Influence of Different Diets on Growth and Development of Eastern Honey Bee (Apis cerana)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Bee Rearing and Feeding
2.2. Food Consumption, Body Weight and Survival Analysis
2.3. Diet Preferences
2.4. Hypopharyngeal Gland Measurements
2.5. Assessment of Total Midgut Proteolytic Enzyme Activity
2.6. Statistical Analysis
3. Results
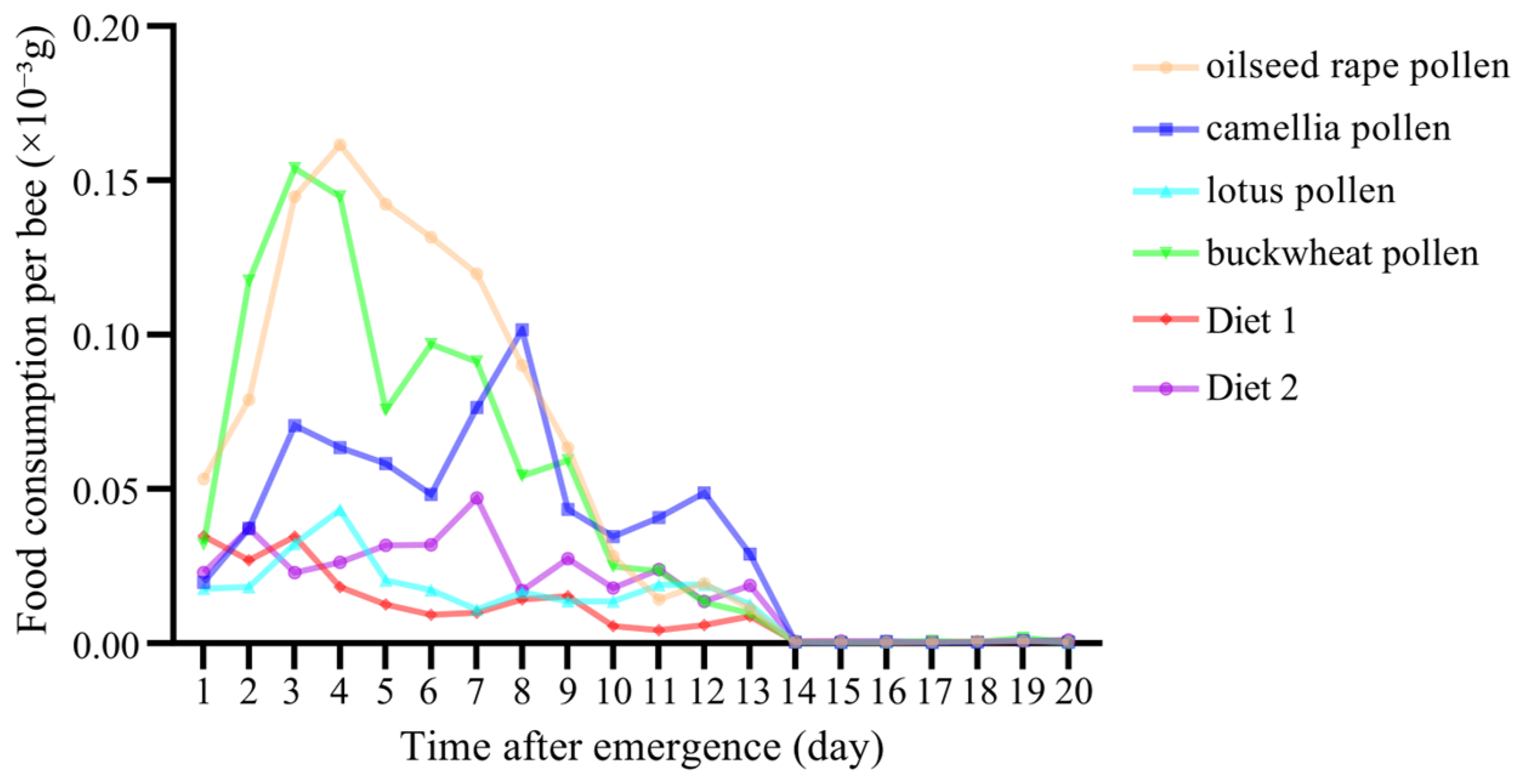
3.1. Diet Consumption
3.2. Diet Preference
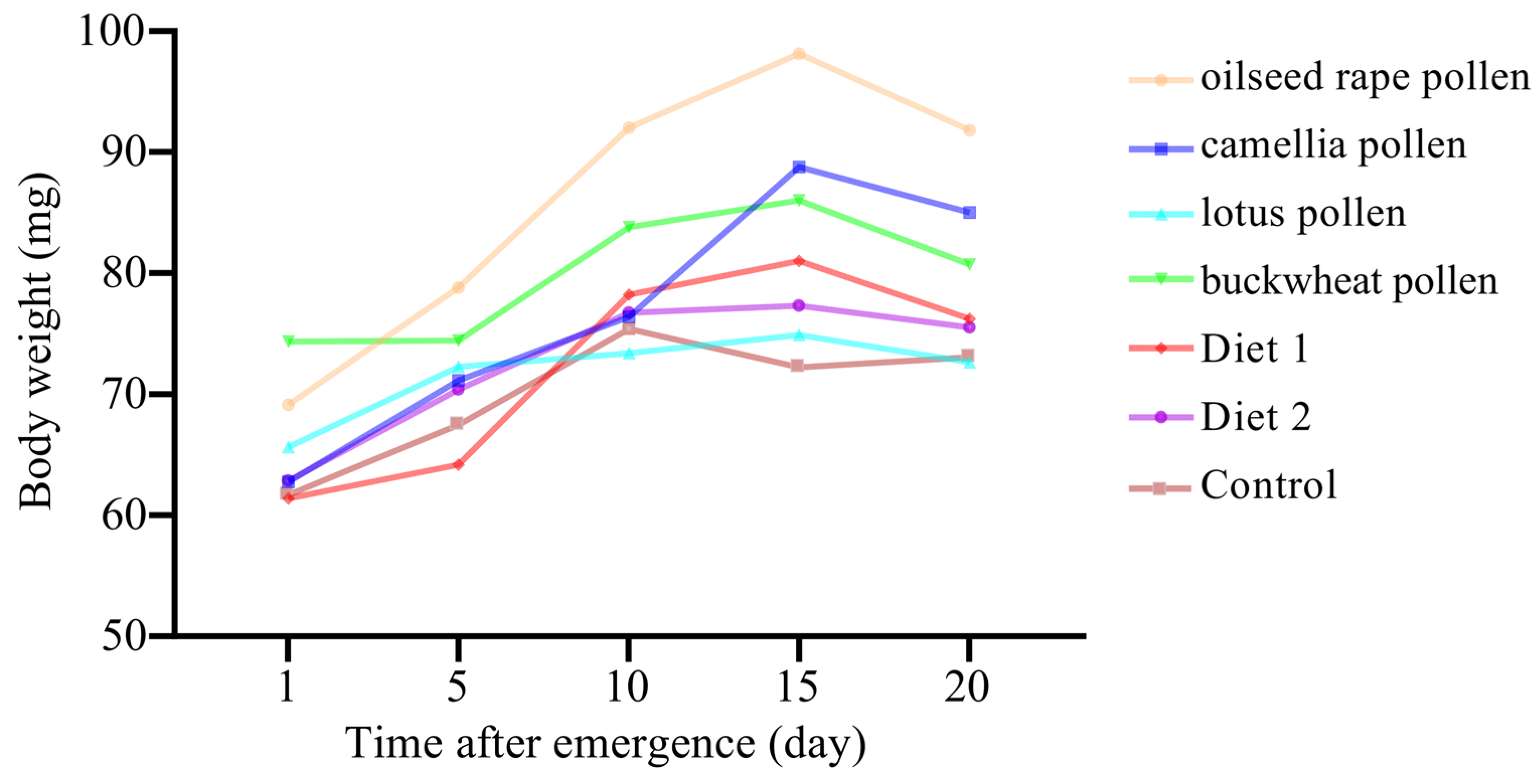
3.3. Body Weight
3.4. Survival Probability
3.5. Hypopharyngeal Gland Development
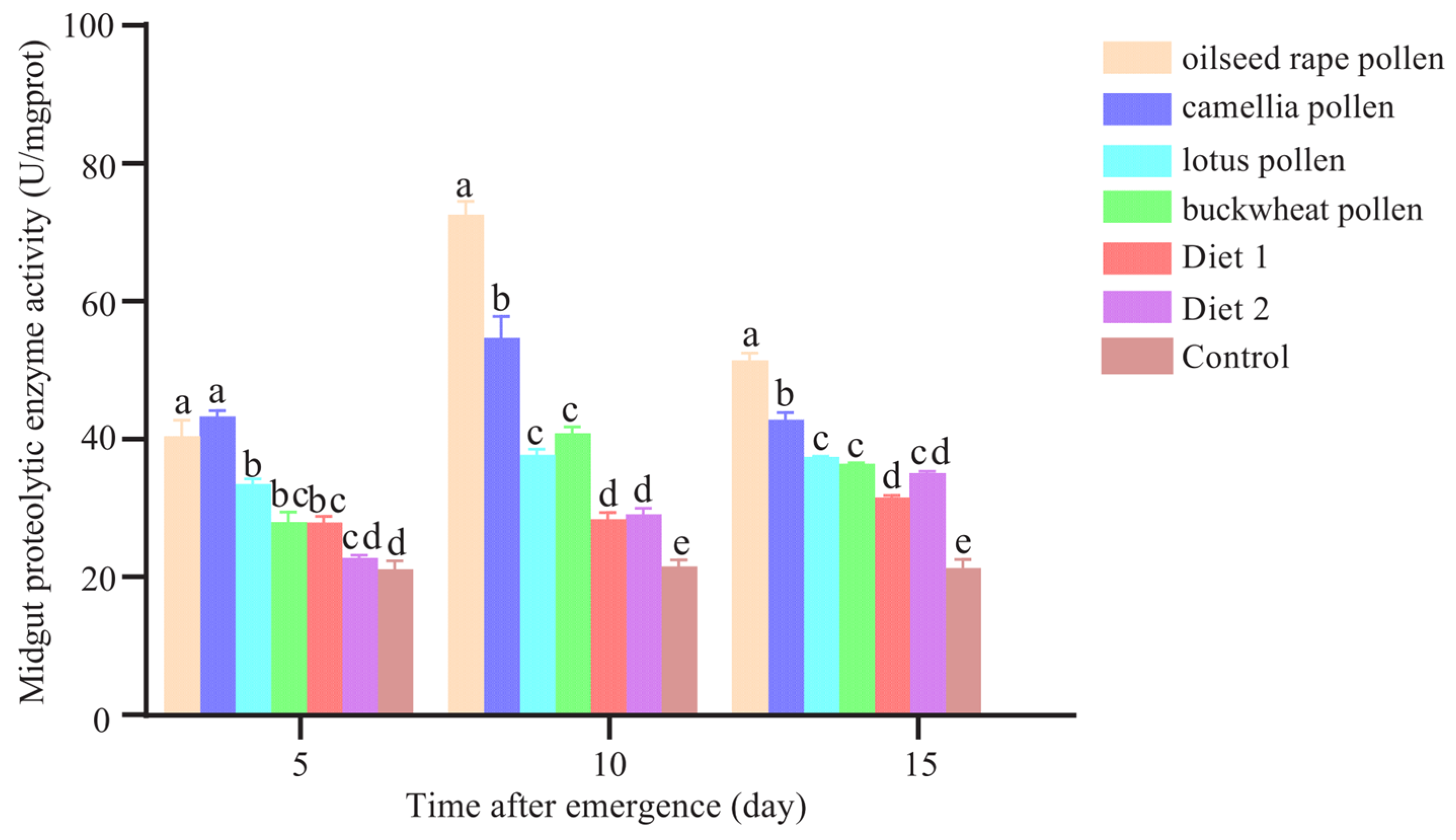
3.6. Total Midgut Proteolytic Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung, K.L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.E.D.; Barbosa, C.D.; Lourenço, A.P. Pollen nutrition in honey bees (Apis mellifera): Impact on adult health. Apidologie 2016, 47, 15–25. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Otto, C.R.V.; Roth, C.L.; Carlson, B.L.; Smart, M.D. Land-use change reduces habitat suitability for supporting managed honey bee colonies in the Northern Great Plains. Proc. Natl. Acad. Sci. USA 2016, 113, 10430–10435. [Google Scholar] [CrossRef]
- Topitzhofer, E.; Lucas, H.; Chakrabarti, P.; Breece, C.; Bryant, V.; Sagili, R.R. Assessment of pollen diversity available to honey bees (Hymenoptera: Apidae) in major cropping systems during pollination in the Western United States. J. Econ. Entomol. 2019, 112, 2040–2048. [Google Scholar] [CrossRef]
- Noordyke, E.R.; Ellis, J.D. Reviewing the efficacy of pollen substitutes as a management tool for improving the health and productivity of western honey bee (Apis mellifera) colonies. Front. Sustain. Food Syst. 2021, 5, 772897. [Google Scholar] [CrossRef]
- Mattila, H.R.; Otis, G.W. Influence of pollen diet in spring on development of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 2006, 99, 604–613. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Wongsiri, S.; Seeley, T.D. Asian Honey Bees: Biology, Conservation, and Human Interactions, 1st ed.; Harvard University Press: Cambridge, UK, 2009; pp. 52–64. [Google Scholar]
