Adaptability of Yuanjiang River Valley Danaus genutia to Different Host Plants in Yunan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Establishment of Near-Natural Experimental Population
2.3. Oviposition Preference of Adults on Different Plants
2.4. Larval Feeding Preference on Different Plants
2.5. Establishment of D. genutia Age-Stage, Two-Sex Life Table
2.6. Data Analysis
3. Results
3.1. Oviposition Preference of D. genutia Adults on Different Plants
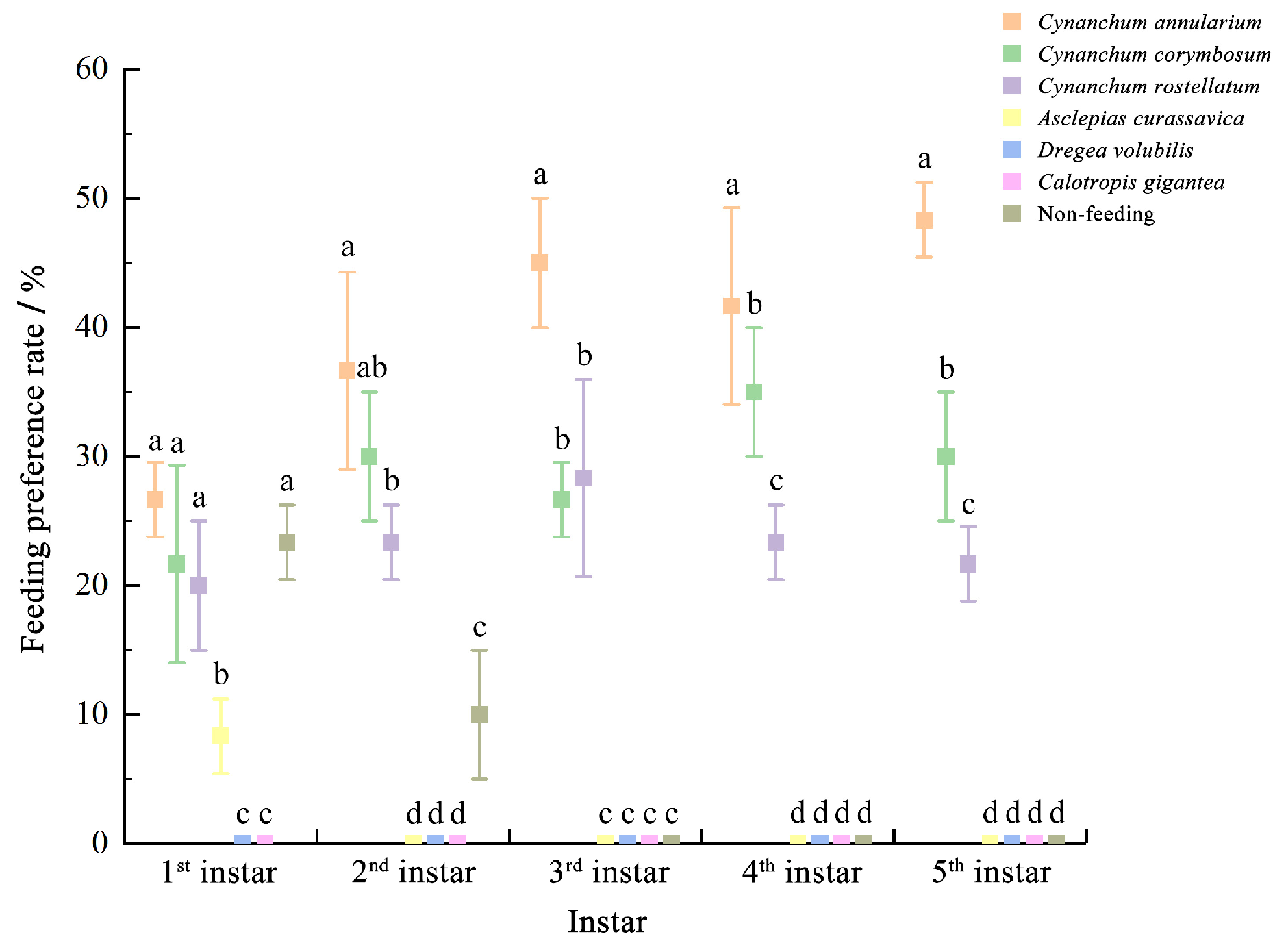
3.2. Feeding Preference of D. genutia Larvae on Different Plants
3.3. Life Table Parameters of D. genutia on Two Host Plants
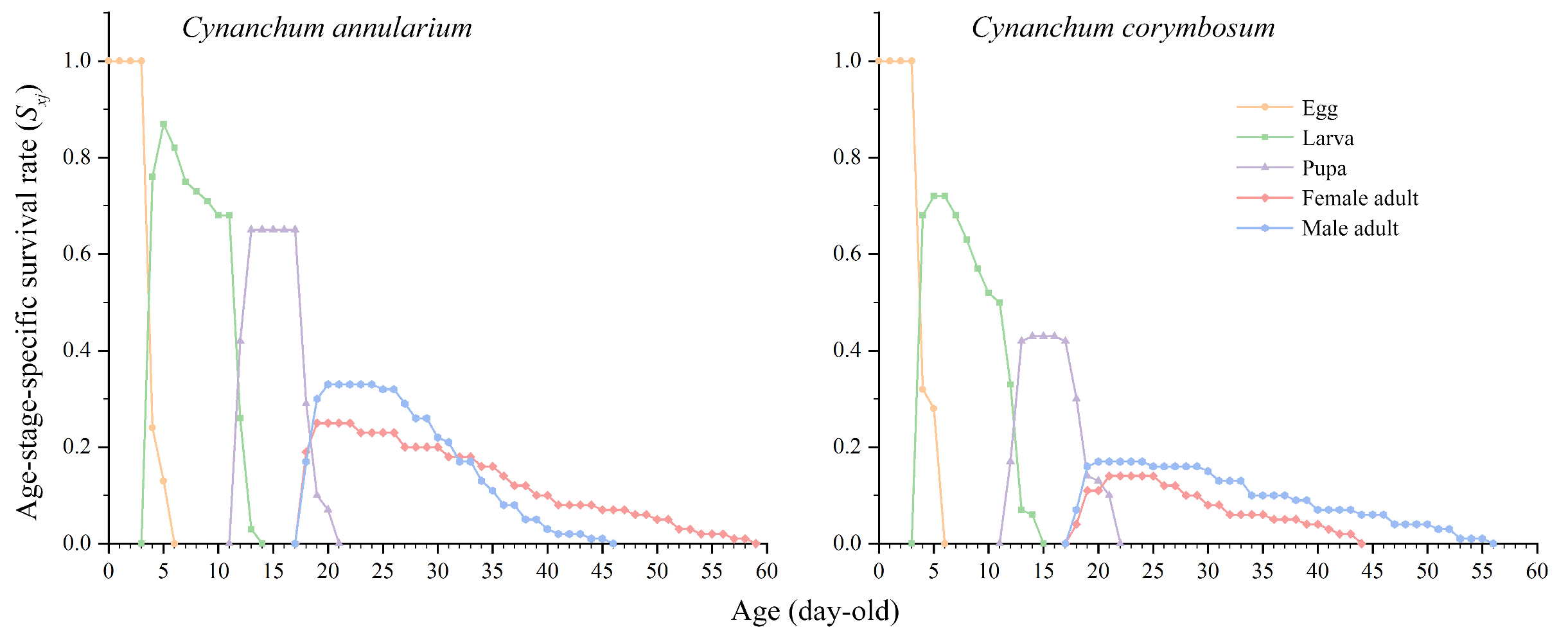
3.3.1. Growth and Development Stages and Population Parameters of D. genutia
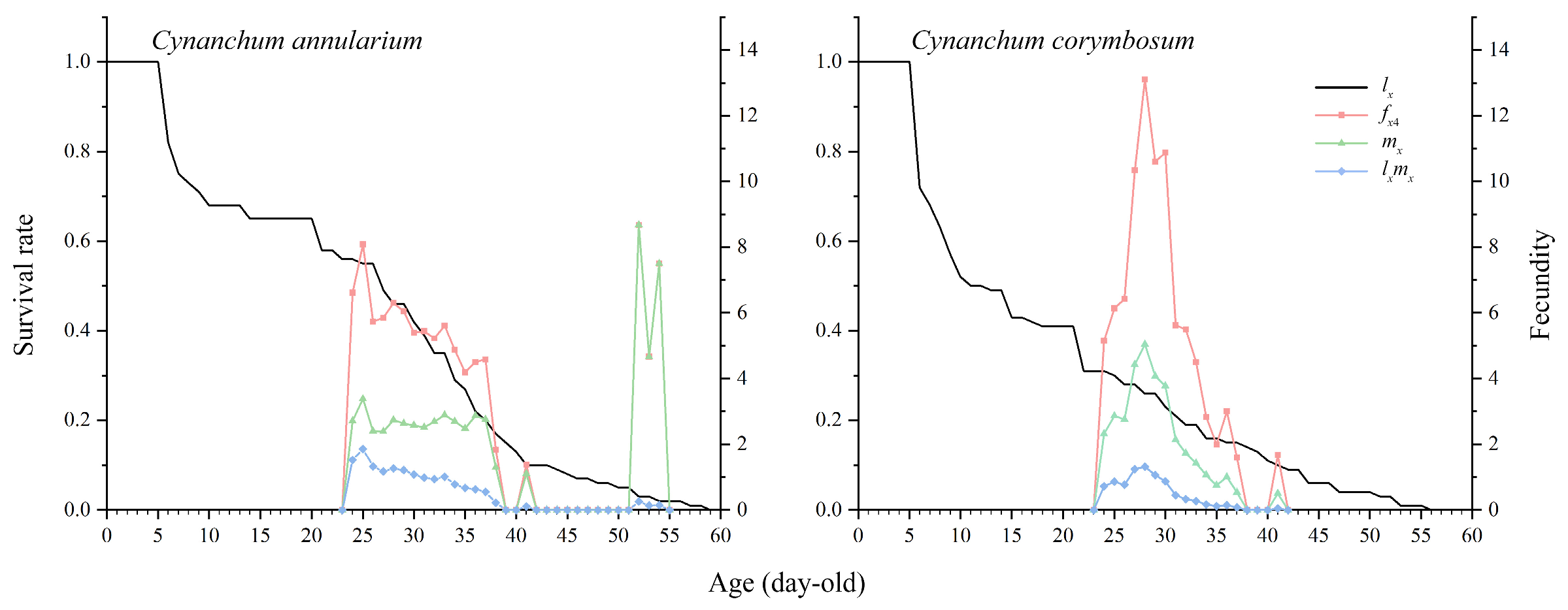
3.3.2. Survival Rate and Fecundity of D. genutia
3.3.3. Expected Lifespan of D. genutia
3.3.4. Reproductive Value of D. genutia
3.3.5. Life Table Parameters of D. genutia
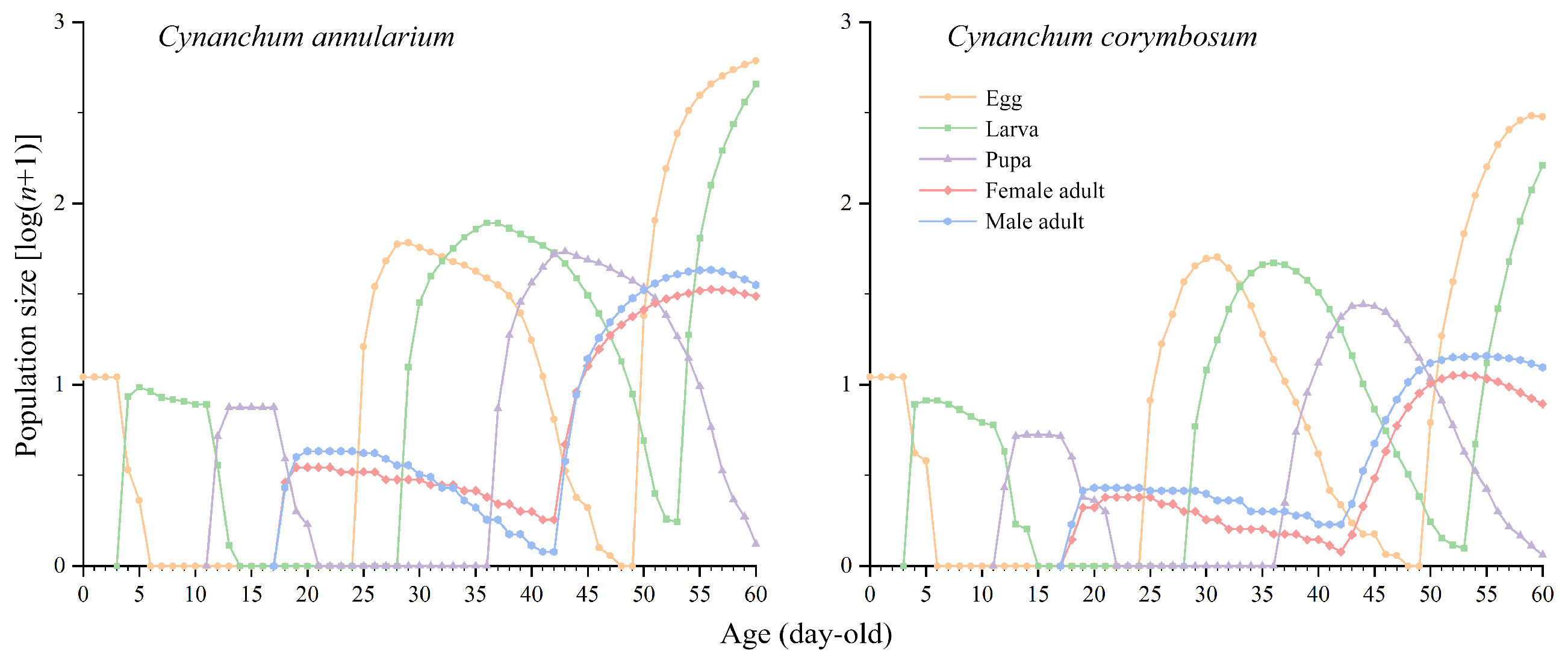
3.3.6. Population Prediction of D. genutia
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y. Danaidae. Monograph of Chinese Butterflies (Revised Edition), 2nd ed.; Henan Scientific and Technological Publishing House: Zhengzhou, China, 2000; pp. 270–271. [Google Scholar]
