Cuticular and Exuvial Biomass and Nitrogen Economy During Assimilation and Growth of the American Grasshopper, Schistocerca americana
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Experimental Protocol
2.2. Experimental Procedures
2.3. Statistics
3. Results
3.1. Morphological Size Changes and Development
3.2. Development Time
3.3. Exuvial Biomass and Nitrogen Losses During Assimilation and Growth
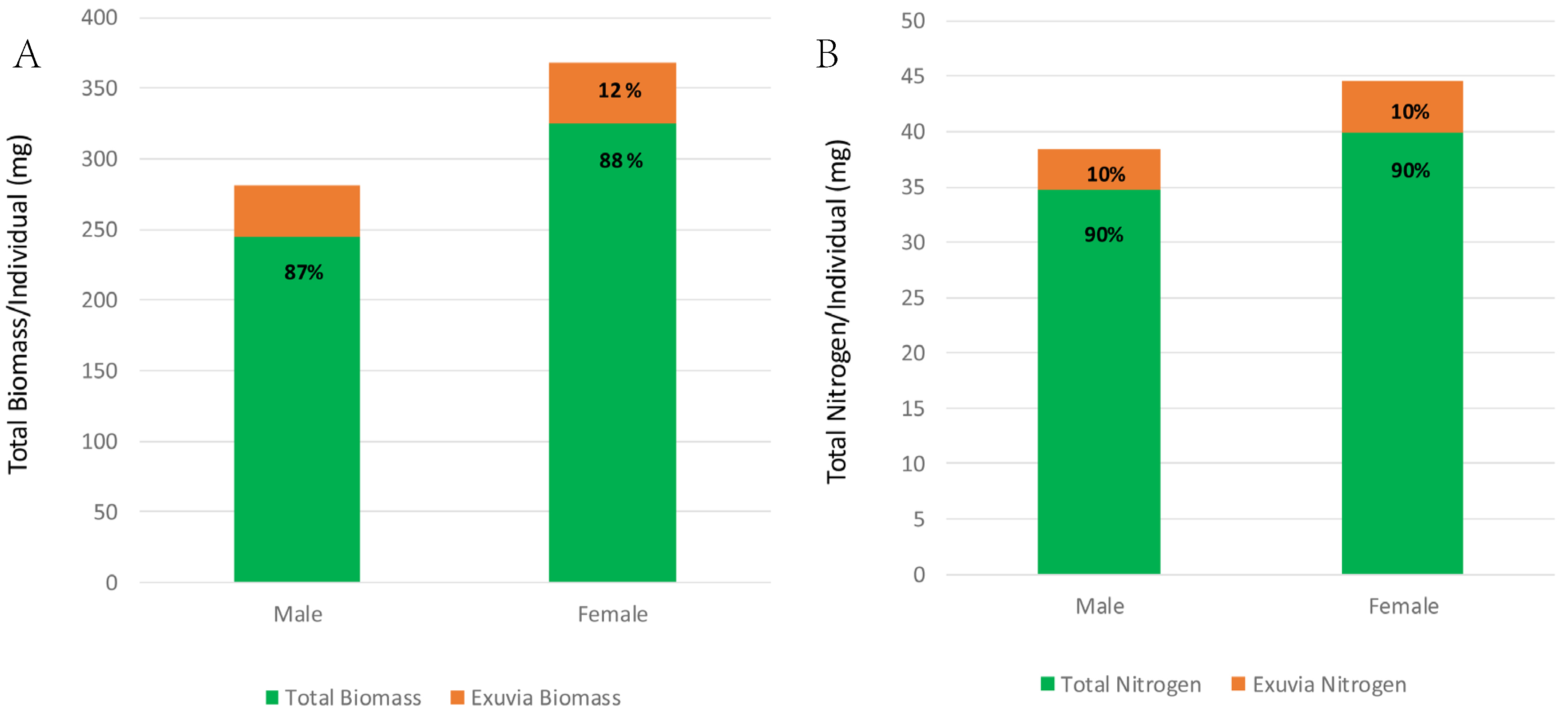
3.4. Total Biomass and Nitrogen Assimilation and Growth Compared with Exuvial Biomass and Nitrogen Losses
4. Discussion
4.1. Energetics and Energy Budgets
4.2. Conservation of Cuticular Nitrogen During Insect Molt Cycles
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Downer, R.G.H. Physiological and environmental considerations in insect bioenergetics. In Energy Metabolism in Insects; Downer, R.G.H., Ed.; Plenum Press: New York, NY, USA, 1981; pp. 1–17. [Google Scholar]
- Tomlinson, S.; Arnall, S.G.; Munn, A.; Bradshaw, S.D.; Maloney, S.K.; Dixon, K.W.; Didham, R.K. Applications and implications of ecological energetics. Trends Ecol. Evol. 2014, 29, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Slansky, F., Jr.; Rodriguez, J.G. Nutritional ecology of insects, mites, spiders, and related invertebrates: An overview. In Nutritional Ecology of Insects, Mites, Spiders, and Related Invertebrates; Slansky, F., Jr., Rodriguez, J.G., Eds.; Wiley Interscience, John Wiley & Sons: New York, NY, USA, 1987; pp. 1–69. [Google Scholar]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O.; Roespstorff, P. Aspects of cuticular sclerotization in the locust Schistocerca gregaria, and the beetle, Tenebrio molitor. Insect Biochem. Mol. Biol. 2007, 37, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Klocke, D.; Schmitz, H. Water as a major modulator of the mechanical properties of insect cuticle. Acta Biomater. 2011, 7, 2935–2942. [Google Scholar] [CrossRef]
- Moussian, B. Arthropod cuticle. In Arthropod Biology and Evolution; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 171–196. [Google Scholar] [CrossRef]
- Clark, A.J.; Triblehorn, J.D. Mechanical properties of the cuticles of three cockroach species that differ in their wind-evoked escape behavior. PeerJ 2014, 2, e501. [Google Scholar] [CrossRef]
- Cohen, E. Chitin biochemistry: Synthesis, hydrolysis and inhibition. Adv. Insect Physiol. 2010, 38, 5–74. [Google Scholar]
- Dirks, J.H.; Taylor, D. Fracture toughness of locust cuticle. J. Exp. Biol. 2012, 215, 1502–1508. [Google Scholar] [CrossRef]
- Locke, M. The Wigglesworth Lecture: Insects for studying fundamental problems in biology. J. Insect Physiol. 2001, 47, 495–507. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Arakane, Y.; Noh, M.Y.; Mun, S.; Merzendorfer, H.; Boehringer, C.; Wellmeyer, B.; Yang, Q.; Qu, M.; Liu, L. Chapter One Chitin in insect cuticle. In Advances in Insect Physiololy; Sugumaran, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 62, pp. 1–110. [Google Scholar] [CrossRef]
