Influence of Temperature, Humidity, and Photophase on the Developmental Stages of Spodoptera litura (Lepidoptera: Noctuidae) and Prediction of Its Population Dynamics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
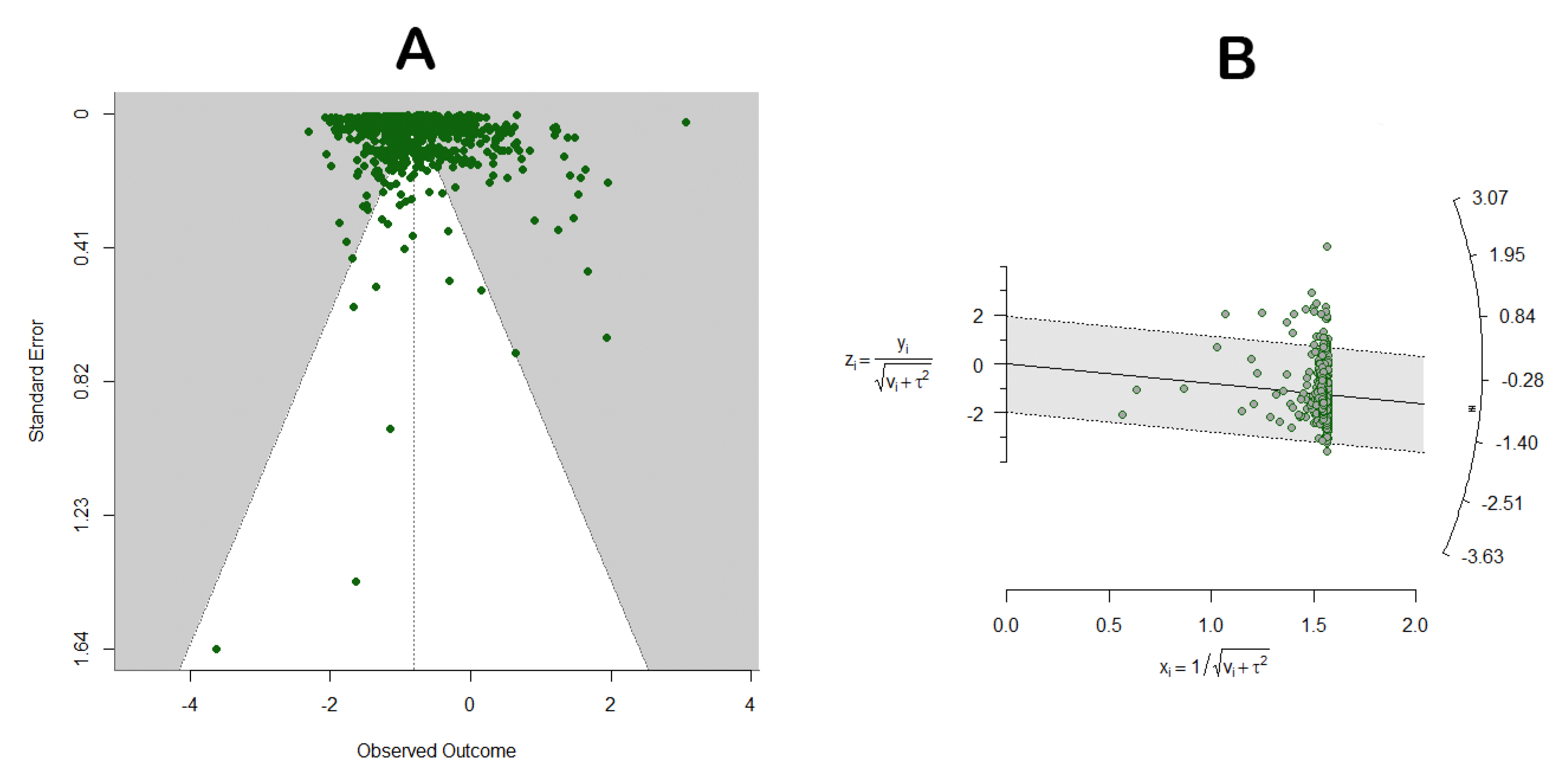
3.1. Literature Search and Screening Results
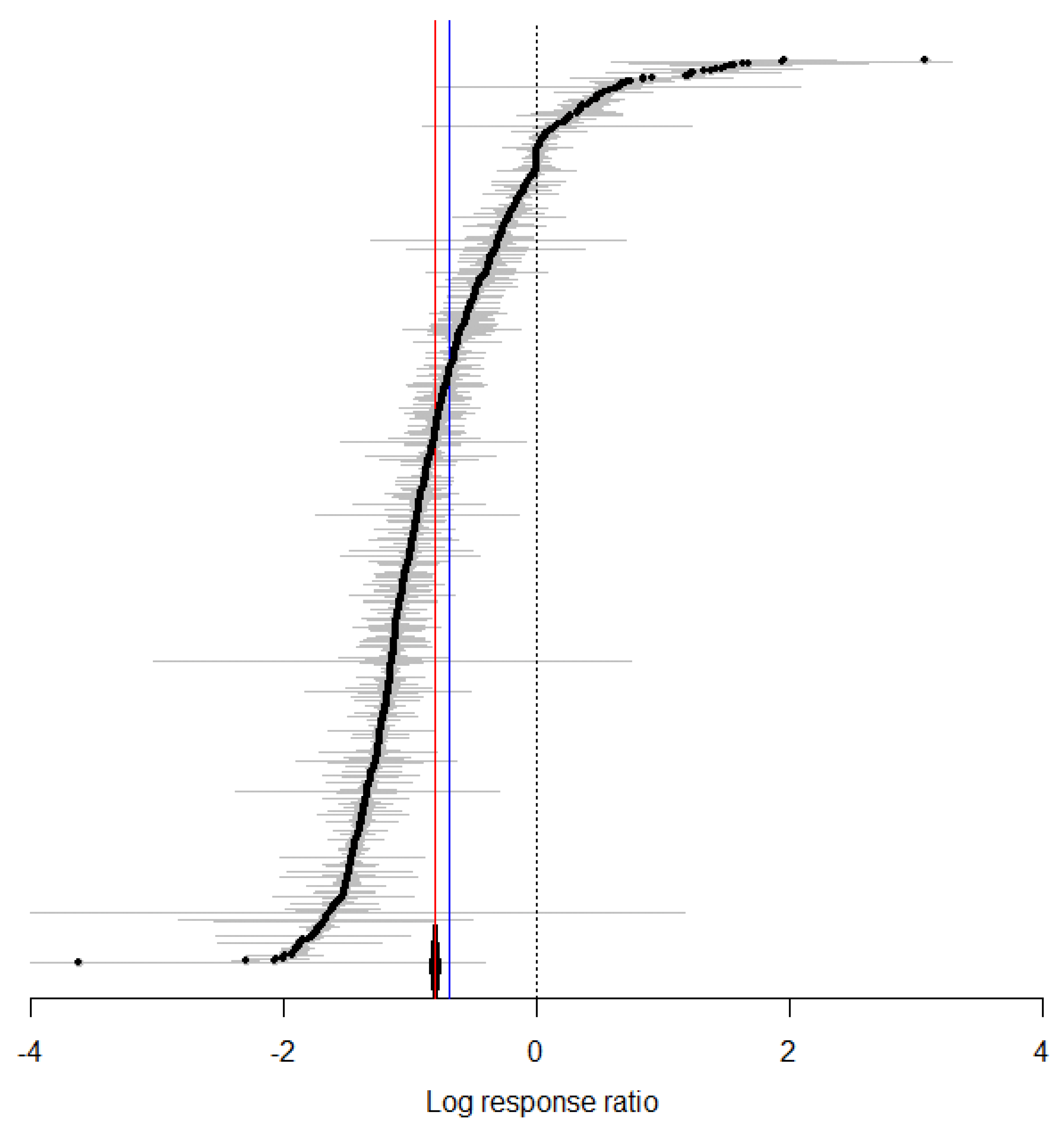
3.2. Overall Effect of Temperature on the Biological Traits of S. litura
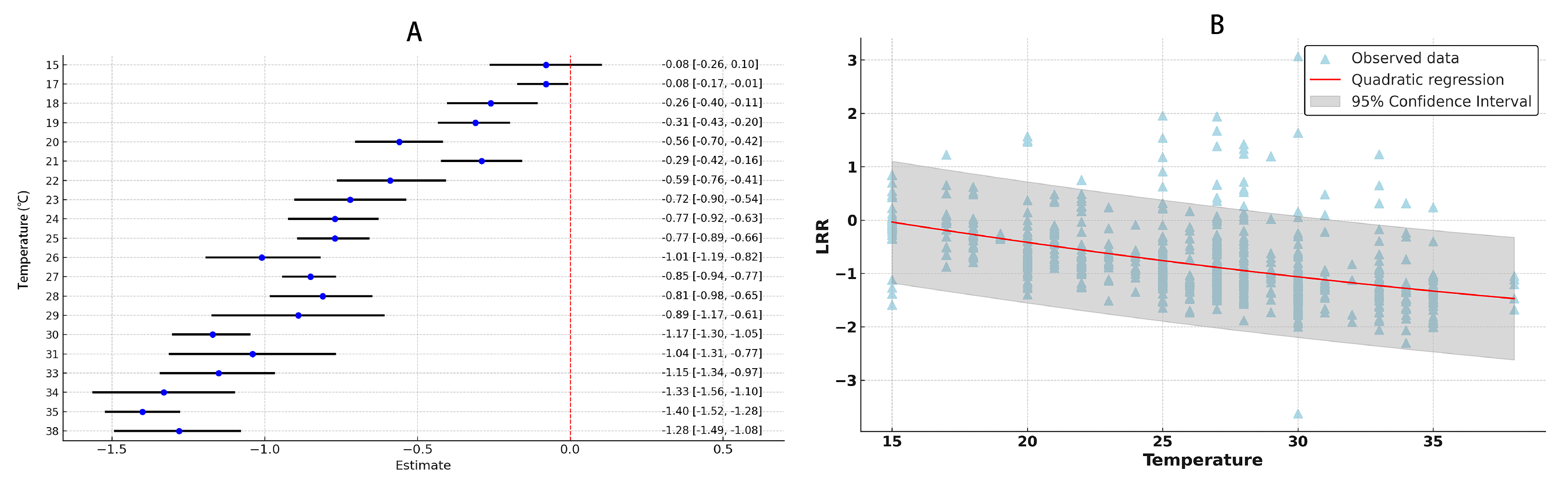
3.3. The Impact of Temperature on Development Period
3.4. Temperature Effects on Developmental Duration at Different Larval Stages
3.5. The Effect of Temperature Changes on the Lifespan of Adult S. litura
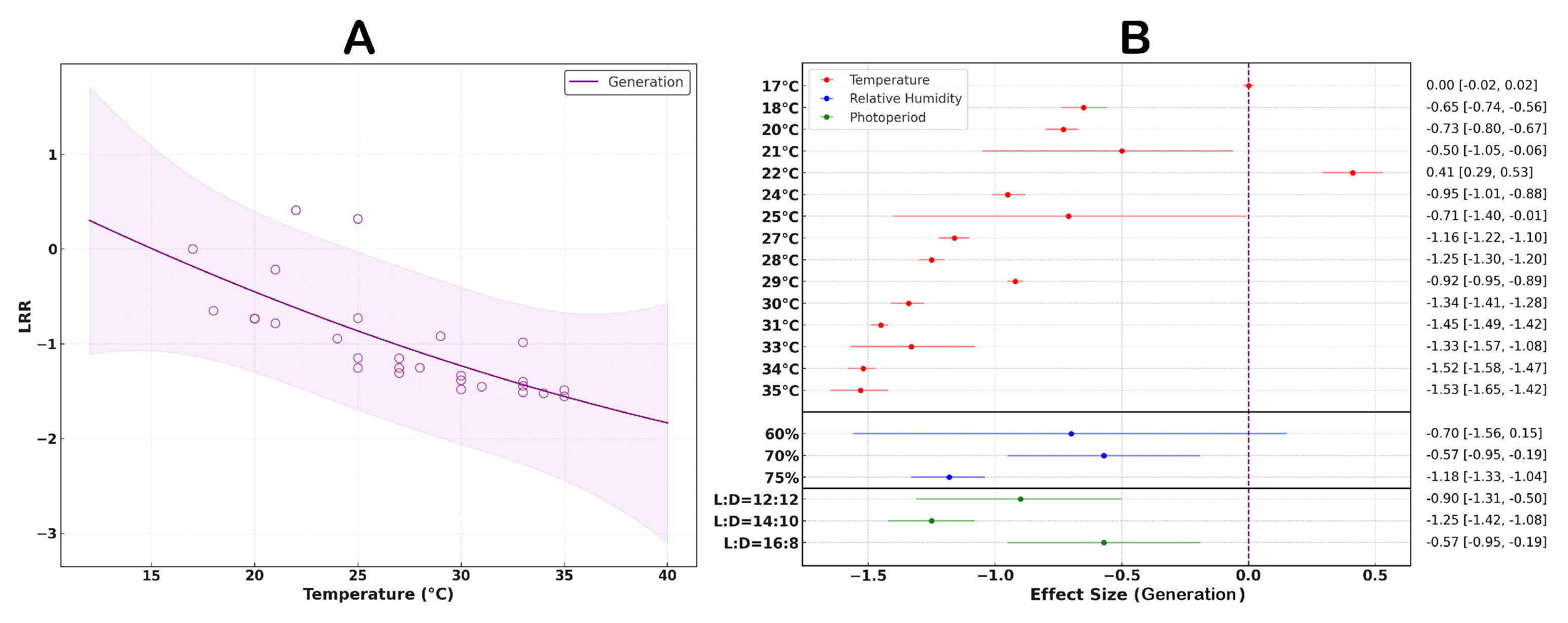
3.6. The Effect of Temperature on the Generation Cycle of S. litura
3.7. Model Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haq, M.; Mia, M.T.; Rabbi, M.F.; Ali, M.A. Incidence and severity of rice diseases and insect pests in relation to climate change. In Climate Change and Food Security in South Asia; Springer: Berlin/Heidelberg, Germany, 2011; pp. 445–457. [Google Scholar]
- Hakala, K.; Hannukkala, A.; Huusela-Veistola, E.; Jalli, M.; Peltonen-Sainio, P. Pests and diseases in a changing climate a major challenge for Finnish crop production. Agric. Food Sci. 2011, 20, 3–14. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Poudel, A.; Aryal, S. The impact of climate change on insect pest biology and ecology: Implications for pest management strategies, crop production, and food security. J. Agric. Food Res. 2023, 14, 100733. [Google Scholar]
- Sharma, H.C. Climate change effects on insects: Implications for crop protection and food security. J. Crop Improv. 2014, 28, 229–259. [Google Scholar] [CrossRef]
