Insecticidal and Repellent Activity of Plant Powders on the Weevil (Sitophilus zeamais) in Stored Corn Grains in a Rural Community of Oaxaca, Mexico
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
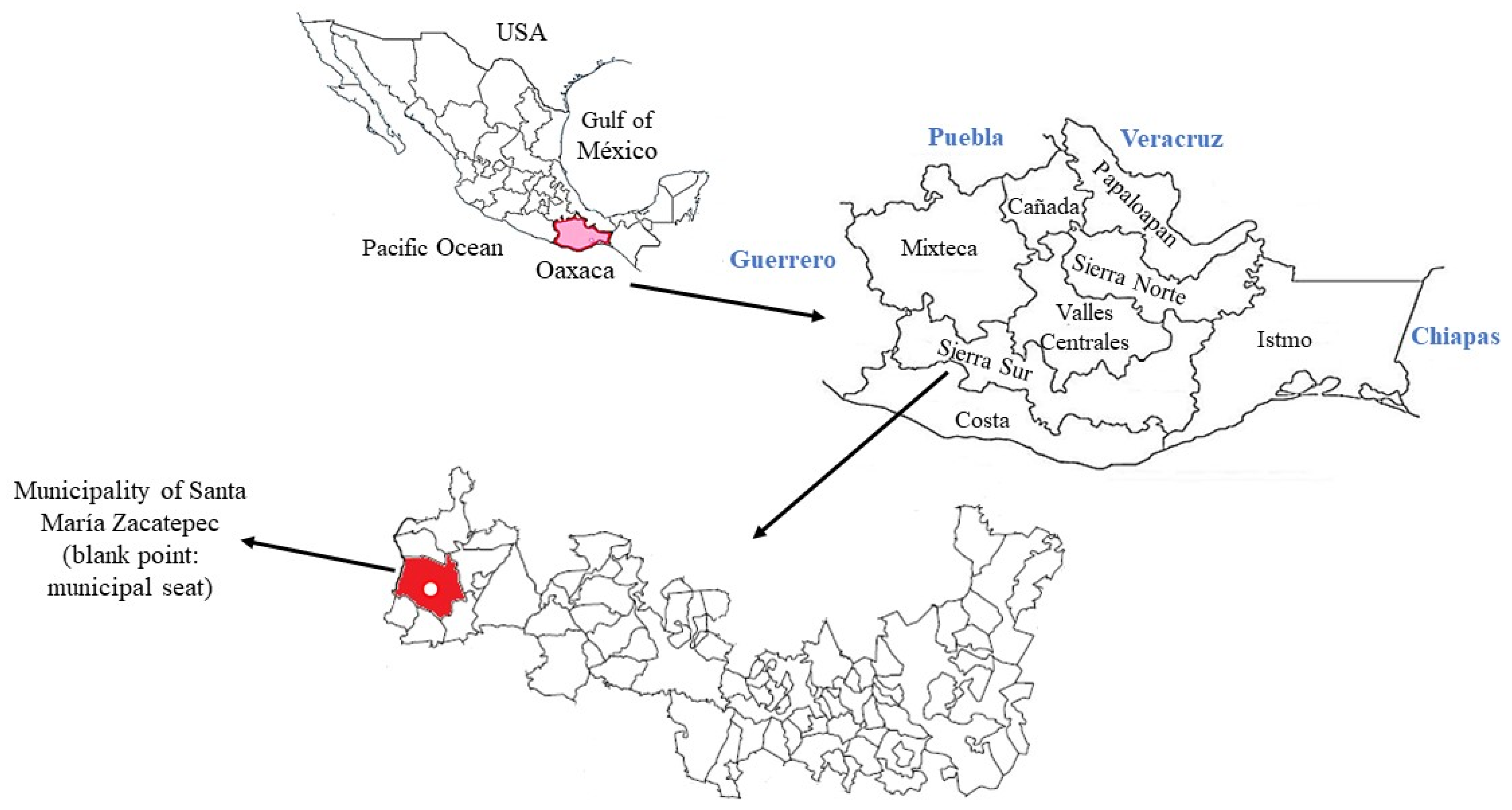
2.1. Place Where the Study Was Carried Out
2.2. Obtaining and Preparing Plant Material
2.3. Obtaining and Preparing Corn
2.4. Obtaining and Preparing the Insect
2.5. Mortality Test
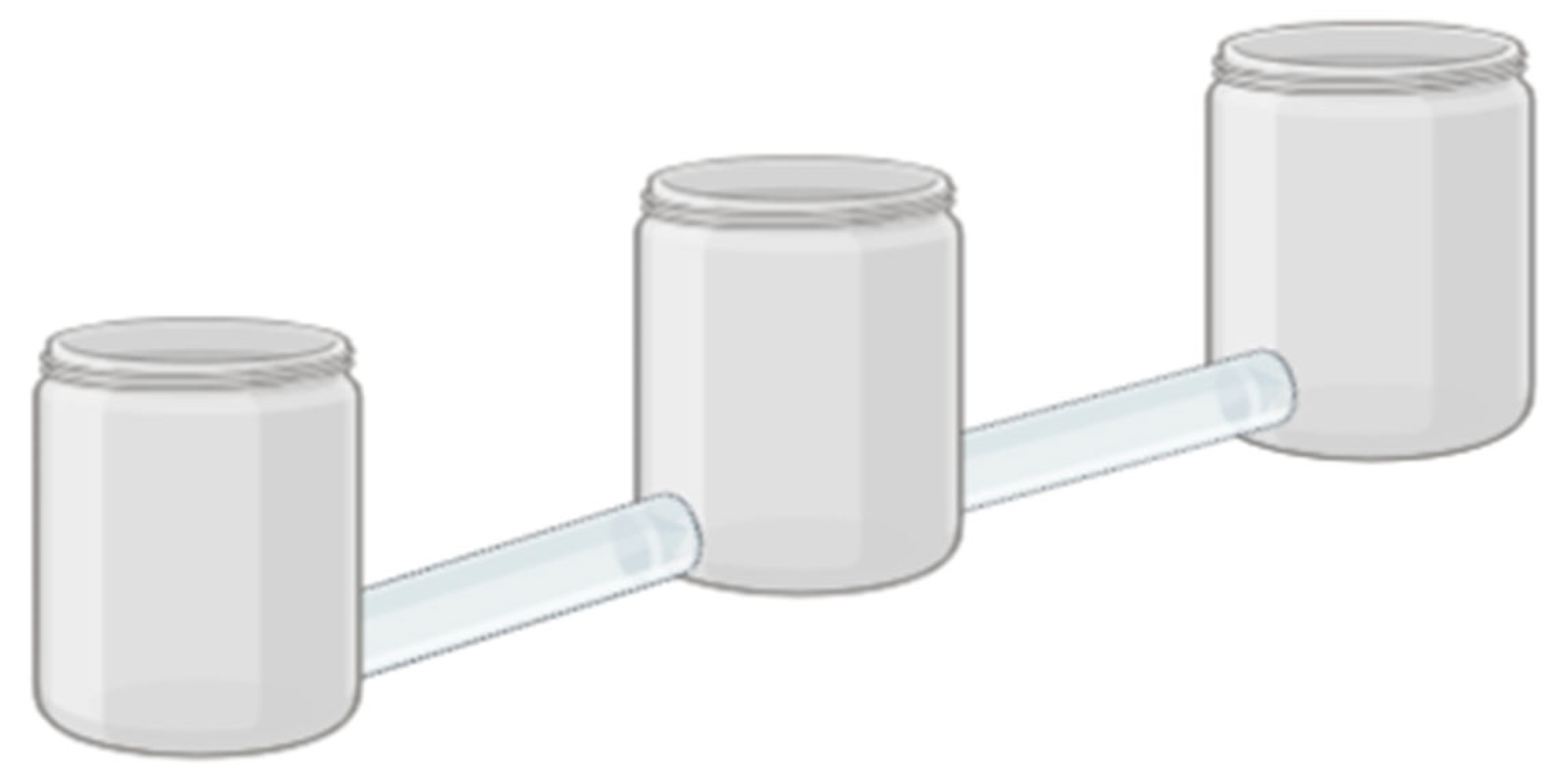
2.6. Repellency Test
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Insecticidal Effect
3.2. Repellency Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Sec. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). El Maíz, Patrimonio Biocultural de México. Miradas Desde Nuestras Escuelas. Centro de Educación y Capacitación para el Desarrollo Sustentable (Cecadesu), Primera ed.; SEMARNAT: Ciudad de Mexico, Mexico, 2022; 240p, Available online: https://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/libros2018/CD007487.pdf (accessed on 12 May 2024).
