0.05). The greatest difference in frozen brood removal was in the first generation of LVG colonies, when it was 35% higher than that of the HVG colonies.
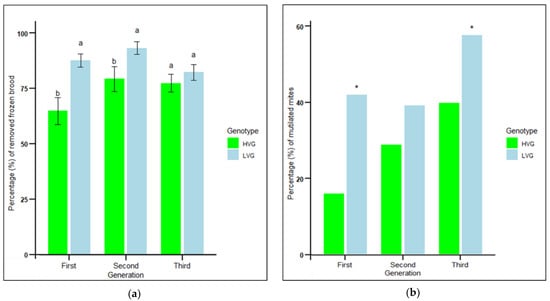
Figure 1.
Mean percentage of removed frozen brood ± SEM in 24 h (a) and mean percentage of dented and/or mutilated mites recovered from bottom boards (b) of colonies during the three generations of selection for high and low Varroa growth (HVG and LVG, respectively). Different literals for the percentage of removed frozen brood indicate significant differences between genotypes within each generation based on analysis of variance and Fisher-protected LSD tests of arcsine-square root transformed data (p < 0.05). Untransformed values are shown. Asterisks for percentage of dented and/or mutilated mites indicate significant differences between genotypes within each generation based on contingency table analyses with corrected chi-squares (p < 0.05).
Grooming behavior measured by the mean percent dented and/or mutilated mites from LVG colonies was significantly higher than that from HVG colonies in the first and third generations (χ2 = 47.1, n = 1323, p < 0.0001; χ2 = 5.7, n = 230, p < 0.05, respectively), but not in the second generation (χ2 = 3.5, n = 965, p > 0.05) (Figure 1b). Mite damage rates from LVG colonies in the first and third generations were 61% and 30% greater than those of HVG colonies (Figure 1b), respectively, which was associated with 47% and 66% lower Varroa infestation levels in adult bees and brood, respectively, as reported previously [12]. The greatest difference was in the first generation, where mite damage was 42% in LVG compared to 16% in HVG colonies. There were no significant effects of genotype × generation interaction (p > 0.05). For both hygienic and grooming behaviors, there was no evidence that each round of selection progressively increased the traits, which appeared to differ by generation, possibly by random variation or environmental factors each year. Therefore, all subsequent examinations of traits of individual LVG and HVG bees were only performed on the third generation of selection.
3.2. Grooming Behavior at the Individual Level
The proportion of individuals performing intense grooming was significantly higher for bees from LVG colonies (0.42) than for bees from HVG colonies (0.36) (χ2 = 6.26, p < 0.05) (Figure 2a). However, there was a significantly shorter grooming starting time for intense but not light groomers with HVG-intense groomers being significantly slower than LVG-intense groomers, but not for light groomers (F1,1532 = 1.55, p < 0.0001) (Figure 2b). The fastest response was with LVG-intense groomers (4.32 s), followed by HVG-intense groomers (4.66 s), then LVG-light groomers (12.58 s), and the slowest was with HVG-light groomers (13.7 s). There were significant effects for genotype x intensity interaction (p < 0.05) with LVG bees that performed intense grooming starting to groom faster than HVG bees.
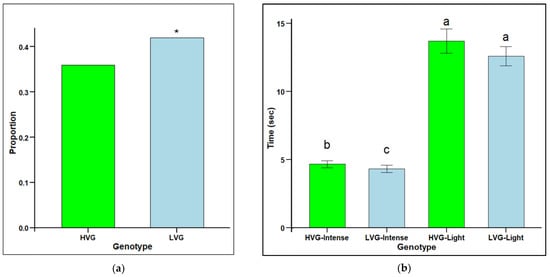
Figure 2.
Grooming intensity (proportion of individuals grooming over 180 s (a) and grooming starting time (mean time (s) to observe first grooming instances ± SEM) (b) for worker honey bees of the third generation of selection for high Varroa growth (HVG) and low Varroa growth (LVG). Asterisk (*) for the proportion of groomers indicate significant differences between bee types (p < 0.05) and are based on contingency table analyses with corrected chi-squares (a). Different literals for time to groom indicate significant differences between bee types (p < 0.05) and are based on ANOVA and Fisher-protected LSD tests of log-transformed data (b). Untransformed values are presented.
3.3. Cellular Immunity
Haemocyte concentration was significantly higher in the haemolymph of non-parasitized LVG bees (25.1 × 103 haemocytes per µL) compared to non-parasitized HVG bees (11.1 × 103 haemocytes per µL), as well as in the haemolymph of parasitized LVG bees (18.0 × 103 haemocytes per µL) compared to parasitized HVG (3.5 × 103 haemocytes per µL) bees (F1,38 = 5.0, p < 0.05) (Figure 3). Parasitism significantly lowered haemocyte concentration in HVG bees, but not in LVG bees. There were no significant interaction effects of genotype x treatment (p > 0.05).
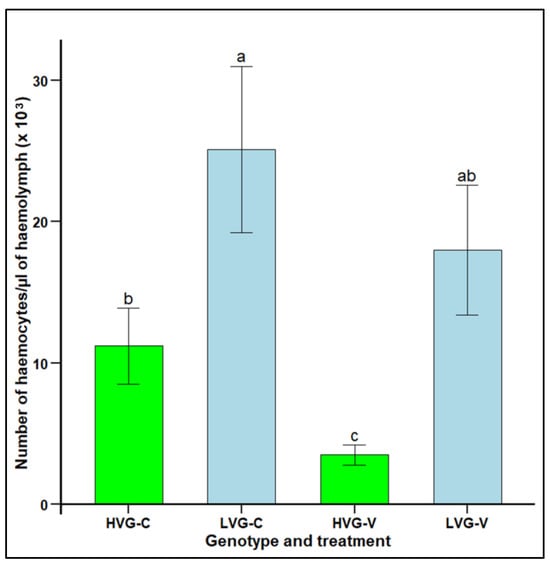
Figure 3.