- Zakria, M.; Zaib, M.S.; Abbas, K.; Sarmad, M.; Zaka, S.M.; Noor-ul-Ane, M. Influence of temperature on the development and reproduction of Spodoptera litura (Fabricius) on castor bean: Implications for its use as a trap crop. Arthropod-Plant Interact. 2022, 16, 505–515. [Google Scholar]
- Ahmad, M.; Arif, M.I.; Ahmad, M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007, 26, 809–817. [Google Scholar]
- Ahmad, M.; Ghaffar, A.; Rafiq, M. Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J. Agric. Biol. 2013, 1, 23–28. [Google Scholar]
- Lu, Y.; Yuan, M.; Gao, X.; Kang, T.; Zhan, S.; Wan, H.; Li, J. Identification and validation of reference genes for gene expression analysis using quantitative PCR in Spodoptera litura (Lepidoptera: Noctuidae). PLoS ONE 2013, 8, e68059. [Google Scholar]
- Yoon, S.; Lee, W. Methodological analysis of bioclimatic variable selection in species distribution modeling with application to agricultural pests (Metcalfa pruinosa and Spodoptera litura). Comput. Electron. Agric. 2021, 190, 106430. [Google Scholar]
- Maharjan, R.; Hong, S.; Ahn, J.; Yoon, Y.; Jang, Y.; Kim, J.; Lee, M.; Park, K.; Yi, H. Temperature and host plant impacts on the development of Spodoptera litura (Fabricius)(Lepidoptera: Noctuidae): Linear and nonlinear modeling. Insects 2023, 14, 412. [Google Scholar] [CrossRef]
- Miyashita, K. Effects of constant and alternating temperatures on the development of Spodoptera litura F.: Lepidoptera: Noctuidae. Appl. Entomol. Zool. 1971, 6, 105–111. [Google Scholar] [CrossRef]
- Rao, M.S.; Prasad, T.V. Temperature based phenology model for predicting establishment and survival of Spodoptera litura (Fab.) on groundnut during climate change scenario in India. J. Agrometeorol. 2020, 22, 24–32. [Google Scholar]