- Rangel, M.A.; Burgos-Díaz, J.A.; Tucuch-Haas, J.I.; Benítez-Riquelme, I.; García-Zavala, J.J. Susceptibilidad de polaciones nativas de maíz y preferencia del gorgojo en Yucatán, México. Rev. Mex. Cienc. Agrícolas 2020, 11, 1469–1479. [Google Scholar] [CrossRef]
- Odjo, S.; Bongianino, N.; González, R.J.; Cabrera, S.M.L.; Palacios, R.N.; Burgueño, J.; Verhulst, N. Effect of storage technologies on postharvest insect pest control and seed germination in mexican maize landraces. Insects 2022, 13, 878. [Google Scholar] [CrossRef]
- Corrêa, A.S.; Oliveira, L.O.; Braga, L.S.; Guedes, R.N.C. Distribution of the related weevil species Sitophilus oryzae and S. zeamais in Brazil. Insect Sci. 2012, 20, 763–770. [Google Scholar] [CrossRef]
- Oliveira, C.L.; Celante, M.S.; Rodrigues, C.P.R.; Pereira, C.E. Storage of corn seeds infested by weevil. Arq. Inst. Biol. 2021, 88, e00572020. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, N.; González, J.; Vilchis, R.; Enyanche, F.; Odjo, S. Poscosecha para pequeños productores de maíz en México ¿Dónde estamos, adónde queremos llegar, cuál es nuestro papel en el camino? Enlace Rev. Agric. Conserv. 2017, 41, 30–33. [Google Scholar]
- Arrahman, A.; Saenong, M.S. Controlling maize weevil in corn plants by improving cultivation technology and postharvest handling. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012034. [Google Scholar] [CrossRef]
- Rodríguez, P.C. Intoxicación por fosfuro de aluminio. Med. Leg. Costa Rica 2022, 39, 20–31. [Google Scholar]
- Torres, R.I.A.; Silva, R.; Gomes, S.A.; Milet-Pinheiro, P.; Guedes, P.P.M.; Ferraz, N.D.M.A.; Silva, M.V.; Napoleão, T.H.; Santos, C.M.T. Chemical characterization and insecticidal effect against Sitophilus zeamais (maize weevil) of essential oil from Croton rudolphianus leaves. Crop Prot. 2020, 129, 105043. [Google Scholar] [CrossRef]
- Boate, U.R.; Otayor, R.A. Review on the bio-insecticidal properties of some plant secondary metabolites: Types, formulations, modes of action, advantages and limitations. Asian J. Res. Zool. 2020, 3, 27–60. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Kortbeek, R.W.J.; van der Gragt, M.; Bleeker, P.M. Endogenous plant metabolites against insects. Eur. J. Plant Pathol. 2019, 154, 67–90. [Google Scholar] [CrossRef]
- Cheraghi, N.M.; Farzaei, M.H.; Karimpour, R.E.; Amin, G.; Khanavi, M.; Akbarzadeh, T.; Shams-Ardekani, M.R. An evidence-based review on medicinal plants used as insecticide and insect repellent in traditional Iranian medicine. Iran. Red Crescent Med. J. 2016, 18, e22361. [Google Scholar] [CrossRef]
- Aros, J.; Silva, A.G.; Fischer, S.; Figueroa, I.; Rodríguez, M.J.C.; Lagunes, T.A.; Castañeda, R.G.; Aguilar, M.L. Actividad insecticida del aceite esencial del paico Chenopodium ambrosioides L. sobre Sitophilus zeamais Motschulsky. Chil. J. Agric. Anim. Sci. 2019, 35, 282–292. [Google Scholar] [CrossRef]
- Jbilou, R.; Matteo, R.; Bakrim, A.; Bouayad, N.; Rharrabe, K. Potential use of Origanum vulgare in agricultural pest management control: A systematic review. J. Plant Dis. Prot. 2024, 131, 347–363. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W. A systematic review of botany, phytochemicals and pharmacological properties of “Hoja santa” (Piper auritum Kunth). Z. Naturforschung C. 2020, 76, 93–102. [Google Scholar] [CrossRef]
- El Baghazaoui, R.; Belmalha, S.; Boutagayout, A.; Nassiri, L.; El Alami, S.; Savoie, J.-M.; Bouiamrine, E.H. Insecticidal properties and chemical characterization of Laurus nobilis L. essential oils from two regions of Morocco against Callosobruchus maculatus (Coleoptera: Bruchinae). Agriculture 2024, 14, 1150. [Google Scholar] [CrossRef]