Haemocyte concentration per µL of haemolymph (mean ± SEM) of adult workers from honey bee colonies of the third generation of selection for high and low Varroa growth (HVG and LVG, respectively). Bees of both genotypes were exposed (V) or not exposed (C) to V. destructor parasitism in cages under a controlled environment. Different literals indicate significant differences between genotypes based on analysis of variance and Fisher-protected LSD tests of log-transformed data. Untransformed values are shown.
3.4. Humoral Immunity
The relative expression (RE) of defensin 2 (AmDef-2) in LVG and HVG bees was significantly higher in non-parasitized LVG (4.33 RE) compared to non-parasitized HVG (0.77 RE) bees, as well as between parasitized LVG (11.02 RE) compared to parasitized HVG (4.08 RE) bees (F1,20 = 4.2672, p < 0.05; Figure 4a). However, there were no significant differences when comparing non-parasitized to parasitized bees within either the LVG or HVG genotypes (F1,20 = 0.7343, p > 0.05). There were significant interaction effects of genotype x treatment (p < 0.05).
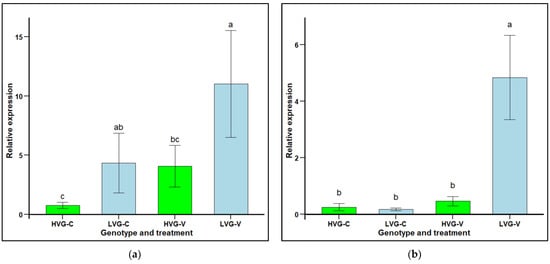
Figure 4.
Relative gene expression (RE mean ± SEM) of the immune-related genes, defensin 2 (AmDef-2; (a) and hymenoptaecin 2 (AmHym-2; (b), of pooled worker honey bees (n = 15) from six colonies selected for high and six colonies selected for low Varroa growth (HVG and LVG, respectively) that were (V) or not (C) challenged with V. destructor. The expression of the target genes was calculated using the Livak 22−ΔΔ method, relative to the reference gene, AmRPS5, and the non-challenged HVG genotype was used as a calibrator. Different literals represent significant differences based on analyses of variance and Fisher protected LSD tests of log transformed data. Untransformed values are presented.
Hymenoptaecin 2 (AmHym-2) relative expression in LVG and HVG bees was not significantly different between non-parasitized LVG (0.17 RE) and HVG (0.25) bees (F1,22 = 0.74, p > 0.05), but was significantly higher in parasitized LVG (4.84 RE) than HVG (0.46 RE) bees (F1,22 = 6.40, p < 0.05; Figure 4b). Comparing non-parasitized to parasitized bees within a genotype, there was significantly higher expression for parasitized LVG bees with a 4.67 RE increase, but not for parasitized HVG bees with only a 0.21 RE increase. There were significant interaction effects of genotype × treatment (p < 0.05).
3.5. DWV Infection Levels
DWV infection levels in LVG and HVG bees were significantly lower in non-parasitized LVG (4.63 copies × 106 per µg RNA) than non-parasitized HVG (5.77 copies × 106 per µg RNA) bees, as well as significantly lower in parasitized LVG (6.19 copies × 106 per µg RNA) than parasitized HVG (8.31 copies × 106 per µg RNA) bees (F3,92 = 5.5288, p < 0.05; Figure 5). Parasitism resulted in higher DWV levels for both LVG and HVG bees, but the increase was only 1.56 versus 2.54 copies × 106 per µg RNA in LVG and HVG bees, respectively. There were no significant interaction effects of genotype x treatment (p > 0.05).
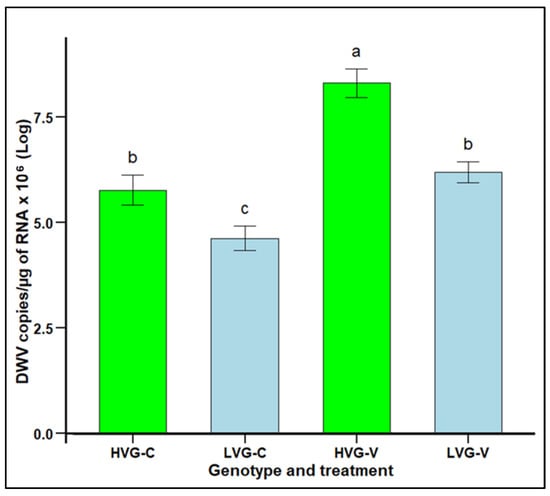
Figure 5.
DWV infection levels (mean DWV copies/µg of RNA x 106 ± SEM) of worker honey bees of the third generation of selection for high and low Varroa growth (HVG and LVG, respectively) that were exposed (V) or not exposed (C) to V. destructor. Different literals indicate significant differences between genotypes based on analysis of variance and Fisher-protected LSD tests of log-transformed data.
4. Discussion
Although it was reported that selection of colonies for high or low Varroa population growth resulted in LVG colonies with almost 90% less Varroa growth than HVG colonies and significantly lower Varroa infestation levels by the third generation, the mechanisms of resistance were not examined [12]. One mechanism of resistance to Varroa is hygienic behavior. Increased resistance to Varroa has been shown to result from increased hygienic behavior where bees detect Varroa infested broods in cells and then remove them [10,30,44,45]. Low Varroa infestation levels in colonies have also been linked to a specialized type of hygienic behavior, Varroa sensitive hygiene (VSH), in which bees are also able to identify the cells where the mite is reproducing, restraining Varroa reproduction and population growth [18,20,46]. This behavior has been observed at high frequencies in honey bee colonies selected for VSH [20].