- Wang, H.T.; Li, L.F.; Chen, H.Y.; Zhang, X.F.; Chen, L.D.; Zhao, H.X. Progress in pollination by Apis cerana cerana. J. Environ. Entomol. 2022, 44, 84–91. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Tang, J.; Ma, C.Y.; Shi, W.; Chen, X.; Liu, Z.G.; Wang, H.H.; Chen, C. A national survey of managed honey bee colony winter losses (Apis mellifera L.) in China. Diversity 2020, 12, 318. [Google Scholar] [CrossRef]
- Luo, Y.X.; Chen, L.D. The current status of Apis cerana beekeeping in China. Apic. China 2018, 69, 22–24. [Google Scholar] [CrossRef]
- Liu, N.N.; Liu, H.M.; Ju, Y.; Li, X.A.; Li, Y.; Wang, T.J.; He, J.M.; Niu, Q.S.; Xing, X.M. Geometric morphology and population genomics provide insights into the adaptive evolution of Apis cerana in Changbai Mountain. BMC Genom. 2022, 23, 64. [Google Scholar] [CrossRef]
- Qu, X.Y.; Zhang, X.R.; Zhang, G.Q.; Qin, H.R.; Zhang, H.X.; Tian, H.Y.; Chen, X. Investigations on beekeeping and breeding of Apis cerana in China. Life 2025, 15, 9. [Google Scholar] [CrossRef]
- Zhang, C.M. The importance of balanced nutrition of honey bee. J. Bee 2023, 43, 38–41. [Google Scholar]
- Yang, K.; Wu, D.; Ye, X.Q.; Liu, D.H.; Chen, J.C.; Sun, P.L. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Wei, W.T.; Hu, F.L. Beekeeping industry in China. Bee World 2011, 88, 41–44. [Google Scholar] [CrossRef]
- Qi, D.D.; Lu, M.L.; Li, J.K.; Ma, C. Metabolomics reveals distinctive metabolic profiles and marker compounds of camellia (Camellia sinensis L.) bee pollen. Foods 2023, 12, 2661. [Google Scholar] [CrossRef]
- Saffari, A.; Kevan, P.G.; Atkinson, J.L. Palatability and consumption of patty-formulated pollen and pollen substitutes and their effects on honeybee colony performance. J. Apic. Sci. 2010, 54, 63–71. [Google Scholar] [CrossRef]
- Manning, R. Artificial feeding of honeybees based on an understanding of nutritional principles. Anim. Prod. Sci. 2018, 58, 689–703. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, K.; Peng, W.J. Research progress of bee artifical feed and its nutritional value. Apic. China 2019, 70, 13–17. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chen, C.; Niu, Q.S.; Qi, W.Z.; Yuan, C.Y.; Su, S.K.; Liu, S.D.; Zhang, Y.S.; Zhang, X.W.; Ji, T.; et al. Survey results of honey bee (Apis mellifera) colony losses in China (2010–2013). J. Apic. Res. 2016, 55, 29–37. [Google Scholar] [CrossRef]
- Jehlik, T.; Kodrik, D.; Kristufek, V.; Koubova, J.; Sabova, M.; Danihlik, J.; Tomcala, A.; Frydrychova, R.C. Effects of Chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 2019, 50, 564–577. [Google Scholar] [CrossRef]
