Reproductive Success of Trichogramma ostriniae over Trichogramma dendrolimi in Multi-Generational Rearing on Corn Borer Eggs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasitoids
2.2. Host
Asian Corn Borer (ACB), Ostrinia furnacalis
2.3. Comparative Reproductive Success of Trichogramma Species Across Generations
2.4. Statistical Analysis
3. Results
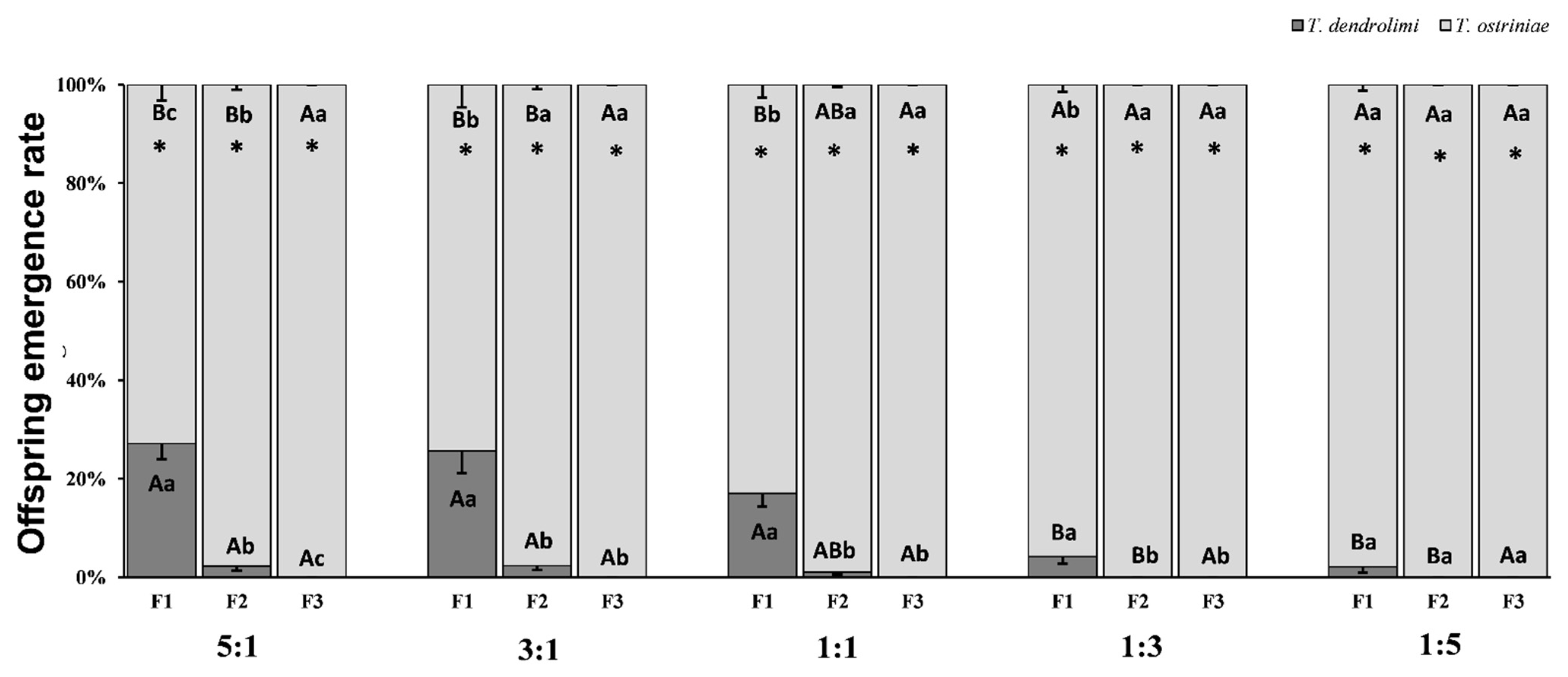
3.1. Effect of Parasitoid Ratio Variations on Offspring Emergence Across Generations in Trichogramma Species
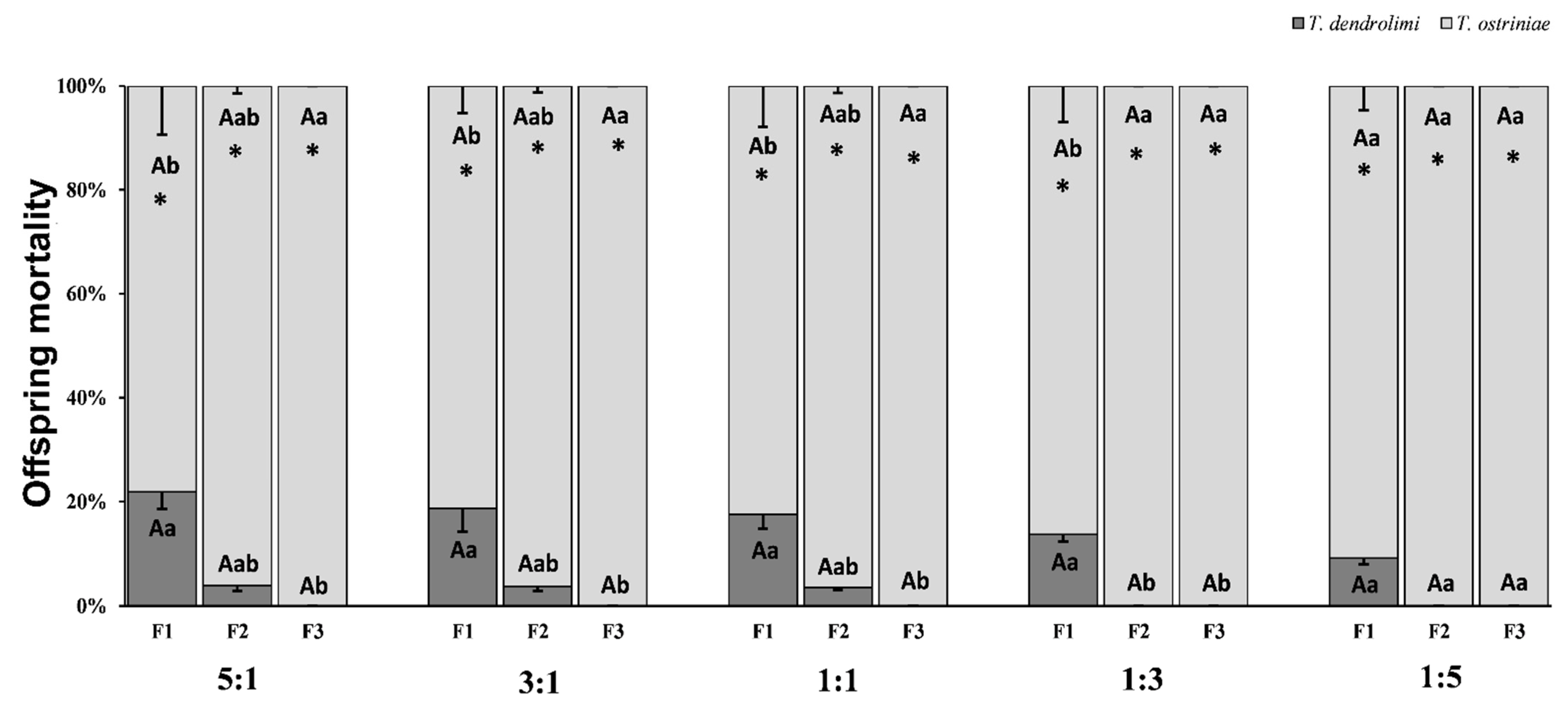
3.2. Effect of Parasitoid Ratio Variations on Offspring Mortality Across Generations in Trichogramma Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nafus, D.M.; Schreiner, I.H. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Trop. Pest Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Afidchao, M.M.; Musters, C.J.M.; de Snoo, G.R. Asian corn borer (ACB) and non-ACB pests in GM corn (Zea mays L.) in the Philippines. Pest Manag. Sci. 2013, 69, 792–801. [Google Scholar] [CrossRef]
- Fang, G.; Zhang, Q.; Cao, Y.; Wang, Y.; Qi, M.; Wu, N.; Qian, L.; Zhu, C.; Huang, Y.; Zhan, S. The draft genome of the Asian corn borer yields insights into ecological adaptation of a devastating maize pest. Insect Biochem. Mol. Biol. 2021, 1, 103638. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Z.; Klein, M.G.; Sheng, C.F.; Li, Y.; Shao, D.X.; Li, Q.Y. Use of pheromone timed insecticide applications integrated with mating disruption or mass trapping against Ostrinia furnacalis (Lepidoptera: Pyralidae) in sweet corn. Environ. Entomol. 2013, 42, 1390–1399. [Google Scholar] [CrossRef]
- Mutuura, A.; Munroe, E. Taxonomy and distribution of the European corn borer and allied species: Genus Ostrinia (Lepidoptera: Pyralidae). Mem. Entomol. Soc. Can. 1970, 102, 1–112. [Google Scholar] [CrossRef]
- CABI. Ostrinia furnacalis (Asian Corn Borer); CABI Compendium: Milpitas, CA, USA, 2022. [Google Scholar]
- Liu, K.Q.; Wang, L.X.; Zhang, T.T.; Bai, S.X.; Wang, K.Q.; Wang, Z.Y.; He, K.L.; Hutchison, W.D. Voltine ecotypes of the Asian corn borer and their response to climate warming. Insects 2021, 12, 232. [Google Scholar] [CrossRef]
- Xie, H.C.; Li, D.S.; Zhang, H.G.; Mason, C.E.; Wang, Z.Y.; Lu, X.; Cai, W.Z.; He, K.L. Seasonal and geographical variation in diapause and cold hardiness of the Asian corn borer, Ostrinia furnacalis. Insect Sci. 2015, 22, 578–586. [Google Scholar] [CrossRef]
- Guo, J.; He, K.; Meng, Y.; Hellmich, R.L.; Chen, S.; Lopez, M.D.; Lauter, N.; Wang, Z. Asian corn borer damage is affected by rind penetration strength of corn stalks in a spatiotemporally dependent manner. Plant Direct. 2022, 6, e381. [Google Scholar] [CrossRef]