The rate of hygienic behavior of LVG colonies was significantly higher than that of HVG colonies in the first two generations but not in the third generation with the difference between LVG and HVG colonies becoming progressively less with each generation. It appears that this trait may have been indirectly selected initially acting as one contributing factor to resistance but possibly becoming less of a contributor with each generation. In a previous study selecting for low Varroa growth, greater hygienic behavior was not found to be related to Varroa resistance [47]. However, directly selecting for increased hygienic behavior reduces Varroa populations in honey bee colonies, with bees removing 72% more killed brood and resulting in 60% lower mite infestation [48]. Other examples selecting for hygienic behavior resulted in 78% [21] and 52% lower mite infestation levels [46]. The inconsistent relationship between an increase in hygienic behavior with low Varroa population growth in this study indicates that it is only one contributor to resistance and other mechanisms may be involved.
Varroa resistance in honey bees has also been reported to result from increased grooming behavior where bees remove Varroa from their own bodies or that of other bees [1]. Grooming behavior at the colony level was indirectly assessed by analyzing the percentage of damaged (dented and/or mutilated) mites recovered from colonies [17]. Mite damage rate in LVG colonies was significantly higher than that in HVG colonies in the first and third generations, but not in the second generation, and thus, it was not consistently associated with low Varroa growth. For previous studies selecting for LVG that examined grooming behavior, Rinderer et al. [49] found 33% more damaged mites associated with 73% lower Varroa infestation levels, whereas Lodesani et al. [50] found that mite damage varied by generation with no correlation between damaged mite numbers and lower Varroa infestation levels. However, direct selection for increased grooming behavior can provide effective Varroa resistance, such as 70% more damaged mites with 34% lower Varroa infestation levels [16], 64% more damaged mites and 55% lower Varroa infestation levels [51], and 43% more damaged mites and 64% lower Varroa infestation levels [52]. Like hygienic behavior, grooming behavior appears to contribute to resistance as it was associated with the LVG genotype in two of the three generations of selection but not in the second generation. Therefore, additional mechanisms must be involved in the resistance to Varroa of the LVG genotype.
One reason that grooming behavior was not consistently linked with low Varroa population growth in all three generations of this study could be that grooming behavior is a polygenic trait, as indicated by nine protein markers being associated with it [53]. Thus, it is possible that different sets of grooming behavior genes may have been more strongly selected in certain generations. This may also explain why there is a considerable range in the relationship between the level of damaged mites and infestation levels reported between different studies. It is also possible that a proportion of the mutilated and/or dented mites collected from the colonies were also a result of VSH or uncapping/recapping behavior as it has been demonstrated that bees injure mites when they remove them from cells [54]. This study did not distinguish between the ways that Varroa could have been injured and so the numbers could reflect both VSH and grooming behavior. Future studies on LVG and HVG genotypes are warranted to investigate the relative contribution of each of these behaviors to Varroa damage.
Grooming behavior was also assessed at the individual level by measuring grooming intensity and grooming starting time in the third generation. LVG bees had a significantly greater proportion of intense groomers relative to light groomers, and intense-grooming LVG bees started grooming sooner than intense- and light-grooming HVG bees. A higher proportion of intense groomers can contribute to Varroa resistance as intense groomers can dislodge over 80% of mites from their bodies compared to light groomers [15,55]. These results agree with the significantly greater number of damaged mites collected from LVG colonies in comparison with HVG colonies. None of the previous studies selecting for LVG examined grooming starting time or grooming intensity [11,12,14,47,49,50,56]. In this study, 14% more LVG bees performed grooming events within 3 min of a stimulus than HVG bees. By comparison, studies directly selecting genotypes for increased grooming behavior resulted in 42% [47] and 32% [16] more bees performing grooming events within 3 min of a stimulus compared to non-selected genotypes. The higher grooming intensity and faster grooming starting time in the third generation of LVG bees supports grooming behavior as one of the multiple mechanisms contributing to the LVG phenotype. Studies conducted in Europe did not find a correlation between mite fall, damaged mites, or hygienic behavior, and the survival of Varroa-infested colonies [14]. However, other studies conducted in the Americas and the Middle East have shown a relationship between these traits and colony health and survivorship [16,17,31,45]. Perhaps the relative contribution of different traits to the resistance of bees to Varroa and their effect on colony survivorship varies depending on the environment or some other factor. Therefore, more studies conducted in different regions of the world are needed to determine whether these traits are useful to increase colony survivorship in different environments.
One potential behavioral mechanism of resistance that was not assessed in this study was recapping, which involves young bees detecting infested brood, opening the cells, occasionally removing the mites, and re-sealing the cells [57]. This behavior may reduce mite fertility and fecundity because mite reproduction and mortality of mite offspring is higher in recapped cells than in mite-infested cells that are not opened [58]. Recapping could be an important mechanism of resistance against V. destructor in some honey bee populations. For example, a population of honey bees that has survived Varroa infestations without acaricide treatments for more than 20 years in Cuba had high rates of recapping behavior of mite-infested cells, with the removal of over 80% of mites from parasitized cells [59]. Thus, artificially parasitizing capped broods of HVG and LVG colonies to assess recapping would be useful to determine if recapping is increased in LVG colonies. The contribution of recapping to Varroa resistance in honey bee colonies warrants further investigation before incorporating this trait into selective breeding programs.
Another potential behavioral mechanism of resistance that was not assessed in this study was mite non-reproduction (MNR) or suppressed mite reproduction (SMR), in which a percentage of Varroa foundress females do not reproduce or have low fecundity in parasitized brood [60,61]. MNR and SMR have been shown in honey bee populations demonstrating resistance to Varroa [62,63,64]. Additionally, low mite fecundity is consistently found in presumed Varroa-resistant populations of honey bees [64,65,66]. The mechanisms that cause MNR are not clear, but they may be behavioral, as VSH is responsible for a significant proportion of low mite fertility, as hygienic bees remove reproducing mites from capped cells [18,46]. The value of this trait was evidenced during an eight-year breeding program that selected for a bee genotype with lower mite reproduction, and that resulted in a 60% reduction in Varroa populations [60]. It is possible that the MNR and recapping traits had been indirectly selected by the overall trait of LVG, which remains to be investigated.