- Kasali, F.M.; Tusiimirem, J.; Kadima, J.N.; Agaba, A.G. Ethnomedical uses, chemical constituents, and evidence-based pharmacological properties of Chenopodium ambrosioides L.: Extensive overview. Future J. Pharm. Sci. 2021, 7, 153. [Google Scholar] [CrossRef]
- Singletary, K. Oregano: Overview of the literature on health benefits. Nutr. Today 2010, 45, 129–138. [Google Scholar] [CrossRef]
- Sotelo, L.C.; Tagle, E.L.J.; Aniceto, T.C.; Galeana, H.J.; Condori, C.S.; Flores, F.G.; Salinas, S.D.O. Estudio etnofarmacológico y fitoquímico de las plantas medicinales de mayor uso en Julián Blanco, Guerrero, México. Acta Agrícola Y Pecu. 2022, 8, e0081012. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Plan Municipal de Desarrollo. Santa María Zacatepec, Honorable Ayuntamiento Constitucional: Putla, Oaxaca, 2022; 261p, Available online: https://sisplade.oaxaca.gob.mx/bm_sim_services/PlanesMunicipales/2022_2024_/447.pdf (accessed on 16 May 2024).
- Gobierno de México. Santa María Zacatepec. Municipio de Oaxaca. Available online: https://www.economia.gob.mx/datamexico/es/profile/geo/santa-maria-zacatepec#economy (accessed on 16 May 2024).
- Consejo Nacional de Evaluación de la Política de Desarrollo Social. Oaxaca. Santa María Zacatepec. 2020. Available online: https://sistemas.coneval.org.mx/DATAMUN/dato-actualizado?c=2020&e=20&m=20447&sg=4&g=26&owli= (accessed on 16 May 2024).
- Lagunes, T.A.; Rodríguez, H.C. Búsqueda de Tecnología Apropiada para el Combate de Plagas del maíz Almacenado en Condiciones Rústicas; CONACYT/Colegio de Postgraduados: Montecillo, México, 1989; 150p. [Google Scholar]
- Juárez, F.B.I.; Jasso, P.Y.; Aguirre, R.J.R.; Jasso, P.I. Efecto de polvos de asteráceas sobre el gorgojo del maíz (Sitophilus zeamais Motsch). Polibotánica 2010, 30, 123–135. [Google Scholar]
- Torres, C.; Silva, G.; Tapia, M.; Rodríguez, J.C.; Urbina, A.; Figueroa, I.; Lagunes, A.; Santillan, O.C.; Robles, B.A.; Aguilar, M.S. Insecticidal properties of Laurelia sempervirens powder to Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) control. Bol. Latinoam. Caribe Plant Med. Aromat. 2015, 14, 48–59. [Google Scholar]
- Granados, E.C.A.; Alonso, H.N.; Ortega, O.B.; Reyes, E.M.; Chan, B.M.; Camacho, C.J.C. Polvos botánicos de Senecio salignus (Asteraceae) y Solanum diversifolium (Solanaceae) como alternativa ecológica de control de Sitophilus zeamais (coleoptera: Curculionidae). Entomol. Mex. 2017, 4, 403–408. [Google Scholar]
- Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Almacenamiento y conservación de granos y semillas. Subsecretaría de Desarrollo Rural. Dirección General de Apoyos para el Desarrollo Rural. Ficha técnica. Available online: https://somossemilla.org/wp-content/uploads/2017/06/Almacenamiento-de-semillas.pdf (accessed on 11 February 2025).
- Gómez, H.H.A.; González, M.O.; González, C.J.C. Vegetales pulverizados para el manejo de Sitophilus zeamais Motschulsky en almacenamiento. Rev. Mex. Cienc. Agrícolas 2018, 9, 787–798. [Google Scholar]
- Moghadamnia, A.A. An update on toxicology of aluminum phosphide. DARU J. Pharm. Sci. 2012, 20, 25. [Google Scholar] [CrossRef]
- Orlando, C.A. Fosfina, un Fumigante Seguro y eficaz. D2. Manual de Sistemas Cuarentenarios para Plagas Agrícolas; Facultad de Agronomía y Zootecnia: Tucumán, Argentina, 2016; 161p, Available online: https://www.eeaoc.org.ar/wp-content/uploads/2018/12/sc20.pdf (accessed on 5 March 2025).