- Chen, J.; Ou, X.H.; Liu, B.; Zhao, Y.S.; Guo, C.C.; Liu, Q.; Li, S.Q.; Hu, S.H. Insect resource and diversity of Yuanjiang Nature Reserve in the Dry-hot valley of Yunnan. In Proceedings of the Seventh National Symposium on the Conservation and Sustainable Use of Biodiversity in China, Changchun, China, 3–5 August 2006; pp. 337–346. [Google Scholar]
- Igarashi, S.; Fukuda, H. The Life Histories of Asian Butterflies; Tokai University Press: Tokyo, Japan, 2000; Volume 2, pp. 413–414. [Google Scholar]
- Chen, X.M.; Zhou, C.L.; Shi, J.Y.; Shi, L.; Yi, C.H. Ornamental Butterflies in China; China Forestry Publishing House: Beijing, China, 2008; pp. 43, 209–210. [Google Scholar]
- Li, C.L. Yunnan Butterfly; China Forestry Publishing House: Beijing, China, 1995; p. 77. [Google Scholar]
- Shi, J.Y.; Wang, M.X.; Yao, J.; Pu, Z.Y.; Shi, R.H.; Lei, P. Design, Construction and Management of Butterfly Garden, 1st ed.; Science Press: Beijing, China, 2013; p. 492. [Google Scholar]
- Atluri, J.B.; Rayalu, M.B.; Deepika, D.S. Life history and larval performance of the common tiger butterfly, Danaus genutia Cramer (Lepidoptera; Rhopalocera; Danaidae). Bioscan 2010, 5, 511–515. [Google Scholar]
- Meyer, C.E. Notes on the life history of Danaus genutia alexis (Waterhouse and Lyell) (Lepidoptera: Nymphalidae). Aust. Entomol. 1995, 22, 137–139. [Google Scholar]
- Chen, Z.; Cao, Y.; Zhou, Y.Q.; Zhou, C.L.; Chen, X.M.; Shi, L. Biological characteristics of an experimental population of the common tiger butterfly, Danaus genutia (Lepidoptera: Nymphalidae). Chin. J. Appl. Entomol. 2017, 54, 279–291. [Google Scholar]