Cellular immunity, based on the concentration of haemocytes in the haemolymph, was significantly higher in LVG than in HVG bees both with and without Varroa parasitism. As increased cellular immunity has never been related to Varroa resistance; this was unexpected. Because newly emerged bees were determined to not have Varroa parasitism prior to being placed in cages in this study, it could be proposed that increased haemocyte concentrations in bees without Varroa shows that a basal function has been indirectly selected during the development of LVG colonies. Having higher concentrations of haemocytes in LVG bees free of pathogens and parasites could benefit bees because haemocytes in insects are involved in many processes, including molting and development, hypoxia survival, vitellogenin production, iron transport, lipoprotein synthesis, apoptotic cell clearing, and other non-immune activities [67]. Thus, many beneficial traits may have been increased in LVG bees due to higher haemocyte concentrations. In addition, only the decline in haemocyte concentration with Varroa parasitism was significant in HVG bees. Like non-parasitized bees, parasitized LVG bees had more haemocytes than parasitized HVG bees, which could contribute to resistance by allowing them to reduce Varroa feeding as haemocytes are involved in the production of reactive oxygen and nitrogen species, which can be toxic [25]. In addition, more haemocytes in LVG bees could increase clotting and wound healing, including wounds created by Varroa when piercing the bee exoskeleton [24], and more haemocytes could participate in humoral immune pathways for AMP production [68]. Thus, increased haemocytes could be contributing to resistance in multiple ways. It is unknown if the higher haemocyte concentrations in healthy LVG bees were due to greater production of haemocytes during development, greater storage of haemocytes, or a longer haemocyte lifespan, but higher concentrations could allow LVG bees to be better able to compensate for the loss of haemocytes during Varroa parasitism. Fruit fly resistance to wasp parasitism has been linked to higher constitutive haemocyte production [69], but this is the first report which demonstrates that the same mechanism could possibly be involved in honey bee resistance to Varroa parasitism. Thus, higher cellular immunity appears to be a novel resistance mechanism to Varroa, and it is likely contributing, along with the behavioral resistance in the LVG phenotype. In addition, as this is a constitutive trait, future research could examine if LVG bees are also resistant to other pathogens, such as Spiroplasma melliferum, where haemocytes are associated with resistance [70] and Nosema spp., where haemocytes increase aggregation [71].
Humoral immunity of LVG and HVG bees exposed to V. destructor parasitism was estimated from the expression of the AMP immune-related genes, AmDef-2 and AmHym-2. The expression of genes for AMPs in bees are activated by different signaling pathways, which would be the Toll pathway for defensins, and Imd pathway for hymenoptaecins [22,37]. There were no significant differences in the expression of both genes in non-parasitized LVG and HVG bees, but the expression of AmHym-2 was significantly higher in parasitized LVG than parasitized HVG bees, indicating a triggering of the Imd pathway. However, there was considerable variation in the expression of AmDef-2, and it is possible that a significant difference would have been found with additional replications. None of the previous studies selecting bees for LVG [11,12,14,47,49,50,56] examined humoral immunity through gene expression. However, the upregulation of immune-related genes has been observed in studies of adult bees parasitized by V. destructor, such as both AmDef-2 and AmHym-2 [72,73]. In addition, the expression of AmDef-1 and AmHym-1 was higher in parasitized Varroa-resistant genotypes compared to susceptible genotypes, indicating that there is genetic variation in defense gene expression among honey bees related to Varroa resistance [1,4,74]. Increased expression of AMPs could be a response to stresses, such as cell damage caused by Varroa feeding and by DWV replication, or it could contribute to resistance to the parasite. Thus, in addition to hygienic behavior, grooming behavior, and cellular immunity, these results show that humoral immunity appears to be contributing to Varroa resistance of LVG bees. However, it is important to examine the expression of more genes regulated by the Toll or Imd pathways, as well as genes related to the JAK/STAT and JNK pathways that also regulate bee immunity [37]. Since AMPs are also important in resistance to a variety of honey bee pathogens, such as Paenibacillus larvae [37] and Spiroplasma melliferum [70], increased humoral resistance in the LVG bees may also indicate that they have enhanced resistance to a variety of bee diseases.
DWV levels in caged bees of the third generation varied between genotypes, with LVG bees having significantly lower infection levels than HVG bees in both control and Varroa-exposed bees. Viral infection levels were highest when bees of both genotypes were challenged with the mite. Clearly, Varroa exposure triggers DWV proliferation as has been demonstrated in other studies [1,9]. The only other study that selected bees for LVG that assessed DWV levels was that of Emsen et al. [75], who found 47% lower DWV levels compared with HVG bees, which was very similar to this study. Additionally, bee genotypes with resistance to Varroa have been shown to have lower infection levels of DWV than susceptible genotypes [76]. Viral resistance is a part of humoral immunity, and the lower DWV titers observed in bees from Varroa-resistant genotypes (selected or natural) compared to susceptible genotypes indicates antiviral mechanisms can differ by bee genotype [1,75,77]. Possible potential mechanisms of viral resistance could be less feeding by V. destructor on LVG bees, reducing the amounts of DWV transmitted, increasing resistance to DWV multiplication, such as RNAi [29] or other unknown mechanisms. Penn et al. [78] suggested that RNAi affecting DWV replication does occur but they did not find a strong correlation between Varroa-resistant genotypes of honey bees with DWV loads. Thus, humoral immunity as detected by higher AMP gene expression and lower DWV replication are contributing to the LVG phenotype, in addition to higher hygienic behavior, grooming behavior, and cellular immunity.