- Basualdo, M.; Barragan, S.; Vanagas, L.; Garcia, C.; Solana, H.; Rodriguez, E.; Bedascarrasbure, E. Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef]
- Schmidt, J.O.; Thoenes, S.C.; Levin, M.D. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 1987, 80, 176–183. [Google Scholar] [CrossRef]
- Di-Pasquale, G.; Salignon, M.; Le-Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Höcherl, N.; Siede, R.; Illies, I.; Gätschenberger, H.; Tautz, J. Evaluation of the nutritive value of maize for honey bees. J. Insect Physiol. 2012, 58, 278–285. [Google Scholar] [CrossRef]
- Danihlík, J.; Skrabisová, M.; Lenobel, R.; Sebela, M.; Omar, E.; Petrivalsky, M.; Crailsheim, K.; Brodschneider, R. Does the pollen diet influence the production and expression of antimicrobial peptides in individual honey bees? Insects 2018, 9, 79. [Google Scholar] [CrossRef]
- Gregorc, A.; Sampson, B.; Knight, P.R.; Adamczyk, J. Diet quality affects honey bee (Hymenoptera: Apidae) mortality under laboratory conditions. J. Apic. Res. 2019, 58, 492–493. [Google Scholar] [CrossRef]
- Sagona, S.; Coppola, F.; Nanetti, A.; Tafi, E.; Palego, L.; Betti, L.; Giannaccini, G.; Felicioli, A. Effects of two commercial protein diets on the health of two imago ages of Apis mellifera L. reared in laboratory. Animals 2022, 12, 968. [Google Scholar] [CrossRef]
- Amro, A.; Younis, M.; Ghania, A. Physiological effects of some pollen substitutes diets on caged honey bee workers (Apis mellifera L.). Int. J. Environ. 2020, 9, 87–99. [Google Scholar] [CrossRef]
- Hoover, S.E.R.; Higo, H.A.; Winston, M.L. Worker honey bee ovary development: Seasonal variation and the influence of larval and adult nutrition. J. Comp. Physiol. B 2006, 176, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Human, H.; Nicolson, S.W.; Strauss, K.; Pirk, C.W.W.; Dietemann, V. Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata). J. Insect Physiol. 2007, 53, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Manning, R.; Dods, K.; Baer, B.; Blache, D. Nurse bees regulate the larval nutrition of developing workers (Apis mellifera) when feeding on various pollen types. J. Econ. Entomol. 2024, 117, 683–695. [Google Scholar] [CrossRef]