- Pearl, R.; Parker, S.L. Experimental studies on the duration of life. I. Introductory discussion of the duration of life in Drosophila. Am. Nat. 1921, 55, 481–509. [Google Scholar] [CrossRef]
- Morris, R.F.; Miller, C.A. The development of life tables for the spruce budworm. Can. J. Zool. 1954, 32, 283–301. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett)(Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlıha, R.; Smith, C.L.; Kavousi, A.; Salih, M.; Özgökçe, A.G.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, Two-Sex Life Table: An Introduction to Theory, Data Analysis, and Application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Chi, H.; Fu, J.W.; You, M.S. Age-stage, two-sex life table and its application in population ecology and integrated pest management. Acta Entomol. Sin. 2019, 62, 255–262. [Google Scholar]
- Ma, M.; Ren, F.X.; Wang, Y.; Kong, W.N.; Wei, M.F.; Chi, H.; Özgökçe, M.S.; Ma, R.Y. Group-reared life table: Rationales of data analysis and methods of converting to individually-reared life table. Acta Entomol. Sin. 2024, 67, 1147–1162. [Google Scholar]
- Cui, J.; Yin, J.X.; Tian, X.Y.; Gao, Y.; Shi, S.S.; Li, W.B. Age–Stage, Two-Sex Life Table Analysis of Riptortus pedestris (Hemiptera: Alydidae) Across Different Soybean Varieties. Insects 2024, 15, 952. [Google Scholar] [CrossRef]