5. Conclusions
Honey bee genotypes selected for low or high V. destructor population growth (LVG and HVG, respectively) were examined for multiple potential Varroa resistance mechanisms. Rates of hygienic and grooming behaviors in LVG colonies were significantly higher than those in HVG colonies in two of the three generations. For individual bees in the third generation, grooming start time was significantly shorter and the proportion of individuals performing intense grooming higher in the LVG than in the HVG genotype. Cellular immunity was greater as well based on significantly higher haemocyte concentrations in non-parasitized and Varroa-parasitized LVG bees. Humoral immunity was greater with Varroa-parasitized LVG bees having significantly higher expression of the antimicrobial peptide gene, hymenoptaecin 2. In addition, antiviral resistance may be involved as there were significantly lower levels of DWV in Varroa-parasitized LVG bees. While selection for LVG and HVG bees was solely based on Varroa population growth, it appears that components of behavioral, cellular, and humoral mechanisms were all selected, potentially contributing to this resistance, rather than just one or two resistance mechanisms. Thus, LVG resistance appears to be a multi-gene trait related to multiple resistance mechanisms. Since all the potential mechanisms examined in this study appeared to contribute to the resistance of LVG bees, future research could examine additional resistance mechanisms as it is possible that even more traits and, thus, more genes are involved. Due to the simplicity of the methodology used to select LVG colonies, based on mite fall, it could be easily implemented by queen breeders. In contrast, selecting for particular resistance mechanisms, such as higher haemocyte concentration or lower virus levels, would be less simple for queen breeders, although this could result in other novel bee genotypes. The approach used in this study has the potential advantage of including multiple resistance mechanisms that could have additive or synergistic effects and be more stable as Varroa would have to overcome several modes of resistance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/insects16040385/s1: Table S1. Name of honey bee and deformed wing virus genes, abbreviations, Gene ID, accession number, forward and reverse primers, and length of the amplicons (bp) used in this study; Table S2. gBlock synthetic gene fragments (300 bp) used in this study.
Author Contributions
Conceptualization, E.G.-N., P.H.G. and A.D.l.M.; methodology, A.D.l.M., N.M. and E.G.-N.; formal analyses, T.P. and A.D.l.M.; resources, E.G.-N.; investigation, A.D.l.M. and N.M.; data curation, T.P.; writing, A.D.l.M., E.G.-N., P.H.G. and T.P.; supervision, E.G.-N. and P.H.G.; funding acquisition, E.G.-N. and A.D.l.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially funded by the Ontario Ministry of Agriculture, Food and Rural Affairs, Grant No. ND2017-3142. It was also partially funded by a Canadian Bee Research Grant from the Canadian Honey Council.
Data Availability Statement
The data from this study will be provided by the corresponding author upon reasonable request. The data are not publicly available due to future publications and collaborations related to the breeding lines.
Acknowledgments
We thank the staff and volunteers from the Honey Bee Research Center, University of Guelph, Technology Transfer Program, Ontario Beekeepers’s Association, and Ontario Bee Breeders Association. In particular, we wish to thank Berna Emsen, Daniel Borges, Yiza Bernal, Roberto Pelaez, Darinka Garduno, Wissarut Sukhaket, Joelle Harrison, Emmy Proctor, Kathryn Knowles, Max Dewaele, Monique Dulong, Sarah Guzman, Fred Ferguson, Pia Marquardt, Wendy Shipsides, Kate Petreny, Catherine VanderHeyden, Mark Bolzon, and Johann Peters for their assistance with laboratory and field work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| A. mellifera | Apis mellifera |
| AmDef-2 | Apis mellifera defensin 2 |
| AmHym-2 | Apis mellifera hymenoptaecin 2 |
| AmRPS5 | Apis mellifera 40S ribosomal protein S5 |
| AMP | Antimicrobial peptides |
| bp | Base pairs |
| C | Control treatment |
| CA | Canada |
| cDNA | Complementary desoxyribonucleic acid |
| cm | Centimeters |
| CO2 | Carbon dioxide |
| Ct | Cycle threshold value |
| DB identifier | Database gene identifier DE Germany |
| dsH2O | Deionized sterile water |
| DWV | Deformed wing virus |
| gc | Guanine cytosine content |
| Gene ID | BeeBase gene identifiers |
| h | Hours |
| H2O | Water |
| HBRC | Honey Bee Research Center |
| HVG | High Varroa population growth |
| HVG-C | High Varroa population growth bees without Varroa (control) |
| HVG-V | High Varroa population growth bees with Varroa |
| JP | Japan |
| L | Liter |
| Log | Logarithm |
| LSD test | Least Significant Difference test |
| LVG | Low Varroa population growth |
| LVG-C | Low Varroa population growth bees without Varroa (control) |
| LVG-V | Low Varroa population growth bees with Varroa |
| mg | Milligrams |
| min | Minutes |
| mL | Milliliters |
| mm | Millimeters |
| MNR | Mite non-reproduction |
| µg | Microgram |
| µL | Microliter |
| N | North |
| ng | Nanograms |
| nM | Nanomole |
| PCR | Polymerase chain reaction |
| RE | Relative expression |
| RH | Relative humidity |
| RNA | Ribonucleic acid |
| RNAi | Ribonucleic acid interference |
| rpm | Revolutions per minutes |
| RT-qPCR | Quantitative real time PCR |
| s | Seconds |
| SEM | Standard error of the mean |
| SMR | Suppressed mite reproduction |
| SOV | Suppressed in ovo virus infection |
| USA | United States of America |
| V | Varroa exposed bees treatment |
| V. destructor | Varroa destructor |
| VSH | Varroa sensitive hygiene |
| W | West |
References
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Varroa destructor and its impacts on honey bee biology. Front. Bee Sci. 2023, 1, 1272937. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, J.; Wei, Q.; Liu, F.; Cui, L.; Rueppell, O.; Xu, S. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nat. Commun. 2024, 15, 725. [Google Scholar] [CrossRef]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Effect of Varroa destructor, wounding and Varroa homogenate on gene expression in brood and adult honey bees. PLoS ONE 2017, 12, e0169669. [Google Scholar] [CrossRef]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 2018, 117, 1175–1183. [Google Scholar] [CrossRef]
- Reyes-Quintana, M.; Espinosa-Montaño, L.G.; Prieto-Merlos, D.; Koleoglu, G.; Petukhova, T.; Correa-Benítez, A.; Guzman-Novoa, E. Impact of Varroa destructor and deformed wing virus on emergence, cellular immunity, wing integrity and survivorship of Africanized honey bees in Mexico. J. Invertebr. Pathol. 2019, 164, 43–48. [Google Scholar] [CrossRef]