- Pang, C.X.; Dong, K.; Guo, Y.Q.; Ding, G.L.; Lu, Y.M.; Guo, Z.B.; Wu, J.; Huang, J.X. Effects of three types of pollen on the growth and development of honey bee larvae (Hymenoptera, Apidae). Front. Ecol. Evol. 2022, 10, 870081. [Google Scholar] [CrossRef]
- Zheng, B.L.; Wu, Z.F.; Xu, B.H. The effects of dietary protein levels on the population growth, performance, and physiology of honey bee workers during early spring. J. Insect Sci. 2014, 14, 191. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Wardell, G.; Ahumada-Segura, F.; Rinderer, T.; Danka, R.; Pettis, J. Comparisons of pollen substitute diets for honey bees: Consumption rates by colonies and effects on brood and adult populations. J. Apic. Res. 2008, 47, 265–270. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 2000, 222, 187–209. [Google Scholar] [CrossRef]
- Somerville, D.C.; Nicol, H.I. Mineral content of honeybee-collected pollen from southern New South Wales. Aust. J. Exp. Agric. 2002, 42, 1131–1136. [Google Scholar] [CrossRef]
- Kim, H.; Frunze, O.; Maigoro, A.Y.; Lee, M.L.; Lee, J.H.; Kwon, H.W. Comparative study of the effect of pollen substitute diets on honey bees during early spring. Insects 2024, 15, 101. [Google Scholar] [CrossRef]
- Kim, H.; Maigoro, A.Y.; Lee, J.H.; Frunze, O.; Kwon, H.W. The improving effects of probiotic-added pollen substitute diets on the gut microbiota and individual health of honey bee (Apis mellifera L.). Microorganisms 2024, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K. The protein balance of the honey bee worker. Apidologie 1990, 21, 417–429. [Google Scholar] [CrossRef]
- Crailsheim, K. Interadult feeding of jelly in honeybee (Apis mellifera L.) colonies. J. Comp. Physiol. B 1991, 161, 55–60. [Google Scholar] [CrossRef]
- De-Jong, E.W.; DeGrandi-Hoffman, G.; Chen, Y.P.; Graham, H.; Ziolkowski, N. Effects of diets containing different concentrations of pollen and pollen substitutes on physiology, Nosema burden, and virus titers in the honey bee (Apis mellifera L.). Apidologie 2019, 50, 845–858. [Google Scholar] [CrossRef]
- Jung, C.; Yoon-Hwan, B. Production and characteristics of winter generation honey bees, Apis mellifera: Discussion with overwintering failure. J. Apic. 2022, 37, 265–274. [Google Scholar] [CrossRef]