- Lastushkina, E.; Telichko, O.; Syrmolot, O.; Belova, T. Using insecticides for the protection of maize plants against the Asian corn borer. In BIO Web of Conferences, Proceedings of the II International Conference on Current Issues of Breeding, Technology and Processing of Agricultural Crops, and Environment (CIBTA-II-2023), Uzbekistan, Russia, 3–5 July 2023; EDP Sciences: Les Ulis, France, 2023; Volume 71, p. 01101. [Google Scholar]
- Zhou, D.R.; Wang, Y.S.; Li, W.D. Studies on the identification of the dominant corn borer species in China. Acta. Phytophyl. Sin. 1988, 15, 145–152. [Google Scholar]
- Zhenying, W.; Xin, L.; Kanglai, H.; Darong, Z. Review of history, present situation and prospect of the Asian maize borer research in China. J. Shenyang Agric. Univ. 2000, 31, 402–412. [Google Scholar]
- Wojciechowska, M.; Stepnowski, P.; Gołębiowski, M. The use of insecticides to control insect pests. Invertebr. Surviv. J. 2016, 13, 210–220. [Google Scholar]
- Tai, H.; Zhang, F.; Xiao, C.; Tang, R.; Liu, Z.; Bai, S.; Wang, Z. Toxicity of chemical pesticides commonly used in maize to Trichogramma ostriniae (Hymenoptera: Trichogrammatidae), an egg parasitoid of Asian corn borer. Ecotoxicol. Environ. Saf. 2022, 241, 113802. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Ann. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.K.; Mohanraj, P.; Lakshmi, B.L. Chapter 5—Trichogrammatids. In Ecofriendly Pest Management for Food Security; Omkar, I., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 139–181. [Google Scholar]
- Wang, Z.Y.; He, K.L.; Zhang, F.; Lu, X.; Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Cont. 2014, 68, 136–144. [Google Scholar] [CrossRef]
- Parra, J.R.P. Mass Rearing of Natural Enemies. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2301–2305. [Google Scholar]
- Iqbal, A.; Chen, Y.M.; Hou, Y.Y.; Ruan, C.C.; Desneux, N.; Khan, M.Q.; Zang, L.S. Rearing Trichogramma ostriniae on the factitious host Antheraea pernyi via multiparasitism with Trichogramma chilonis facilitates enhanced biocontrol potential against Ostrinia furnacalis. Biol. Cont. 2021, 156, 104567. [Google Scholar] [CrossRef]
- Ghaemmaghami, E.; Fathipour, Y.; Bagheri, A.; Talebi, A.A.; Reddy, G.V. Changes in functional and numerical responses of the parasitoid wasp Trichogramma brassicae (Hymenoptera: Trichogrammatidae) over 45 generations of rearing on Ephestia kuehniella. Ann. Entomol. Soc. Am. 2022, 115, 326–335. [Google Scholar] [CrossRef]