- Genersch, E.; Aubert, M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. 2010, 41, 54. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Eu, Y.-J.; Whyard, S.; Currie, R.W. Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double-stranded RNA ingestion. Insect Mol. Biol. 2012, 21, 446–455. [Google Scholar] [CrossRef]
- Martin, S.J.; Brettell, L.E. Deformed wing virus in honeybees and other insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.J.; De Guzman, L.I. Breeding for resistance to Varroa destructor in North America. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- De la Mora, A.; Emsen, B.; Morfin, N.; Borges, D.; Eccles, L.; Kelly, P.G.; Goodwin, P.H.; Guzman-Novoa, E. Selective breeding for low and high Varroa destructor growth in honey bee (Apis mellifera) colonies: Initial results of two generations. Insects 2020, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- De La Mora, A.; Goodwin, P.H.; Emsen, B.; Kelly, P.G.; Petukhova, T.; Guzman-Novoa, E. Selection of honey bee (Apis mellifera) genotypes for three generations of low and high population growth of the mite Varroa destructor. Animals 2024, 14, 3537. [Google Scholar] [CrossRef] [PubMed]
- Boecking, O.; Spivak, M. Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie 1999, 30, 141–158. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for resistance to Varroa destructor in Europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Emsen, B.; Unger, P.; Espinosa-Montaño, L.G.; Petukhova, T. Genotypic variability and relationships between mite infestation levels, mite damage, grooming intensity, and removal of Varroa destructor mites in selected strains of worker honey bees (Apis mellifera L.). J. Invertebr. Pathol. 2012, 110, 314–320. [Google Scholar] [CrossRef]
- Morfin, N.; Given, K.; Evans, M.; Guzman-Novoa, E.; Hunt, G.J. Grooming behavior and gene expression of the Indiana “mite-biter” honey bee stock. Apidologie 2020, 51, 267–275. [Google Scholar] [CrossRef]
- Hunt, G.; Given, K.J.; Tsuruda, J.M.; Andino, G.K. Breeding mite-biting bees to control Varroa. Bee Cult. 2016, 144, 41. [Google Scholar]
- Harbo, J.R.; Harris, J.W. Suppressed mite reproduction explained by the behaviour of adult bees. J. Apic. Res. 2005, 44, 21–23. [Google Scholar] [CrossRef]
- Ibrahim, A.; Spivak, M. The relationship between Suppression of Mite Reproduction (SMR) and hygienic behavior. Am. Bee J. 2004, 144, 406. [Google Scholar]
- Harris, J.W. Bees with Varroa Sensitive Hygiene preferentially remove mite infested pupae aged ≤ five days post capping. J. Apic. Res. 2007, 46, 134–139. [Google Scholar] [CrossRef]
- Danka, R.G.; Harris, J.W.; Dodds, G.E. Selection of VSH-derived “Pol-Line” honey bees and evaluation of their Varroa-resistance characteristics. Apidologie 2016, 47, 483–490. [Google Scholar] [CrossRef]
- Morfin, N.; Anguiano-Baez, R.; Guzman-Novoa, E. Honey bee (Apis mellifera) immunity. Vet. Clin. N. Am. Food Anim. Pract. 2021, 37, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Kanbar, G.; Engels, W. Ultrastructure and bacterial infection of wounds in honey bee (Apis mellifera) pupae punctured by Varroa mites. Parasitol. Res. 2003, 90, 349–354. [Google Scholar] [CrossRef]
- Nakhleh, J.; El Moussawi, L.; Osta, M. The melanization response in insect immunity. In Advances in Insect Physiology (Insect Immunity); Ligoxygakis, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 52, pp. 83–109. [Google Scholar]
- Millanta, F.; Sagona, S.; Mazzei, M.; Forzan, M.; Poli, A.; Felicioli, A. Phenoloxidase activity and haemolymph cytology in honeybees challenged with a virus suspension (deformed wings virus DWV) or phosphate buffered suspension (PBS). Cienc. Rural 2019, 49, e20180726. [Google Scholar] [CrossRef]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Swevers, L.; Sun, J. Antimicrobial peptides as potential antiviral factors in insect antiviral immune response. Front. Immunol. 2020, 11, 2030. [Google Scholar] [CrossRef]
- De Graaf, D.C.; Laget, D.; De Smet, L.; Claeys Boúúaert, D.; Brunain, M.; Veerkamp, R.F.; Brascamp, E.W. Heritability estimates of the novel trait ‘suppressed in ovo virus infection’ in honey bees (Apis mellifera). Sci. Rep. 2020, 10, 14310. [Google Scholar] [CrossRef]
- De Smet, L.; Ravoet, J.; Wenseleers, T.; De Graaf, D.C. Expression of key components of the RNAi machinery are suppressed in Apis mellifera that suffer a high virus infection. Entomol. Sci. 2017, 20, 76–85. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Performance of hygienic honey bee colonies in a commercial apiary. Apidologie 1998, 29, 291–302. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Corona, M.; Alburaki, M.; Reynaldi, F.J.; Invernizzi, C.; Fernández De Landa, G.; Maggi, M. Honey bee populations surviving Varroa destructor parasitism in Latin America and their mechanisms of resistance. Front. Ecol. Evol. 2024, 12, 1434490. [Google Scholar] [CrossRef]
- Morfin, N.; Espinosa-Montaño, L.G.; Guzman-Novoa, E. A Direct assay to assess self-grooming behavior in honey bees (Apis mellifera L.). Apidologie 2020, 51, 892–897. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for Varroa research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Murphy, D.B.; Davidson, M.W. Fundamentals of Light Microscopy and Electronic Imaging, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera L.) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030. [Google Scholar] [CrossRef]
- Walsh, A.T.; Triant, D.A.; Le Tourneau, J.J.; Shamimuzzaman, M.; Elsik, C.G. Hymenoptera genome database: New genomes and annotation datasets for improved GO enrichment and orthologue analyses. Nucleic Acids Res. 2022, 50, D1032–D1039. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.-L.; Jiang, H.; Kanost, M.; Thompson, G.I.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Morfin, N.; Macías-Macías, J.O.; Guzman-Novoa, E. Viral quantification in bee samples using synthetic DNA sequences with Real-Time PCR (qPCR). In Virus-Host Interactions: Methods and Protocols; Aquino De Muro, M., Ed.; Springer: New York, NY, USA, 2023; Volume 2610. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef]
- Bustin, S. Absolute quantification of mRNA using Real-Time Reverse Transcription Polymerase Chain Reaction assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Forsgren, E.; de Miranda, J.R.; Isaksson, M.; Wei, S.; Fries, I. Deformed wing virus associated with Tropilaelaps mercedesae infesting European honey bees (Apis mellifera). Exp. Appl. Acarol. 2009, 47, 87–97. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: R-Project, 2019. Available online: https://www.R-project.org/ (accessed on 1 June 2024).