- Zhong, T.; Gong, L.; Pan, Y.; Li, J.; Lu, A.; Liu, L.; Wu, H.; Zhao, Z.; Wang, L. Performance of Spodoptera litura (Lepidoptera: Noctuidae) in responses to different amplitudes of alternating temperatures across permissive warm temperature regimes. J. Econ. Entomol. 2024, 117, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Lu, Z.; Chen, L.; Yu, W.; Zhang, S. Effect of temperature and food on Spodoptera litura population. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2000, 11, 111–114. [Google Scholar]
- Pham, T.A.; Hwang, S. High temperatures reduce nutrients and defense compounds against generalist Spodoptera litura F. in Rorippa dubia. Arthropod-Plant Interact. 2020, 14, 333–344. [Google Scholar] [CrossRef]
- Karmakar, P.; Pal, S. Influence of temperature on food consumption and utilization parameters of the common cutworm, Spodoptera litura (Fab)(Lepidoptera: Noctuidae). J. Entomol. Zool. Stud. 2017, 5, 92–95. [Google Scholar]
- Harvey, J.A.; Tougeron, K.; Gols, R.; Heinen, R.; Abarca, M.; Abram, P.K.; Basset, Y.; Berg, M.; Boggs, C.; Brodeur, J. Scientists’ warning on climate change and insects. Ecol. Monogr. 2023, 93, e1553. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Cooper, C.; Booth, A.; Varley-Campbell, J.; Britten, N.; Garside, R. Defining the process to literature searching in systematic reviews: A literature review of guidance and supporting studies. Bmc Med. Res. Methodol. 2018, 18, 85. [Google Scholar] [CrossRef]
- Dinet, J.; Favart, M.; Passerault, J.M. Searching for information in an online public access catalogue (OPAC): The impacts of information search expertise on the use of Boolean operators. J. Comput. Assist. Learn. 2004, 20, 338–346. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [PubMed]
- Corbeil, R.R.; Searle, S.R. Restricted maximum likelihood (REML) estimation of variance components in the mixed model. Technometrics 1976, 18, 31–38. [Google Scholar] [CrossRef]