- Yadav, Y.; Shukla, V.; Deswal, P. Effect of aluminium phosphide powder residue on earthworm Eisenia fetida. Int. J. Res. Anal. Rev. 2019, 6, 779–783. [Google Scholar]
- Abbott, W.S. Classic paper: Abbott’s formula. A method of computing the effectiveness of an insecticide. J. Am. Control. Assoc. 1987, 3, 302–303. [Google Scholar]
- Silva, L.B.; da Silva, J.C.; Pavan, B.E.; Pereira, F.F.; Maggioni, K.; Andrade, L.H.; Candido, A.C.S.; Peres, M.T.L.P. Insecticide irritability of plant extracts against Sitophilus zeamais. Afr. J. Agric. Res. 2013, 8, 978–983. [Google Scholar] [CrossRef]
- Ail-Catzim, C.E.; García-López, A.M.; Troncoso-Rojas, R.; González-Rodríguez, R.E.; Sánchez-Segura, Y. Insecticidal and repellent effect of extract of Pluchea sericea (Nutt.) on adults Bemisia tabaci (Genn.). Rev. Chapingo Ser. Hortic. 2015, 21, 33–41. [Google Scholar] [CrossRef]
- Gómez, F.C.; Ramírez, L.M.B.; Gaona, E.F. Efecto insecticida del polvo de Chenopodium ambrosioides L. y carbonato de calcio en el control de Sitophilus zeamais en granos de maíz. Investig. Agrar. 2016, 18, 116–120. [Google Scholar] [CrossRef]
- Ibrahim, A. Effects of malathion dust and mexican tea powder (Chenopodium ambrosoides) combinations on the maize weevil, Sitophilus zeamais Mostch. (Coleoptera: Curculionidae) on maize. Sci. Technol. Arts Res. J. 2015, 4, 57–62. [Google Scholar] [CrossRef]
- Ntonifor, N.; Forbanka, D.; Mbuh, J.V. Potency of Chenopodium ambrosioides powders and its combinations with wood ash on Sitophilus zeamais in stored maize. J. Entomol. 2011, 8, 375–383. [Google Scholar] [CrossRef]
- Bautista, S.N.A.; Góngora, G.C.J.; Chan, C.R.J.; Ballina, G.H.S.; González, M.D.; Ruíz, S.E. Efecto de polvos vegetales en el gorgojo mexicano (Zabrotes subfasciatus Boheman) y su daño a granos almacenados de frijol lima (Phaseolus lunatus L.). Trop. Subtrop. Agroecosystems 2021, 24, 1–7. [Google Scholar] [CrossRef]
- Agüero, C.M.; Valdés, H.; Pozo, V.E. Eficacia del polvo de Piper auritum Kunth con variantes de secado contra Sitophilus oryzae (L.) (Coleoptera; Curculionidae). Cent. Agrícola 2020, 47, 38–44. [Google Scholar]
- Caballero, G.K.; Olivero, V.J.; Pino, B.N.; Stashenko, E.E. Chemical composition and bioactivity of Piper auritum and P. multiplinervium essential oils against the red flour beetle, Tribolium castaneum (Herbst). Bol. Latinoam. Caribe Plant Med. Aromat. 2014, 13, 10–19. [Google Scholar]
- Granados, E.C.; Pérez, P.R.; Bautista, M.N.; Alonso, H.N.; Sánchez, G.J.A.; Martínez, T.S.H.; Sánchez, M.S. Insecticidal effect of botanical extracts on developmental stages of Bactericera cockerelli (Sulc) (Hemiptera: Triozidae). Southwest. Entomol. 2015, 40, 97–110. [Google Scholar] [CrossRef]
- Kasrati, A.; Sakar, E.H.; Aljaiyash, A.; Hirri, A.; Tamegart, L.; Abbad, I.; Alaoui, J.C. Chemical profiling, insecticidal, and phytotoxic effect of essential oils from leaves and inflorescence of Moroccan Chenopodium ambrosioides (L.). Plants 2024, 8, 483. [Google Scholar]