- Gallot, A.; Rispe, C.; Leterme, N.; Gauthier, J.-P.; Jaubert-Possamai, S.; Tagu, D. Cuticular proteins and seasonal photoperiodism in aphids. Insect Biochem. Mol. Biol. 2010, 40, 235–240. [Google Scholar] [CrossRef]
- Elias-Neto, M.; Nasciment, A.L.O.; Bonetti, A.M.; Nascimento, F.S.; Mateus, S.; Garofalo, C.A.; Bitondi, M.M.G. Heterochrony of cuticular differentiation in eusocial corbiculate bees. Apidologie 2014, 45, 397–408. [Google Scholar] [CrossRef]
- Kakkar, G.; Chouvenc, T.; Su, N.-Y. Postecdysis sclerotization of mouthparts of the Formosan subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Ent. 2016, 109, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Mira, A. Exuvia eating: A nitrogen meal? J. Insect Physiol. 2000, 46, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Sieglaff, D.H.; Pereira, R.M.; Capinera, J.L. Microbial control of Schistocerca americana (Orthoptera: Acrididae) by Metarhizium flavoviride (Deuteromycotina) instar dependent mortality and efficacy of ultra-low volume application under greenhouse conditions. J. Econ. Entomol. 1998, 91, 76–85. [Google Scholar] [CrossRef]
- Schmidt, R.R. Nitrogen and phosphorous metabolism during synchronous growth of Chlorella pyrenoidosa. Exp. Cell. Res. 1961, 23, 209–217. [Google Scholar] [CrossRef]
- Mullins, D.E.; Keil, C.B.; White, R.H. Maternal and paternal nitrogen investment in Blattella germanica (L.) (Dictyoptera; Blattellidae). J. Exp. Biol. 1992, 162, 55–72. [Google Scholar] [CrossRef]
- Ledder, G. The basic dynamic energy budget model and some implications. Lett. Biomath. 2014, 1, 221–233. [Google Scholar] [CrossRef]
- Llandres, A.L.; Marques, G.M.; Manio, J.L.; Kooijman, S.A.L.M.; Kearney, M.R.; Casas, J. A dynamic energy budget for the whole life-cycle of holometabolous insects. Ecol. Monogr. 2015, 85, 353–371. [Google Scholar] [CrossRef]
- van der Vaart, E.; Johnston, A.S.A.; Sibly, R.M. Predicting how many animals will be where: How to build, calibrate and evaluate individual-based models. Ecol. Model. 2016, 326, 113–123. [Google Scholar] [CrossRef]
- Sibly, R.M.; Grim, V.; Martin, B.T.; Johnston, A.S.A.; Kulakowska, K.; Topping, C.J.; Calows, P.; Nabe-Nielsen, J.; Thorbek, P.; DeAngelis, D.L. Representing the acquisition and use of energy by individuals in agent-based models of animal populations. Methods Ecol. Evol. 2013, 4, 151–161. [Google Scholar] [CrossRef]
- LaSalle, M.W.; Cruz, A.C.; Miller, G. Energy lost to the exuviae during molting of Lycosa watsoni Certsch (Araneae: Lycosidae). Fla. Entomol. 1984, 67, 465–471. [Google Scholar] [CrossRef]
- Lease, H.M.; Wolf, B.O. Exoskeletal chitin scales isometrically with body size in terrestrial insects. J. Morphol. 2010, 271, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Vshivkova, T.A. Food energy expenditures in the Gypsy Moth Lymantria dispar L. (Lepidoptera, Lymantriidae) at different stages of ontogeny. Biol. Bull. 2003, 30, 476–481. [Google Scholar] [CrossRef]
- Carefoot, T.H. Energy partitioning in the Desert locust, Schistocerca gregaria (Forsk.). Acrida 1977, 6, 85–107. [Google Scholar]