- Kosimov, Z.O. Methods for estimating integrated variance: Jump robustness issues in high frequency time series. Econ. Math. Methods 2024, 60, 107–117. [Google Scholar] [CrossRef]
- Garamszegi, L.Z.; Markó, G.; Herczeg, G. A meta-analysis of correlated behaviours with implications for behavioural syndromes: Mean effect size, publication bias, phylogenetic effects and the role of mediator variables. Evol. Ecol. 2012, 26, 1213–1235. [Google Scholar]
- Clark, T.S.; Linzer, D.A. Should I use fixed or random effects? Political Sci. Res. Methods 2015, 3, 399–408. [Google Scholar]
- Lajeunesse, M.J. Bias and correction for the log response ratio in ecological meta-analysis. Ecology 2015, 96, 2056–2063. [Google Scholar] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar]
- Thornton, A.; Lee, P. Publication bias in meta-analysis: Its causes and consequences. J. Clin. Epidemiol. 2000, 53, 207–216. [Google Scholar]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar]
- Rao, M.S.; Manimanjari, D.; Rao, A.C.R.; Swathi, P.; Maheswari, M. Effect of climate change on Spodoptera litura Fab. on peanut: A life table approach. Crop Prot. 2014, 66, 98–106. [Google Scholar]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar]
- Régnière, J.; Powell, J.; Bentz, B.; Nealis, V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J. Insect Physiol. 2012, 58, 634–647. [Google Scholar]
- Jung, J.M.; Byeon, D.H.; Jung, S.; Lee, W.H. Effect of climate change on the potential distribution of the common cutworm (Spodoptera litura) in South Korea. Entomol. Res. 2019, 49, 519–528. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, X.; Xie, B.; Ali, A.; Wu, K. Seasonal pattern of Spodoptera litura (Lepidoptera: Noctuidae) migration across the Bohai Strait in northern China. J. Econ. Entomol. 2015, 108, 525–538. [Google Scholar] [CrossRef]
- Sahu, B.; Pachori, R.; Navya, R.N.; Patidar, S. Extent of damage by Spodoptera litura on cabbage. J. Entomol. Zool. Stud. 2020, 8, 1153–1156. [Google Scholar]