- Retschnig, G.; Rich, J.; Crailsheim, K.; Pfister, J.; Perreten, V.; Neumann, P. You are what you eat: Relative importance of diet, gut microbiota and nestmates for honey bee, Apis mellifera, worker health. Apidologie 2021, 52, 632–646. [Google Scholar] [CrossRef]
- Noordyke, E.R.; Van-Santen, E.; Ellis, J.D. Tracing the fate of pollen substitute patties in western honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 2021, 114, 1421–1430. [Google Scholar] [CrossRef]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Manaswi, A.; Noordyke, E.; Prouty, C.; Ellis, J.D. Western honey bee (Apis mellifera L.) attraction to commercial pollen substitutes and wildflower pollen in vitro. J. Appl. Entomol. 2023, 147, 244–247. [Google Scholar] [CrossRef]
- Lee, M.R.; Choi, Y.S.; Kim, D.W.; Lee, M.Y. Age-dependent hypopharyngeal gland development and morphometric characteristics in the cross-bred lineage of honeybees reared for high royal jelly production. J. Asia-Pac. Entomol. 2019, 22, 699–704. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Khaibari, A.M.; Omar, M.O. Consumption rate of some proteinic diets affecting hypopharyngeal glands development in honeybee workers. Saudi J. Biol. Sci. 2011, 18, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Hung, Y.T.; Wu, M.C. Mechanistic exploration of royal jelly production in caged honey bees (Apis mellifera). Sci. Rep. 2024, 14, 30277. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y.P.; Huang, E.; Huang, M.H. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 2010, 56, 1184–1191. [Google Scholar] [CrossRef]
- D’Emilio, C.; Valenzano, V.; Maiolino, P.; Power, K.; Martano, M.; Di-Prisco, G. Effects of pollen enriched diet on hypopharyngeal glands’ morphology and morphometry and vitellogenin transcription levels in honey bees. Ital. J. Anim. Sci. 2025, 24, 484–492. [Google Scholar] [CrossRef]
- Mishchenko, O.; Postoienko, V.; Lytvynenko, O.; Ivanyshyn, A.; Afara, K. Influence of the food protein on the development of hypopharyngeal glands, fat body, quality and lifespan of honeybees. Sci. Horiz. 2023, 26, 44–51. [Google Scholar] [CrossRef]
- Li, C.C.; Xu, B.H.; Wang, Y.X.; Feng, Q.Q.; Yang, W.R. Effects of dietary crude protein levels on development, antioxidant status, and total midgut protease activity of honey bee (Apis mellifera ligustica). Apidologie 2012, 43, 576–586. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Snyder, L.; Meador, C.; Ayotte, T. Honey bee (Apis mellifera) nurses do not consume pollens based on their nutritional quality. PLoS ONE 2018, 13, e0191050. [Google Scholar] [CrossRef]