- Chu, S.S.; Hu, J.F.; Liu, Z.L. Composition of essential oil of chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest Manag. Sci. 2011, 67, 714–718. [Google Scholar] [CrossRef]
- Valdivia, A.A.L.; Rubio, F.Y.; Camacho, C.C.; Brea, M.O.; Matos, T.M.; Sosa, C.M.; Pérez, H.Y. Phytochemical and antibacterial properties of Piper auritum Kunth. Av. Investig. Agropecu. 2018, 22, 77–90. [Google Scholar]
- Lam, G.A.; Ayora, T.T.; Garrido, R.E.R.; Ruíz, V.V.M.; Guzmán, A.J.M.; Cristóbal, A.J. Chemical composition and antifungal activity of essential oils extracted from Pimenta dioica and Piper auritum leaves grown in Mexico. Cogent Food Agric. 2024, 10, 2356935. [Google Scholar] [CrossRef]
- Pérez, H.R.G.; Reyes, G.C.; Grijalva, A.R.; Chávez, P.M.; Espadas, M.C.; Hernández, G.M. Usos tradicionales y prácticas de manejo de Piper auritum en comunidades maya rurales de Yucatán. Bot. Sci. 2023, 101, 1049–1069. [Google Scholar] [CrossRef]
- Zoubiri, S.; Baaliouamer, A. Potentiality of plants as source of insecticide principles. J. Saudi Chem. Soc. 2014, 18, 925–938. [Google Scholar] [CrossRef]
- Ajiboye, O.; Babarinde, S.A.; Adesina, G.O.; Olusoji, O.C.; Adebayo, T.A.; Adelasoye, K.A. Combinations of spintor with botanical powders as toxicants against red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Plant Prot. Res. 2023, 63, 254–262. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
| Treatment | Number of Dead Individuals (n = 20) Mean ± SD | Corrected Mortality (%) Mean ± SD |
|---|---|---|
| Origanum vulgare 1% | 4.3 ± 1.2 | 21.7 ± 5.8 a |
| Laurus nobilis 1% | 6.0 ± 2.0 | 30.0 ± 10.0 ab |
| Piper auritum 1% | 6.7 ± 0.6 | 33.3 ± 2.9 ab |
| Chenopodium ambrosioides 1% | 7.3 ± 3.1 | 36.7 ± 15.3 ab |
| Origanum vulgare 2% | 4.7 ± 1.2 | 23.3 ± 5.8 a |
| Laurus nobilis 2% | 6.0 ± 1.0 | 30.0 ± 5.0 ab |
| Piper auritum 2% | 11.0 ± 2.0 | 55.0 ± 10.0 bc |
| Chenopodium ambrosioides 2% | 12.7 ± 1.5 | 63.3 ± 7.6 cd |
| Origanum vulgare 3% | 11.0 ± 2.6 | 55.0 ± 13.2 bc |
| Laurus nobilis 3% | 11.0 ± 1.0 | 55.0 ± 5.0 bc |
| Piper auritum 3% | 16.7 ± 1.5 | 83.3 ± 7.6 de |
| Chenopodium ambrosioides 3% | 17.0 ± 1.7 | 85.0 ± 8.7 de |
| Aluminum phosphide | 20 ± 0.0 | 100 ± 0.0 e |
| Control without vegetable powder | 0.0 | --- |
| Treatment | No. of Insects Attracted (%) * Mean ± SD | No. of Insects in the Control (%) Mean ± SD | Comparison Treatment Versus Control | Repellency Index (RI) *** Media ± DE | Repellency Effect *** |
|---|---|---|---|---|---|
| Chenopodium ambrosioides 1% ** | 18.3 ± 3.5 (61.0 ± 11.5) bcdA | 11.7 ± 3.5 (39.0 ± 11.5) A | t = 2.325, p = 0.081 NS | 1.2 ± 0.3 | Attractive |
| Piper auritum 1% ** | 23.3 ± 3.1 (78.0 ± 10.1) cdA | 6.7 ± 3.1 (22.0 ± 10.1) B | t = 6.682, p = 0.003 **** | 1.5 ± 0.2 | Attractive |
| Laurus nobilis 1% ** | 25.5 ± 3.0 (83.0 ± 10.0) dA | 5.0 ± 3.0 (17.0 ± 10.0) B | t = 8.165, p = 0.001 **** | 1.7 ± 0.2 | Attractive |