- Li, M.N.; Peng, Z.K.; Guo, C.S.; Xiao, Y.; Yin, F.; Yuan, H.B.; Li, Z.Y.; Zalucki, M.P. Age-Stage, Two-Sex Life Tables of Megalurothrips usitatus (Bagnall) and Frankliniella intonsa (Trybom) on Different Bean Pods Under Laboratory Conditions: Implications for Their Competitive Interactions. Insects 2024, 15, 1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Adaptive mechanisms of Locusta migratoria to Four Different Plants. Ph.D. Dissertation, Shandong Agricultural University, Taian, China, 2023. [Google Scholar]
- Li, X.S.; Zhang, Y.L.; Luo, Y.Q.; Settele, J. Studies on life history, life table, habitat and conservation of Byasa impediens (Lepidoptera: Papilionidae). Acta Ecol. Sin. 2006, 26, 3184–3197. [Google Scholar]
- Chen, R.L.; Cai, W.J.; Yang, H.; Gu, M.B. The study on life table of Cethosia cyane cyane (Drury) experimental population. J. Environ. Entomol. 2015, 37, 44–50. [Google Scholar]
- Liao, H.J.; Liu, W.F.; Du, T.; Shi, L.; Zhou, C.L.; Deng, J. Effects of host plants on oviposition selectivity and population fitness of Euploea core Cramer. Biot. Resour. 2017, 39, 345–351. [Google Scholar]
- Jiang, T.; Fan, C.L.; Wang, T.T.; Wang, X.Y.; Xu, D.Y.; Jiang, Z.E.; Yang, Z.Q.; Tan, D.J.; Lu, Y.C. Feeding and oviposition preferences of Diaphania caesalis Walker on different plants. Plant Prot. 2024, 50, 211–218. [Google Scholar]