- Sangle, P.M.; Satpute, S.B.; Khan, F.S.; Rode, N.S. Impact of climate change on insects. Trends Biosci. 2015, 8, 3579–3582. [Google Scholar]
- Jaworski, T.; Hilszczanski, J. The effect of temperature and humidity changes on insects development their impact on forest ecosystems in the expected climate change. Lesn. Pr. Badaw. 2013, 74, 345. [Google Scholar] [CrossRef]




| TR | CT | n | Variable |
|---|---|---|---|
| 17–34 °C | 17 °C | 43 | 1st instar |
| 17–34 °C | 17 °C | 43 | 2nd instar |
| 17–34 °C | 17 °C | 43 | 3ird instar |
| 17–34 °C | 17 °C | 43 | 4rth instar |
| 17–34 °C | 17 °C | 43 | 5th instar |
| 17–34 °C | 17 °C | 43 | 6th instar |
| 15–35 °C | 15 °C | 51 | Adult longevity (Female) |
| 15–35 °C | 15 °C | 47 | Adult longevity (male) |
| 17–35 °C | 17 °C | 26 | Adult stage |
| 15–38 °C | 15 °C | 105 | Egg |
| 17–33 °C | 17 °C | 16 | Egg to adult |
| 17–35 °C | 17 °C | 28 | Generation |
| 17–33 °C | 17 °C | 8 | Oviposition |
| 17–34 °C | 17 °C | 35 | Pre-oviposition |
| 17–35 °C | 17 °C | 15 | Pre-pupa |
| 15–38 °C | 15 °C | 116 | Pupa |
| 17–35 °C | 17 °C | 31 | Fertility |
| 15–38 °C | 15 °C | 121 | Larval stage |
| 15–38 °C | 15 °C | 857 | Spodoptera litura |
| Variable | Estimate | SE | Z | p | CI.lb | CI.ub | Loglik | AIC | BIC |
|---|---|---|---|---|---|---|---|---|---|
| 1st instar | −1.1675 | 0.0594 | −19.6663 | <0.0001 | −1.2839 | −1.0512 | −19.9416 | 43.8832 | 47.3585 |
| 2nd instar | −0.8725 | 0.0619 | −14.0993 | <0.0001 | −0.9938 | −0.7512 | −21.6789 | 47.3578 | 50.8331 |
| 3ird instar | −0.8763 | 0.0699 | −12.5428 | <0.0001 | −1.0132 | −0.7393 | −26.8192 | 57.6384 | 61.1137 |
| 4th instar | −0.72 | 0.1088 | −6.6187 | <0.0001 | −0.9332 | −0.5068 | −45.3916 | 94.7831 | 98.2584 |