- Schafaschek, T.P.; Rodrigues Hickel, E.; Lopes De Oliveira, C.A.; De Alencar Arnaut De Toledo, V. Infestation and reproduction of Varroa destructor Anderson and Trueman and hygienic behavior in colonies of Apis mellifera L. (Africanized honeybee) with queens of different genetic origins. Sociobiology 2019, 66, 448. [Google Scholar] [CrossRef]
- Seltzer, R.; Kahanov, P.; Kamer, Y.; Hetzroni, A.; Bieńkowska, M.; Hefetz, A.; Soroker, V. The payoffs and tradeoffs of hygienic behavior: A five year field study on a local population of honey bees. J. Apic. Res. 2022, 61, 492–501. [Google Scholar] [CrossRef]
- Ibrahim, A.; Spivak, M. The relationship between hygienic behavior and suppression of mite reproduction as honey bee (Apis mellifera) mechanisms of resistance to Varroa destructor. Apidologie 2006, 37, 31–40. [Google Scholar] [CrossRef]
- Emsen, B.; Petukhova, T.; Guzman-Novoa, E. Factors limiting the growth of Varroa destructor populations in selected honey bee (Apis mellifera L.) colonies. J. Anim. Vet. Adv. 2012, 11, 4519–4525. [Google Scholar]
- Erez, T.; Bonda, E.; Kahanov, P.; Rueppell, O.; Wagoner, K.; Chejanovsky, N.; Soroker, V. Multiple benefits of breeding honey bees for hygienic behavior. J. Invert. Pathol. 2022, 193, 107788. [Google Scholar] [CrossRef]
- Rinderer, T.E.; De Guzman, L.I.; Delatte, G.T.; Stelzer, J.A.; Lancaster, V.A.; Kuznetsov, V.; Beaman, L.; Watts, R.; Harris, J.W. Resistance to the parasitic mite Varroa destructor in honey bees from far-Eastern Russia. Apidologie 2001, 32, 381–394. [Google Scholar] [CrossRef]
- Lodesani, M.; Crailsheim, K.; Moritz, R.F.A. Effect of some characters on the population growth of mite Varroa jacobsoni in Apis mellifera L colonies and results of a bi-directional selection. J. Appl. Entomol. 2002, 126, 130–137. [Google Scholar] [CrossRef]
- Russo, R.M.; Liendo, M.C.; Landi, L.; Pietronave, H.; Merke, J.; Fain, H.; Muntaabski, I.; Palacio, M.A.; Rodríguez, G.A.; Lanzavecchia, S.B.; et al. Grooming behavior in naturally Varroa-resistant Apis mellifera colonies from north-central Argentina. Front. Ecol. Evol. 2020, 8, 590281. [Google Scholar] [CrossRef]
- Nganso, B.T.; Fombong, A.T.; Yusuf, A.A.; Pirk, C.W.W.; Stuhl, C.; Torto, B. Hygienic and grooming behaviors in African and European honeybees—New damage categories in Varroa destructor. PLoS ONE 2017, 12, e0179329. [Google Scholar] [CrossRef]
- Guarna, M.M.; Hoover, S.E.; Huxter, E.; Higo, H.; Moon, K.-M.; Domanski, D.; Bixby, M.E.F.; Melathopoulos, A.P.; Ibrahim, A.; Peirson, M.; et al. Peptide biomarkers used for the selective breeding of a complex polygenic trait in honey bees. Sci. Rep. 2017, 7, 8381. [Google Scholar] [CrossRef]
- Kirrane, M.J.; De Guzman, L.I.; Whelan, P.M.; Frake, A.M.; Rinderer, T.E. Evaluations of the removal of Varroa destructor in Russian honey bee colonies that display different levels of Varroa Sensitive Hygienic activities. J. Insect Behav. 2018, 31, 283–297. [Google Scholar] [CrossRef]
- Hamiduzzaman, M.M.; Emsen, B.; Hunt, G.J.; Subramanyam, S.; Williams, C.E.; Tsuruda, J.M.; Guzman-Novoa, E. Differential gene expression associated with honey bee grooming behavior in response to Varroa mites. Behav. Genet. 2017, 47, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.W.; Harbo, J.R.; Villa, J.D.; Danka, R.G. Variable population growth of Varroa destructor (Mesostigmata: Varroidae) in colonies of honey bees (Hymenoptera: Apidae) during a 10-year period. Environ. Entomol. 2003, 32, 1305–1312. [Google Scholar] [CrossRef]
- Martin, S.J.; Hawkins, G.P.; Brettell, L.E.; Reece, N.; Correia-Oliveira, M.E.; Allsopp, M.H. Varroa destructor reproduction and cell re-capping in mite-resistant Apis mellifera populations. Apidologie 2020, 51, 369–381. [Google Scholar] [CrossRef]
- Oddie, M.; Büchler, R.; Dahle, B.; Kovacic, M.; Le Conte, Y.; Locke, B.; De Miranda, J.R.; Mondet, F.; Neumann, P. Rapid parallel evolution overcomes global honey bee parasite. Sci. Rep. 2018, 8, 7704. [Google Scholar] [CrossRef]
- Rodríguez-Luis, A.; Grindrod, I.; Webb, G.; Piñeiro, A.P.; Martin, S.J. Recapping and mite removal behaviour in Cuba: Home to the world’s largest population of Varroa-resistant European honeybees. Sci. Rep. 2022, 12, 15597. [Google Scholar] [CrossRef]