- Tonğa, A.; Erkek, M.; Ali, J.; Fathipour, Y.; Özder, N. A comparative approach for life history and functional response demonstrates similar survival strategies for Trichogramma evanescens and T. pintoi. Pest Manag. Sci. 2024, 80, 5630–5639. [Google Scholar] [CrossRef]
- Badran, F.; Fathipour, Y.; Bagheri, A.; Attaran, M.; Reddy, G.V. Generation-dependent functional and numerical responses of a naturally fungus-infected colony of Habrobracon hebetor (Hymenoptera: Braconidae) reared on Ephestia kuehniella (Lepidoptera: Pyralidae) in Iran. J. Econ. Entomol. 2021, 114, 62–71. [Google Scholar] [CrossRef]
- Iqbal, A.; Hou, Y.Y.; Chen, Y.M.; Ali, A.; Monticelli, L.S.; Desneux, N.; Zang, L.S. Impact of Trichogramma parasitoid age on the outcome of multiparasitism in the factitious host eggs of Chinese oak silkworm, Antheraea pernyi. J. Pest Sci. 2020, 93, 1347–1357. [Google Scholar] [CrossRef]
- Wang, Z.; He, K.; Yan, S. Large-scale augmentative biological control of Asian corn borer using Trichogramma in China: A success story. In Proceedings of the Second International Symposium on Biological Control of Arthropods, Davos, Switzerland, 12–16 September 2005; pp. 487–494. [Google Scholar]
- Yuan, X.; Li, D.; Deng, W. Research on the Reproduction of Trichogramma chilonis Based on Samia cynthia ricini Eggs: Temperature, Functional Response and Proportional Effect. Insects 2024, 15, 963. [Google Scholar] [CrossRef]
- Li, T.H.; Tian, C.Y.; Zang, L.S.; Hou, Y.Y.; Ruan, C.C.; Yang, X.; Monticelli, L.; Desneux, N. Multiparasitism with Trichogramma dendrolimi on egg of Chinese oak silkworm, Antheraea pernyi, enhances emergence of Trichogramma ostriniae. J. Pest Sci. 2019, 92, 707–713. [Google Scholar] [CrossRef]
- Pinto, J.D. Novel Taxa of Trichogramma from the New World Tropics and Australia (Hymenoptera: Trichogrammatidae). J. N. Y. Entomol. Soc. 1992, 100, 621–633. [Google Scholar]
- Stouthamer, R.; Hu, J.; van Kan, F.J.; Platner, G.R.; Pinto, J.D. The utility of internally transcribed spacer 2 DNA sequences of the nuclear ribosomal gene for distinguishing sibling species of Trichogramma. BioControl 1999, 43, 421–440. [Google Scholar] [CrossRef]
- Myint, Y.Y.; Bai, S.; Zhang, T.; Babendreier, D.; He, K.; Wang, Z. Molecular and morphological identification of Trichogramma (Hymenoptera: Trichogrammatidae) species from Asian corn borer (Lepidoptera: Crambidae) in Myanmar. J. Econ. Entomol. 2021, 114, 40–49. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Sindhura, K.A.; Radhika, D.H.; Damodaram, K.J. Parasitoids as biocontrol agents in India. Curr. Opin. Insect Sci. 2024, 12, 101282. [Google Scholar] [CrossRef]