| 5th instar | −0.8143 | 0.0795 | −10.2486 | <0.0001 | −0.9701 | −0.6586 | −32.1914 | 68.3829 | 71.8582 |
| 6th instar | 0.0597 | 0.0850 | 0.7018 | 0.4828 | −0.1070 | 0.2263 | −35.2275 | 74.4549 | 77.9303 |
| Adult longevity (Female) | −0.9734 | 0.0476 | −20.4557 | <0.0001 | −1.0667 | −0.8801 | −17.2454 | 38.4908 | 42.3148 |
| Adult longevity (male) | −1.1394 | 0.0558 | −20.441 | <0.0001 | −1.2488 | −1.03 | −20.7576 | 45.5151 | 49.1724 |
| Adult stage | −1.0392 | 0.1132 | −9.1778 | <0.0001 | −1.2611 | −0.8173 | −21.6633 | 47.3266 | 49.7644 |
| Egg | −1.2022 | 0.054 | −22.2472 | <0.0001 | −1.3081 | −1.0963 | −85.7709 | 175.5419 | 180.8306 |
| Egg to adult | −0.3387 | 0.0875 | −3.8707 | 0.0001 | −0.5102 | −0.1672 | −5.4890 | 14.9779 | 16.3940 |
| Generation | −0.9917 | 0.1043 | −9.5097 | <0.0001 | −1.1961 | −0.7873 | −22.2479 | 48.4958 | 51.0874 |
| Oviposition | −0.9705 | 0.2411 | −4.0252 | <0.0001 | −1.4434 | −0.4979 | −7.2267 | 18.4534 | 18.3452 |
| Pre−oviposition | −0.092 | 0.083 | −1.1081 | 0.2678 | −0.2546 | 0.0707 | −24.5303 | 53.0607 | 56.1134 |
| Pre−pupa | −1.0001 | 0.1071 | −9.3409 | <0.0001 | −1.2099 | −0.7902 | −7.5383 | 19.0766 | 20.3547 |
| Pupa | −0.9870 | 0.0432 | −22.8742 | <0.0001 | −1.0716 | −0.9025 | −74.7748 | 153.5496 | 159.0394 |
| Fertility | 0.4685 | 0.1958 | 2.3926 | 0.0167 | 0.0847 | 0.8523 | −46.5657 | 97.1315 | 99.9339 |
| Larval stage | −0.6795 | 0.0426 | −15.9436 | <0.0001 | −0.763 | −0.596 | −79.0335 | 162.0671 | 167.642 |
| Spodoptera litura | −0.8077 | 0.022 | −36.6432 | <0.0001 | −0.8509 | −0.7645 | −849.4961 | 1702.9922 | 1712.4967 |
| Developmental History | Ideal Survival Temperature | Ideal Relative Humidity for Survival | Optimal Light/Dark Duration for Survival |
|---|---|---|---|
| 1st instar | 33 °C | 76% | L:D = 14:10 |
| 2nd instar | 31 °C | 76% | L:D = 12:12 |
| 3ird instar | 30 °C | 83% | L:D = 12:12 |