- Harbo, J.R.; Harris, J.W. An evaluation of commercially produced queens that have the SMR trait. Am. Bee J. 2003, 143, 213–216. [Google Scholar]
- Mondet, F.; Beaurepaire, A.; McAfee, A.; Locke, B.; Alaux, C.; Blanchard, S.; Danka, B.; Le Conte, Y. Honey bee survival mechanisms against the parasite Varroa destructor: A systematic review of phenotypic and genomic research efforts. Int. J. Parasitol. 2020, 50, 433–447. [Google Scholar] [CrossRef]
- Behrens, D.; Huang, Q.; Geßner, C.; Rosenkranz, P.; Frey, E.; Locke, B.; Moritz, R.F.A.; Kraus, F.B. Three QTL in the honey bee Apis mellifera L. suppress reproduction of the parasitic mite Varroa destructor. Ecol. Evol. 2011, 1, 451–458. [Google Scholar] [CrossRef]
- Broeckx, B.J.G.; De Smet, L.; Blacquière, T.; Maebe, K.; Khalenkow, M.; Van Poucke, M.; Dahle, B.; Neumann, P.; Bach Nguyen, K.; Smagghe, G.; et al. Honey bee predisposition of resistance to ubiquitous mite infestations. Sci. Rep. 2019, 9, 7794. [Google Scholar] [CrossRef]
- Nganso, B.T.; Fombong, A.T.; Yusuf, A.A.; Pirk, C.W.W.; Stuhl, C.; Torto, B. Low fertility, fecundity and numbers of mated female offspring explain the lower reproductive success of the parasitic mite Varroa destructor in African honeybees. Parasitology 2018, 145, 1633–1639. [Google Scholar] [CrossRef]
- Medina, L.M.; Martin, S.J. A comparative study of Varroa jacobsoni reproduction in worker cells of honey bees (Apis mellifera) in England and Africanized bees in Yucatan, Mexico. Exp. Appl. Acarol. 1999, 23, 659–667. [Google Scholar]
- Calderón, R.A.; Ureña, S.; Van Veen, J.W. Reproduction of Varroa destructor and offspring mortality in worker and drone brood cells of Africanized honey bees. Exp. Appl. Acarol. 2012, 56, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Haas, E.; Kim, Y. Beyond cellular immunity: On the biological significance of insect hemocytes. Cells 2023, 12, 599. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Kacsoh, B.Z.; Schlenke, T.A. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS ONE 2012, 7, e34721. [Google Scholar] [CrossRef]
- Yang, D.; Zha, G.; Li, X.; Gao, H.; Yu, H. Immune responses in the haemolymph and antimicrobial peptide expression in the abdomen of Apis mellifera challenged with Spiroplasma melliferum CH-1. Microb. Pathog. 2017, 112, 279–287. [Google Scholar] [CrossRef]
- Ni, W.; Bao, J.; Mo, B.; Liu, L.; Li, T.; Pan, G.; Chen, J.; Zhou, Z. Hemocytin facilitates host immune responses against Nosema bombycis. Dev. Comp. Immunol. 2020, 103, 103495. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Hunt, G.J.; Guzman-Novoa, E. Effects of sublethal doses of clothianidin and/or V. destructor on honey bee (Apis mellifera) self-grooming behavior and associated gene expression. Sci. Rep. 2019, 9, 5196. [Google Scholar] [CrossRef]
- Guzman-Novoa, E. Integration of Biotechnologies / Genetic Basis of Disease Resistance in the Honey Bee (Apis mellifera L.). In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 763–767. [Google Scholar]
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; DeGrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera. Insect Sci. 2017, 24, 467–477. [Google Scholar] [CrossRef]
- Emsen, B.; Hamiduzzaman, M.M.; Goodwin, P.H.; Guzman-Novoa, E. Lower virus infections in Varroa destructor-infested and uninfested brood and adult honey bees (Apis mellifera) of a low mite population growth colony compared to a high mite population growth colony. PLoS ONE 2015, 10, e0118885. [Google Scholar] [CrossRef]
- De Souza, F.S.; Allsopp, M.H.; Martin, S.J. Deformed wing virus prevalence and load in honeybees in South Africa. Arch. Virol. 2021, 166, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cuellar, A.K.; De La Mora, A.; Contreras-Escareño, F.; Morfin, N.; Tapia-González, J.M.; Macías-Macías, J.O.; Petukhova, T.; Correa-Benítez, A.; Guzman-Novoa, E. Genotype, but not climate, affects the resistance of honey bees (Apis mellifera) to viral infections and to the mite Varroa destructor. Vet. Sci. 2022, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Penn, H.J.; Simone-Finstrom, M.D.; Chen, Y.; Healy, K.B. Honey bee genetic stock determines deformed wing virus symptom severity but not viral load or dissemination following pupal exposure. Front. Genet. 2022, 13, 909392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).