- Murali-Baskaran, R.K.; Sharma, K.C.; Sridhar, J.; Jain, L.; Kumar, J. Multiple releases of Trichogramma japonicum Ashmead for biocontrol of rice yellow stem borer, Scirpophaga incertulas (Walker). Crop Prot. 2021, 141, 105471. [Google Scholar] [CrossRef]
- Abdel Halim, E.M.; El-Mandarawy, M.R.; Naroz, M.H.; Ahmed, S.S. Studies of certain parameters affecting two parasitoid species, Trichogramma evanescens Westwood and Trichogrammatoidea bactrae Nagaraja (Hymenoptera: Trichogrammatidae), on egg host, Sitotroga cerealella (Olivier) (Gelechiidae: Lepidoptera). Egypt. J. Biol. Pest Con. 2024, 34, 35. [Google Scholar] [CrossRef]
- Sigsgaard, L.; Herz, A.; Korsgaard, M.; Wührer, B. Mass release of Trichogramma evanescens and T. cacoeciae can reduce damage by the apple codling moth Cydia pomonella in organic orchards under pheromone disruption. Insects 2017, 8, 41. [Google Scholar] [CrossRef]
- Tang, L.D.; Sun, J.W.; Dai, P.; Mu, M.Y.; Nkunika, P.O.; Desneux, N.; Zang, L.S. Performance of two dominant trichogrammatid species of fall armyworm from China and Africa under contrasted temperature and humidity regimes. Biol. Control 2023, 179, 105179. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.Y.; Benelli, G.; Desneux, N.; Ali, A.; Zang, L.S. Trichogramma ostriniae is more effective than Trichogramma dendrolimi as a biocontrol agent of the Asian corn borer, Ostrinia furnacalis. Insects 2022, 13, 70. [Google Scholar] [CrossRef]
- Myint, Y.Y.; Bai, S.; Zhang, T.; Babendreier, D.; He, K.; Wang, Z. Ovipositional preference of Trichogramma dendrolimi and Trichogramma ostriniae strains from Myanmar on different host egg ages of Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Biocontrol Sci. Technol. 2022, 32, 700–714. [Google Scholar] [CrossRef]
- Lobdell, C.E.; Yong, T.H.; Hoffmann, M.P. Host color preferences and short-range searching behavior of the egg parasitoid Trichogramma ostriniae. Entomol. Exp. Appl. 2005, 116, 127–134. [Google Scholar] [CrossRef]
- Marchioro, C.A.; Krechemer, F.S.; Foerster, L.A. Assessing the total mortality caused by two species of Trichogramma on its natural host Plutella xylostella (L.) at different temperatures. Neotrop. Entomol. 2015, 44, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Cabello, T.; Gámez, M.; Torres, A.; Garay, J. Possible effects of inter-specific competition on the coexistence of two parasitoid species: Trichogramma brassicae Bezdenko and Chelonus oculator (F.) (Hymenoptera: Trichogrammatidae, Braconidae). Comm. Ecol. 2011, 12, 78–88. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Zhou, S.W.; Yi, H.M.; Sun, Y.; Zhang, J.L.; Chen, G.H.; Du, L.Y.; Zhang, X.M. Assessing parasitism and suitability of three Trichogramma parasitoids Tuta (= Phthorimaea) absoluta (Meyrick) based on parasitoid and host ages. Crop Prot. 2025, 190, 107099. [Google Scholar] [CrossRef]