- Bailey, C.G.; Riegert, P.W. Energy dynamics of Encoptolophus sordidus costalis (Scudder: Orthoptera: Acrididae) in a grassland ecosystem. Can. J. Zool. 1973, 5, 91–100. [Google Scholar] [CrossRef]
- Delvi, M.R.; Pandian, T.J. Ecophysiological studies on the utilization of food in the paddy field grasshopper Oxya velour. Oecologia 1971, 8, 267–275. [Google Scholar] [CrossRef]
- Rychtal, J.; Frynta, D.; Tomek, J.; Varadinova, Z.; Brom, C. Waste recycling can promote group living: A cockroach case study. Lett. Biomath. 2014, 1, 17–22. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do diet and taxonomy influence insect gut bacterial communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef]
- Mikaelyan, A.; Thompson, C.L.; Hofer, M.J.; Brune, A. Deterministic assembly of complex bacterial communities in guts of germ-free cockroaches. Appl. Environ. Microbiol. 2016, 82, 1256–1263. [Google Scholar] [CrossRef]
- Gontang, E.A.; Aylward, F.O.; Carlos, C.; del Rio, T.G.; Chovatia, M.; Fern, A.; Lo, C.-C.; Malfatti, S.A.; Tringe, S.G.; Currie, C.R.; et al. Major changes in microbial diversity and community composition across gut sections of a juvenile Panchlora cockroach. PLoS ONE 2017, 12, e0177189. [Google Scholar] [CrossRef]
- Kakumanu, M.L.; Maritz, J.M.; Carlton, J.M.; Schal, C. Overlapping community compositions of gut and fecal microbiomes in lab-reared and field-collected German cockroaches. Appl. Environ. Microbiol. 2018, 84, e01037-18. [Google Scholar] [CrossRef]
- Pernice, M.; Simpson, S.J.; Ponton, F. Towards an integrated understanding of gut microbiota using insects as model systems. J. Insect Physiol. 2014, 69, 12–18. [Google Scholar] [CrossRef]


| Interval | n | 1 Stadia Interval Average ± SEM Days | 1 Stadia Interval Average ± SEM Days |
|---|---|---|---|
| Males | Females | ||
| 1st Instar | 10 | 6.6 ± 0.2 | 6.2 ± 0.2 |
| 2nd Instar | 10 | 5.2 ± 0.2 | 5.0 ± 0.2 |
| 3rd Instar | 10 | 5.7 ± 0.2 | 5.7 ± 0.2 |
| 4th Instar | 10 | 6.6 ± 0.2 | 6.4 ± 0.2 |
| 5th Instar | 10 | 7.7 ± 0.3 | 8.2 ± 0.4 |
| 6th Instar | 10 | 11.1 ± 0.3 | 12.7 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mullins, D.E.; Gabbert, S.E. Cuticular and Exuvial Biomass and Nitrogen Economy During Assimilation and Growth of the American Grasshopper, Schistocerca americana. Insects 2025, 16, 327. https://doi.org/10.3390/insects16030327
Mullins DE, Gabbert SE. Cuticular and Exuvial Biomass and Nitrogen Economy During Assimilation and Growth of the American Grasshopper, Schistocerca americana. Insects. 2025; 16(3):327. https://doi.org/10.3390/insects16030327
Chicago/Turabian StyleMullins, Donald E., and Sandra E. Gabbert. 2025. "Cuticular and Exuvial Biomass and Nitrogen Economy During Assimilation and Growth of the American Grasshopper, Schistocerca americana" Insects 16, no. 3: 327. https://doi.org/10.3390/insects16030327
APA StyleMullins, D. E., & Gabbert, S. E. (2025). Cuticular and Exuvial Biomass and Nitrogen Economy During Assimilation and Growth of the American Grasshopper, Schistocerca americana. Insects, 16(3), 327. https://doi.org/10.3390/insects16030327