- Jermy, T.; Hanson, F.E.; Dethier, V.G. Induction of specific food preferences in lepidopterous larvae. Entomol. Exp. Appl. 1968, 11, 211–230. [Google Scholar]
- Tang, Q.B.; Wang, C.Z. Leaf disc test used in caterpillar feeding preference study. Chin. Bull. Entomol. 2007, 4, 912–915. [Google Scholar]
- Li, X.H.; Wang, D.J.; Lei, Z.R.; Wang, H.H. Comparison of Life Tables for Experimental Populations of Individual-Rearing and Group-Rearing Frankliniella occidentalis. Sci. Agric. Sin. 2021, 54, 959–968. [Google Scholar]
- Wang, W.Q.; Zheng, Y.Q.; Chen, B.; Phangthavong, S.; Xiao, G.L. Effects of different host plants on the growth, development and fecundity of potato tuber moth Phthorimaea operculella based on the age-stage two-sex life table. J. Plant Prot. 2020, 47, 488–496. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Ntomology 1988, 17, 26–34. [Google Scholar]
- Chang, C.; Huang, C.Y.; Dai, S.M.; Atlihan, R.; Chi, H. Genetically engineered ricin suppresses Bactrocera dorsalis (Diptera: Tephritidae) based on demographic analysis of group-reared life table. J. Econ. Entomol. 2016, 109, 987–992. [Google Scholar] [PubMed]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2024. Available online: https://www.faas.cn/cms/pages/810640950322870004/attachments/Twosex-MSChart.rar (accessed on 14 August 2024).