- Somerville, D.C.; Nicol, H.I. Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east australia and a note on laboratory disparity. Aust. J. Exp. Agric. 2006, 46, 141–149. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeon, H.; Jung, C. Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ. 2020, 44, 4. [Google Scholar] [CrossRef]
- Boch, R. Relative attractiveness of different pollens to honeybees when foraging in a flight room and when fed in the hive. J. Apic. Res. 1982, 21, 104–106. [Google Scholar] [CrossRef]
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Frunze, O.; Lee, J.H.; Kwon, H.W. Enhancing honey bee health: Evaluating pollen substitute diets in field and cage experiments. Insects 2024, 15, 361. [Google Scholar] [CrossRef] [PubMed]
- Pernal, S.F.; Currie, R.W. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 2000, 31, 387–409. [Google Scholar] [CrossRef]
- Omar, E.; Abd-Ella, A.A.; Khodairy, M.M.; Moosbeckhofer, R.; Crailsheim, K.; Brodschneider, R. Influence of different pollen diets on the development of hypopharyngeal glands and size of acid gland sacs in caged honey bees (Apis mellifera). Apidologie 2017, 48, 425–436. [Google Scholar] [CrossRef]
- Yang, K.C.; Peng, Z.W.; Lin, C.H.; Wu, M.C. A new design of bee cage for laboratory experiments: Nutritional assessment of supplemental diets in honey bees (Apis mellifera). Apidologie 2021, 52, 418–431. [Google Scholar] [CrossRef]
- Gao, L.J.; Liu, J.L.; Luo, W.H.; Yang, J.L.; Cao, L.; Ji, C.H.; Wang, R.S.; Ren, Q. Effects of different bee pollens on colony reproduction and worker development of Apis mellifera ligustica. Chin. J. Anim. Nutr. 2019, 31, 4630–4636. [Google Scholar]
- Lee, K.P.; Simpson, S.J.; Clissold, F.J.; Brooks, R.; Ballard, J.W.O.; Taylor, P.W.; Soran, N.; Raubenheimer, D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc. Natl. Acad. Sci. USA 2008, 105, 2498–2503. [Google Scholar] [CrossRef]
- Le-Couteur, D.G.; Solon-Biet, S.; Cogger, V.C.; Mitchell, S.J.; Senior, A.; De-Cabo, R.; Raubenheimer, D.; Simpson, S.J. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell. Mol. Life Sci. 2016, 73, 1237–1252. [Google Scholar] [CrossRef]