- Mashhadi, J.M. Demography and life history of the egg parasitoid, Trichogramma brassicae, on two moths Anagasta kuehniella and Plodia interpunctella in the laboratory. J. Insect Sci. 2009, 9, 51. [Google Scholar]
- Pinto, J.R.; Fernandes, O.A.; Higley, L.G.; Peterson, R.K. Do patterns of insect mortality in temperate and tropical zones have broader implications for insect ecology and pest management? PeerJ 2022, 25, e13340. [Google Scholar] [CrossRef]
- Carey, J.R.; Liedo, P. Mortality dynamics of insects: General principles derived from aging research on the mediterranean fruit fly (Diptera: Tephritidae). Am. Entomol. 1999, 45, 49–55. [Google Scholar] [CrossRef][Green Version]
| Parameter | Source | df | Trichogramma Species | |
|---|---|---|---|---|
| F-Value | p-Value | |||
| Offspring emergence rate (%) | Parasitoid generations | 2 | 1101.536 | <0.0001 |
| Parasitoid ratios | 4 | 603.500 | <0.0001 | |
| Parasitoid generations × Parasitoid ratios | 8 | 475.290 | <0.0001 | |
| Error | 135 | |||
| Parameter | Source | df | Trichogramma Species | |
|---|---|---|---|---|
| F-Value | p-Value | |||
| Offspring mortality (%) | Parasitoid generations | 2 | 25.609 | <0.0001 |
| Parasitoid ratios | 4 | 1.047 | 0.3855 | |
| Parasitoid generations × Parasitoid ratios | 8 | 0.424 | 0.9050 | |
| Error | 135 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Iqbal, A.; Ahmed, K.S.; Zhou, Y.-Y.; Zhang, C. Reproductive Success of Trichogramma ostriniae over Trichogramma dendrolimi in Multi-Generational Rearing on Corn Borer Eggs. Insects 2025, 16, 297. https://doi.org/10.3390/insects16030297
Wang Y, Iqbal A, Ahmed KS, Zhou Y-Y, Zhang C. Reproductive Success of Trichogramma ostriniae over Trichogramma dendrolimi in Multi-Generational Rearing on Corn Borer Eggs. Insects. 2025; 16(3):297. https://doi.org/10.3390/insects16030297
Chicago/Turabian StyleWang, Yu, Asim Iqbal, Kanwer Shahzad Ahmed, Yuan-Yuan Zhou, and Chen Zhang. 2025. "Reproductive Success of Trichogramma ostriniae over Trichogramma dendrolimi in Multi-Generational Rearing on Corn Borer Eggs" Insects 16, no. 3: 297. https://doi.org/10.3390/insects16030297
APA StyleWang, Y., Iqbal, A., Ahmed, K. S., Zhou, Y.-Y., & Zhang, C. (2025). Reproductive Success of Trichogramma ostriniae over Trichogramma dendrolimi in Multi-Generational Rearing on Corn Borer Eggs. Insects, 16(3), 297. https://doi.org/10.3390/insects16030297