- Li, J.Y.; Chen, Y.T.; Fu, J.W.; Shi, M.Z.; Chi, H.; You, M.S. Application of the bootstrap technique and the multinomial theorem in the research of age-stage, two-sex life table. Acta Entomol. Sin. 2022, 65, 1389–1400. [Google Scholar]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. 2024. Available online: https://www.faas.cn/cms/pages/810640950555190004/attachments/TIMING-MSChart.rar (accessed on 14 August 2024).
- Tang, Y.C.; Zhou, C.L.; Chen, X.M. Progress in the Oviposition Behavioral Ecology of Herbivorous Insects. For. Res. 2010, 23, 770–777. [Google Scholar]
- Lian, W.; Ma, Y.H.; Chen, L.H.; Wei, H.Y. Research Progress in the Influence of Host Plants on the Selection Behaviors of Herbivorous Insects. Biol. Disaster Sci. 2022, 45, 299–304. [Google Scholar]
- Lu, Y.H.; Zhang, Y.J.; Wu, K.M. Host plant selection mechanisms and behavioural manipulation strategies of phytophagous insects. Acta Ecol. Sin. 2008, 28, 5113–5122. [Google Scholar]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar]
- Menacer, K.; Cortesero, A.M.; Hervé, M.R. Challenging the preference–performance hypothesis in an above-belowground insect. Oecologia 2021, 197, 179–187. [Google Scholar]
- Janz, N. The relationship between habitat selection and preference for adult and larval food resources in the polyphagous butterfly Vanessa cardui (Lepidoptera: Nymphalidae). J. Insect Behav. 2005, 18, 767–780. [Google Scholar] [CrossRef]
- Boggs, C.L.; Ross, C.L. The effect of adult food limitation on life-history traits in Speyeria mormonia (Lepidoptera, Nymphalidae). Ecology 1993, 74, 433–441. [Google Scholar]
- Scheirs, J.; Bruyn, L.D.; Verhagen, R. Optimization of adult performance determines host choice in a grass miner. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2000, 267, 2065–2069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Ji, L.Z. Chemical ecology of oviposition of herbivorous insects. Chin. J. Ecol. 1994, 13, 39–43. [Google Scholar]
- Renwick, J.A.A.; Chew, F.S. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 1994, 39, 377–400. [Google Scholar] [CrossRef]
- Rausher, M.D. Larval habitat suitability and oviposition preference in three related butterflies. Ecology 1979, 60, 503–511. [Google Scholar] [CrossRef]
- Sadek, M.M. Complementary behaviors of maternal and offspring Spodoptera littoralis: Oviposition site selection and larval movement together maximize performance. J. Insect Behav. 2011, 24, 67–82. [Google Scholar] [CrossRef]
- Janz, N.; Nylin, S. Butterflies and plants: A phylogenetic study. Evolution 1998, 52, 486–502. [Google Scholar] [CrossRef]
- Jiang, M.N. Larval Diet Selection of the Rare Butterfly of Teinopalpus aureus and Genetic Diversity of Two Magnoliaceae Hostplants. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2022. [Google Scholar]