- Schulz, M.; Los, A.; Grzybek, M.; Scibior, R.; Strachecka, A. Piperine as a new natural supplement with beneficial effects on the life-span and defence system of honeybees. J. Agric. Sci. 2019, 157, 140–149. [Google Scholar] [CrossRef]
- Crone, M.K.; Grozinger, C.M. Pollen protein and lipid content influence resilience to insecticides in honey bees (Apis mellifera). J. Exp. Biol. 2021, 224, jeb242040. [Google Scholar] [CrossRef]
- Campos, M.; Markham, K.R.; Mitchell, K.A.; DaCunha, A.P. An approach to the characterization of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Dominguez-Valhondo, D.; Gil, D.B.; Hernandez, M.T.; Gonzalez-Gomez, D. Influence of the commercial processing and floral origin on bioactive and nutritional properties of honeybee-collected pollen. Int. J. Food Sci. Technol. 2011, 46, 2204–2211. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.P.; Rivera, R.; Carroll, M.; Chambers, M.; Hidalgo, G.; De Jong, E.W. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie 2016, 47, 186–196. [Google Scholar] [CrossRef]
- Schmidt, J.O. Feeding preferences of Apis mellifera L. (Hymenoptera: Apidae): Individual versus mixed pollen species. J. Kans. Entomol. Soc. 1984, 57, 323–327. [Google Scholar]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef]
- Brys, M.S.; Skowronek, P.; Strachecka, A. Pollen diet—Properties and impact on a bee colony. Insects 2021, 12, 798. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anagnostopoulos, C.; Anastasiadou, P.; Machera, K. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Sci. Total Environ. 2014, 485, 633–642. [Google Scholar] [CrossRef]
- Sihag, R.C.; Gupta, M. Development of an artificial pollen substitute/supplement diet to help tide the colonies of honeybee (Apis mellifera L.) over the dearth season. J. Apic. Sci. 2011, 55, 15–29. [Google Scholar]
- Li, Y.J.; Zheng, B.L.; Yang, W.R.; Xu, B.H. Research on optimal level of calcium and phosphorus in pollen substitutes. Chin. J. Appl. Entomol. 2012, 49, 1203–1209. [Google Scholar]
- Wei, W.; Chi, X.P.; Zhang, W.X.; Liu, Z.G.; Wang, H.F.; Ma, L.T.; Xu, B.H. Study on wheat germ as protein source for spring multiplication of Apis mellifera L. Shandong Agric. Sci. 2018, 50, 119–125. [Google Scholar] [CrossRef]