| 4th instar | 31 °C | 82% | L:D = 12:12 |
| 5th instar | 30 °C | 76% | L:D = 12:12 |
| Adult longevity (Female) | 33 °C | 65% | L:D = 12:12 |
| Adult longevity (male) | 33 °C | 60% | L:D = 12:12 |
| Adult stage | 35 °C | 75% | L:D = 13:11 |
| Egg | 34 °C | 60% | L:D = 12:12 |
| Egg to adult | 33 °C | 60% | L:D = 12:12 |
| Generation | 35 °C | 75% | L:D = 14:10 |
| Pre-pupa | 30 °C | 65% | L:D = 13:11 |
| Pupa | 30 °C | 75% | L:D = 12:12 |
| Fertility | 34 °C | 75% | L:D = 14:10 |
| Larval stage | 34 °C | 75% | L:D = 12:12 |
| Spodoptera litura | 30−35 °C | 60–83% | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Liu, Z.; Xu, D.; Gan, T.; Deng, X.; Zhang, H.; Zhuo, Z. Influence of Temperature, Humidity, and Photophase on the Developmental Stages of Spodoptera litura (Lepidoptera: Noctuidae) and Prediction of Its Population Dynamics. Insects 2025, 16, 355. https://doi.org/10.3390/insects16040355
Fu C, Liu Z, Xu D, Gan T, Deng X, Zhang H, Zhuo Z. Influence of Temperature, Humidity, and Photophase on the Developmental Stages of Spodoptera litura (Lepidoptera: Noctuidae) and Prediction of Its Population Dynamics. Insects. 2025; 16(4):355. https://doi.org/10.3390/insects16040355
Chicago/Turabian StyleFu, Chun, Zhiqian Liu, Danping Xu, Tingjiang Gan, Xinqi Deng, Honghua Zhang, and Zhihang Zhuo. 2025. "Influence of Temperature, Humidity, and Photophase on the Developmental Stages of Spodoptera litura (Lepidoptera: Noctuidae) and Prediction of Its Population Dynamics" Insects 16, no. 4: 355. https://doi.org/10.3390/insects16040355
APA StyleFu, C., Liu, Z., Xu, D., Gan, T., Deng, X., Zhang, H., & Zhuo, Z. (2025). Influence of Temperature, Humidity, and Photophase on the Developmental Stages of Spodoptera litura (Lepidoptera: Noctuidae) and Prediction of Its Population Dynamics. Insects, 16(4), 355. https://doi.org/10.3390/insects16040355