- Shikano, I.; Akhtar, Y.; Isman, M.B. Relationship between adult and larval host plant selection and larval performance in the generalist moth, Trichoplusia ni. Arthropod-Plant Interact. 2010, 4, 197–205. [Google Scholar] [CrossRef]
- Hu, J.J.; Yuan, G.H.; Li, Y.Y.; Guo, X.R.; Wang, Q.; Guo, S.S.; Li, W.Z. Associative learning in early-instar larvae of Helicoverpa armigera in response to several floral volatiles. Acta Ecol. Sin. 2016, 36, 4204–4210. [Google Scholar]
- Chen, Z.; Zhou, C.L.; Chen, X.M.; Shi, L. Effects of Temperature and Photoperiod on the Development of Immature Stages of Danaus genutia. Sichuan J. Zool. 2018, 37, 420–425. [Google Scholar]
- Watanabe, M.; Sato, K. A spermatophore structured in the bursa copulatrix of the small white Pieris rapae (Lepidoptera, Pieridae) during copulation, and its sugar content. J. Res. Lepid. 1993, 32, 26–36. [Google Scholar] [CrossRef]
- Boggs, C.L. Reproductive strategies of female butterflies: Variation in and constraints on fecundity. Ecol. Entomol. 1986, 11, 7–15. [Google Scholar]
- Kong, H.L.; Liang, X.C.; Luo, Y.Z. Effects of Supplementary Nutriments on the Egg Maturation and the Longevity of the Male Swallowtail Butterfly, Papilio xuthus Linnaeus (Lepidoptera: Papilionidae). J. Yunnan Agric. Univ. 2008, 23, 179–183. [Google Scholar]
- Yang, X.J.; Li, S.; Li, W.Z.; Sheng, Z.Y.; Gao, C.N.; Zhang, S.H.; Yuan, G.H. Effect of different nectar mimics on reproduction of Helicoverpa armigera moths. In Proceedings of the 2020 Central China Insect Academic Symposium, Nanchang, China, 1 October 2020; pp. 295–304. [Google Scholar]
- Gratton, C.; Welter, S.C. Oviposition preference and larval performance of Liriomyza helianthi (Diptera: Agromyzidae) on normal and novel host plants. Environ. Entomol. 1998, 27, 926–935. [Google Scholar]
- Juárez, M.L.; Schöfl, G.; Vera, M.T.; Vilardi, J.C.; Murúa, M.G.; Willink, E.; Hänniger, S.; Heckel, D.G.; Groot, A.T. Population structure of Spodoptera frugiperda maize and rice host forms in S outh A merica: Are they host strains? Entomol. Exp. Appl. 2014, 152, 182–199. [Google Scholar]
- Li, H.; Qin, Y.; Yuan, X.M. Investigation on the traditional production techniques of Yangnai pickled vegetables in Jianshui County. Rural. Econ. Sci. Technol. 2018, 29, 105–106. [Google Scholar]
- Li, Y.Y.; Li, H.L.; Li, J.Y.; Bai, Y.Y.; Yan, G.Y.; Zhang, M.; Li, T.; You, J.P. Study on Pharmacognosy of Yao Medicine Cynanchum Corymbosum Wight. Asia-Pac. Tradit. Med. 2024, 20, 56–60. [Google Scholar]
- Gibbs, M.; Lace, L.A.; Jones, M.J.; Moore, A.J. Intraspecific competition in the speckled wood butterfly Pararge aegeria: Effect of rearing density and gender on larval life history. J. Insect Sci. 2004, 4, 16. [Google Scholar]
- Martin, L.A.; Pullin, A.S. Host-plant specialisation and habitat restriction in an endangered insect, Lycaena dispar batavus (Lepidoptera: Lycaenidae) I. Larval feeding and oviposition preferences. Eur. J. Entomol. 2004, 101, 51–56. [Google Scholar]