| Origanum vulgare 1% ** | 24.3 ± 1.2 (81.0 ± 3.5) dA | 5.7 ± 1.2 (19.0 ± 3.5) B | t = 19.799, p = 0.001 **** | 1.6 ± 0.1 | Attractive |
| Chenopodium ambrosioides 2% ** | 15.3 ± 6.5 (51.0 ± 21.5) bcA | 14.7 ± 6.5 (49.0 ± 21.5) A | t = 0.125, p = 0.906 NS | 1.0 ± 0.5 | Neutral |
| Piper auritum 2% ** | 14.7 ± 3.5 (49.0 ± 11.5) bcA | 15.3 ± 3.5 (51.0 ± 11.5) A | t = −0.232, p = 0.828 NS | 1.0 ± 0.3 | Neutral |
| Laurus nobilis 2% ** | 18.0 ± 2.0 (60.0 ± 7.0) bcdA | 12.0 ± 2.0 (40.0 ± 7.0) B | t = 3.674, p = 0.021 **** | 1.2 ± 0.1 | Attractive |
| Origanum vulgare 2% ** | 16.0 ± 2.0 (53.3 ± 6.5) bcdA | 14.0 ± 2.0 (46.7 ± 6.5) A | t = 1.225, p = 0.288 NS | 1.1 ± 0.1 | Attractive |
| Chenopodium ambrosioides 3% ** | 3.0 ± 1.7 (10.3 ± 5.8) aA | 27.0 ± 1.7 (89.7 ± 5.8) B | t= −16.971, p = 0.001 **** | 0.2 ± 0.1 | Repellent |
| Piper auritum 3% ** | 5.3 ± 2.5 (18.0 ± 8.5) aA | 24.7 ± 2.5 (82.0 ± 8.5) B | t= −9.409, p = 0.001 **** | 0.3 ± 0.2 | Repellent |
| Laurus nobilis 3% ** | 11.7 ± 2.1 (39.0 ± 7.2) abA | 18.3 ± 2.1 (61.0 ± 7.2) B | t= −3.922, p = 0.017 **** | 0.8 ± 0.1 | Repellent |
| Origanum vulgare 3% ** | 11.0 ± 2.0 (36.7 ± 6.5) abA | 19.0 ± 2.0 (63.3 ± 6.5) B | t= −4.899, p = 0.008 **** | 0.7 ± 0.2 | Repellent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Flores, C.; Zapién-Martínez, A.; Sánchez-Cruz, G.; Reyes-Velasco, L.; Segura-Salvador, A.; Vargas-Arzola, J.; Hernández-Osorio, L.A.; Torres-Aguilar, H.; Bernardino-Hernández, H.U. Insecticidal and Repellent Activity of Plant Powders on the Weevil (Sitophilus zeamais) in Stored Corn Grains in a Rural Community of Oaxaca, Mexico. Insects 2025, 16, 329. https://doi.org/10.3390/insects16030329
Peña-Flores C, Zapién-Martínez A, Sánchez-Cruz G, Reyes-Velasco L, Segura-Salvador A, Vargas-Arzola J, Hernández-Osorio LA, Torres-Aguilar H, Bernardino-Hernández HU. Insecticidal and Repellent Activity of Plant Powders on the Weevil (Sitophilus zeamais) in Stored Corn Grains in a Rural Community of Oaxaca, Mexico. Insects. 2025; 16(3):329. https://doi.org/10.3390/insects16030329
Chicago/Turabian StylePeña-Flores, Citlaly, Arturo Zapién-Martínez, Gabriel Sánchez-Cruz, Leobardo Reyes-Velasco, Aristeo Segura-Salvador, Jaime Vargas-Arzola, Luis Alberto Hernández-Osorio, Honorio Torres-Aguilar, and Héctor Ulises Bernardino-Hernández. 2025. "Insecticidal and Repellent Activity of Plant Powders on the Weevil (Sitophilus zeamais) in Stored Corn Grains in a Rural Community of Oaxaca, Mexico" Insects 16, no. 3: 329. https://doi.org/10.3390/insects16030329
APA StylePeña-Flores, C., Zapién-Martínez, A., Sánchez-Cruz, G., Reyes-Velasco, L., Segura-Salvador, A., Vargas-Arzola, J., Hernández-Osorio, L. A., Torres-Aguilar, H., & Bernardino-Hernández, H. U. (2025). Insecticidal and Repellent Activity of Plant Powders on the Weevil (Sitophilus zeamais) in Stored Corn Grains in a Rural Community of Oaxaca, Mexico. Insects, 16(3), 329. https://doi.org/10.3390/insects16030329