- Xue, J.L. Effect of Diet’s Protein Source on Feeding Effect of Honeybee (Apis mellifera L.). Master’s Thesis, Shandong Agricultural University, Taian, China, 2020. [Google Scholar]




| Pollen | Producing Region | Longitude (E) | Latitude (N) | Time of Collection | Protein Content (g/100 g) |
|---|---|---|---|---|---|
| oilseed rape pollen | Huzhu County, Haidong City, Qinghai Province | 101.96° | 36.84° | July | 24.53 ± 0.40 |
| camellia pollen | Yuncheng District, Ya’an City, Sichuan Province | 103.04° | 30.01° | March | 21.50 ± 0.36 |
| lotus pollen | Dingbian County, Yulin City, Shaanxi Province | 107.60° | 37.59° | August | 18.03 ± 0.42 |
| buckwheat pollen | Guangchang County, Fuzhou City, Jiangxi Province | 116.34° | 26.84° | October | 13.43 ± 0.31 |
| Pollen Substitutes | Ingredients | Protein Content (g/100 g) |
|---|---|---|
| Diet 1 | vegetable germ, vegetable proteins, yeast powder, lysine, methionine, etc. | 23.45 ± 0.01 |
| Diet 2 | vegetable proteins, carrot powder, yeast powder, lactic acid bacteria, amino acid, mineral, trace element, multivitamins, etc. | 38.16 ± 0.02 |
| Diet | Total Diet Consumed (g) | Number of Workers |
|---|---|---|
| oilseed rape pollen | 0.257 ± 0.096 ab | 3.3 ± 1.5 c |
| camellia pollen | 0.228 ± 0.034 ab | 2.8 ± 0.3 c |
| louts pollen | 0.117 ± 0.008 b | 0.5 ± 0.9 c |
| buckwheat pollen | 0.357 ± 0.092 a | 13.0 ± 3.4 a |
| Diet 1 | 0.135 ± 0.002 b | 7.8 ± 1.8 b |
| Diet 2 | 0.210 ± 0.018 ab | 0.8 ± 0.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Liang, C.; Zhang, Y.; Huang, J.; Ding, G. Influence of Different Diets on Growth and Development of Eastern Honey Bee (Apis cerana). Insects 2025, 16, 383. https://doi.org/10.3390/insects16040383
Liang R, Liang C, Zhang Y, Huang J, Ding G. Influence of Different Diets on Growth and Development of Eastern Honey Bee (Apis cerana). Insects. 2025; 16(4):383. https://doi.org/10.3390/insects16040383
Chicago/Turabian StyleLiang, Ruonan, Cheng Liang, Yi Zhang, Jiaxing Huang, and Guiling Ding. 2025. "Influence of Different Diets on Growth and Development of Eastern Honey Bee (Apis cerana)" Insects 16, no. 4: 383. https://doi.org/10.3390/insects16040383
APA StyleLiang, R., Liang, C., Zhang, Y., Huang, J., & Ding, G. (2025). Influence of Different Diets on Growth and Development of Eastern Honey Bee (Apis cerana). Insects, 16(4), 383. https://doi.org/10.3390/insects16040383