| Plant | Eggs Received per Day | Percentage of Eggs on the Plant |
|---|---|---|
| Cynanchum annularium | 97.56 ± 8.70 a | 31.48 ± 3.21 a |
| Cynanchum corymbosum | 105.78 ± 7.65 a | 34.09 ± 2.01 a |
| Cynanchum rostellatum | 57.78 ± 6.52 b | 18.62 ± 2.00 b |
| Asclepias curassavica | 35.33 ± 2.40 c | 11.40 ± 0.87 c |
| Dregea volubilis | 3.55 ± 1.57 d | 1.14 ± 0.50 d |
| Calotropis gigantea | 10.11 ± 4.02 d | 3.26 ± 1.26 d |
| Parameters | Host Plant | ||||
|---|---|---|---|---|---|
| n | Cynanchum annularium | n | Cynanchum corymbosum | p | |
| Egg period (d) | 87 | 4.13 ± 0.04 a | 72 | 4.06 ± 0.03 a | 0.11 |
| Larval period (d) | 65 | 8.18 ± 0.05 a | 44 | 8.64 ± 0.08 b | 0.00 * |
| Pupal period (d) | 58 | 6.05 ± 0.07 a | 31 | 5.97 ± 0.14 a | 0.60 |
| Pre-adult period (d) | 58 | 18.43 ± 0.08 a | 31 | 18.87 ± 0.16 b | 0.01 * |
| Pre-adult survival rate | 100 | 0.58 ± 0.05 a | 100 | 0.31 ± 0.05 b | 0.01 * |
| Adult longevity (d) | 58 | 17.57 ± 1.05 a | 31 | 18.74 ± 1.48 a | 0.52 |
| Female adult longevity (d) | 25 | 20.96 ± 2.03 a | 14 | 15.00 ± 1.53 b | 0.02 * |
| Male adult longevity (d) | 33 | 15.00 ± 0.79 a | 17 | 21.82 ± 2.14 b | 0.00 * |
| Sex ratio (Nf/Nm) | 100 | 0.76 ± 0.21 a | 100 | 0.82 ± 0.35 a | 0.79 |
| Number of eggs per female | 25 | 63.44 ± 5.95 a | 14 | 60.35 ± 7.99 a | 0.76 |
| Parameter | Host Plant | ||||
|---|---|---|---|---|---|
| n | Cynanchum annularium | n | Cynanchum corymbosum | p | |
| Gross reproductive rate (GRR) | 100 | 60.92 ± 8.24 a | 100 | 34.41 ± 8.45 b | 0.04 * |
| Intrinsic rate of increase (r)/d−1 | 100 | 0.092 ± 0.007 a | 100 | 0.074 ± 0.010 a | 0.11 |
| Finite rate of increase (λ)/d−1 | 100 | 1.096 ± 0.007 a | 100 | 1.076 ± 0.011 a | 0.11 |
| Net reproduction rate (R0) | 100 | 15.86 ± 3.12 a | 100 | 8.45 ± 2.36 a | 0.06 |
| Mean generation time (T)/d | 100 | 30.10 ± 0.30 a | 100 | 29.03 ± 0.28 b | 0.01 * |
| Doubling time (Td)/d | 100 | 7.54 ± 0.59 a | 100 | 9.43 ± 2.08 a | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Du, T.; Li, Y.; Zhou, C.; Shi, L. Adaptability of Yuanjiang River Valley Danaus genutia to Different Host Plants in Yunan. Insects 2025, 16, 368. https://doi.org/10.3390/insects16040368
Yao J, Du T, Li Y, Zhou C, Shi L. Adaptability of Yuanjiang River Valley Danaus genutia to Different Host Plants in Yunan. Insects. 2025; 16(4):368. https://doi.org/10.3390/insects16040368
Chicago/Turabian StyleYao, Jun, Ting Du, Yangyang Li, Chengli Zhou, and Lei Shi. 2025. "Adaptability of Yuanjiang River Valley Danaus genutia to Different Host Plants in Yunan" Insects 16, no. 4: 368. https://doi.org/10.3390/insects16040368
APA StyleYao, J., Du, T., Li, Y., Zhou, C., & Shi, L. (2025). Adaptability of Yuanjiang River Valley Danaus genutia to Different Host Plants in Yunan. Insects, 16(4), 368. https://doi.org/10.3390/insects16040368






