Impact of Polystyrene Micro- and Nanoplastics on the Biological Traits of the Japanese Carpenter Ant, Camponotus japonicus Mayr (Hymenoptera: Formicidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Housing of C. japonicus
2.2. Preparation of PS-M/NPs Food Media
2.3. Effect of PS-M/NPs on Foraging Behavior of Worker Ants
2.4. Effect of PS-M/NPs on Digging Ability of Worker Ants
2.5. Effect of PS-M/NPs on Body Weight and Survival of Worker Ants
2.6. Data Analysis
3. Results
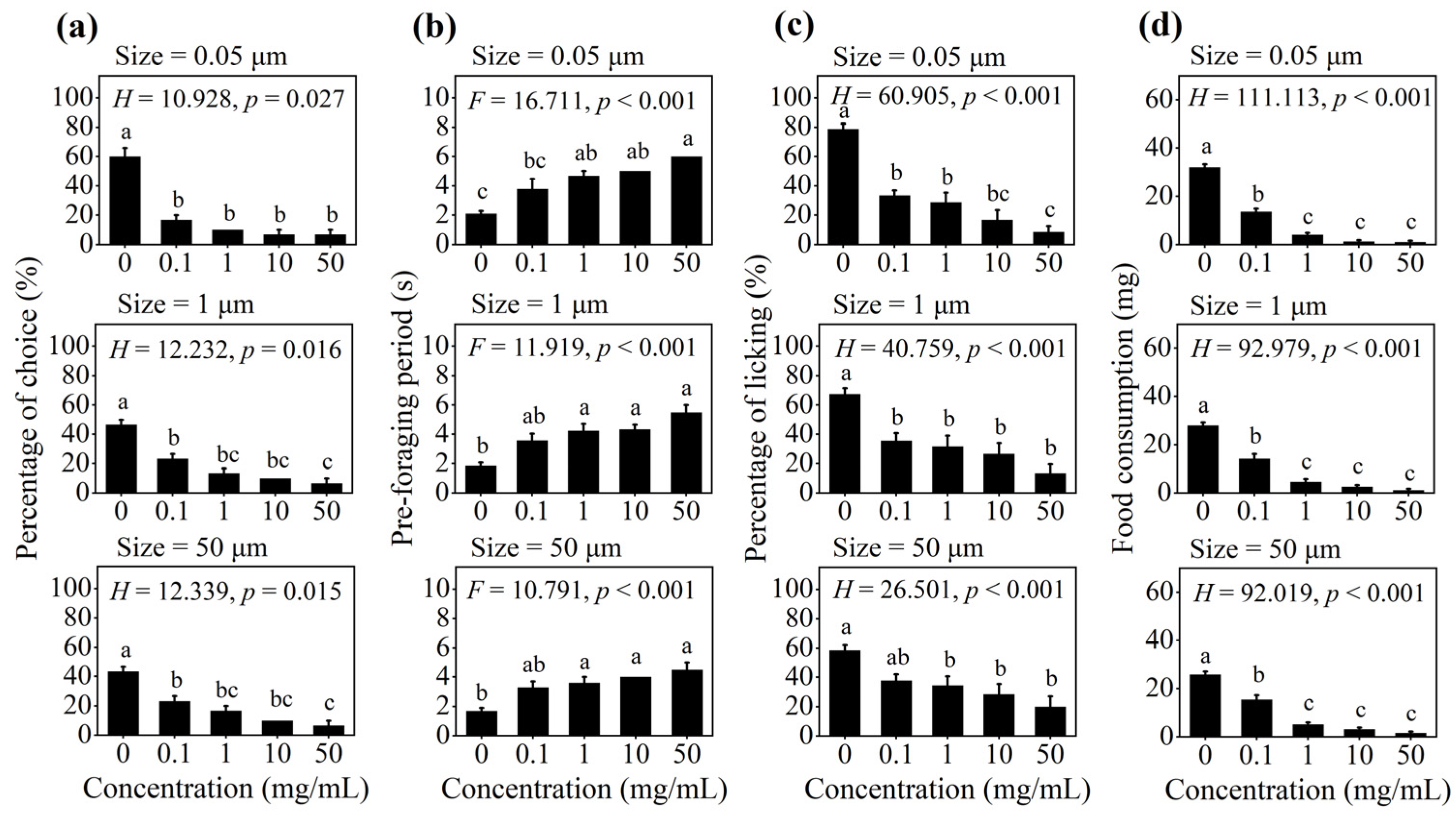
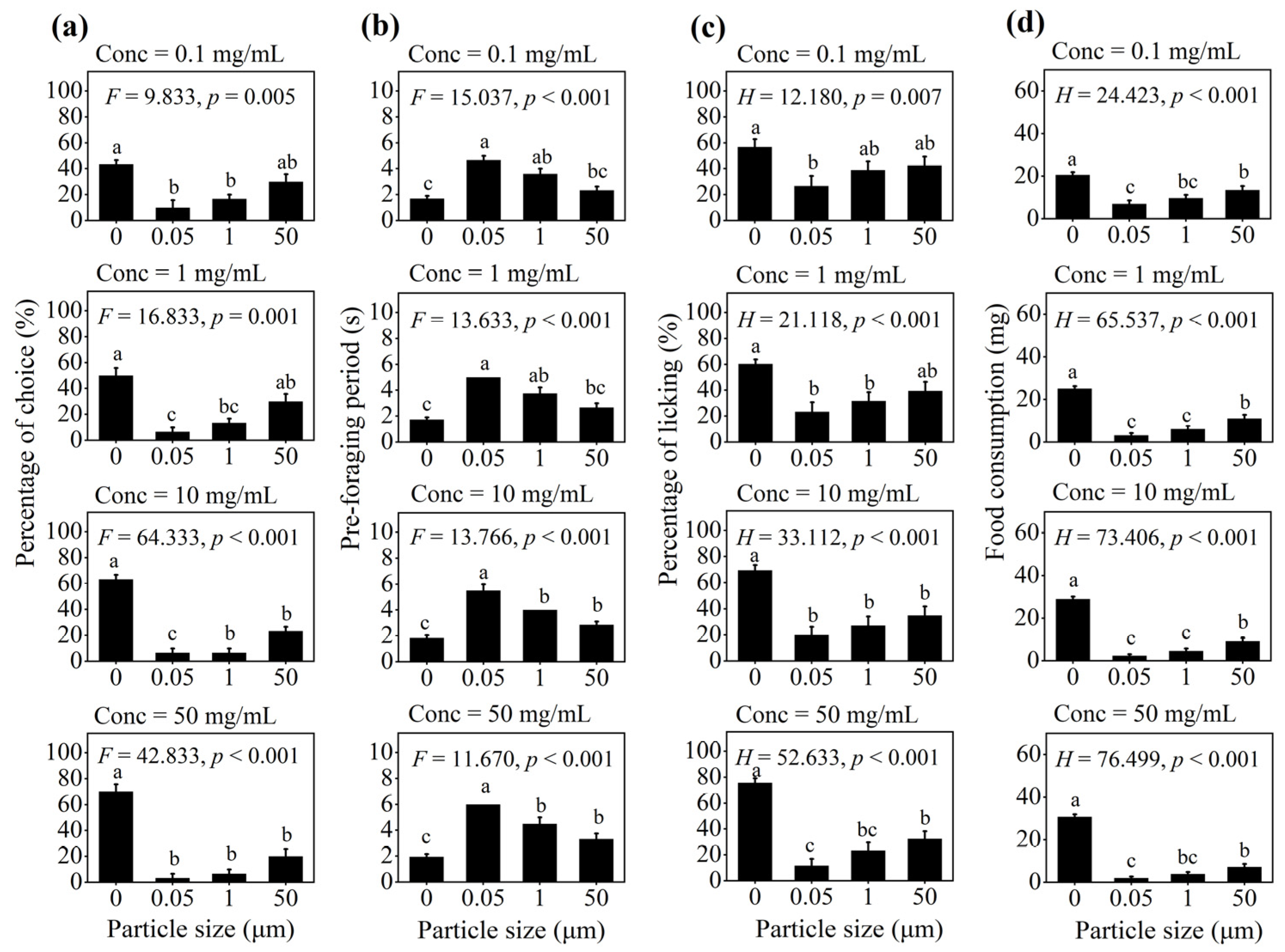
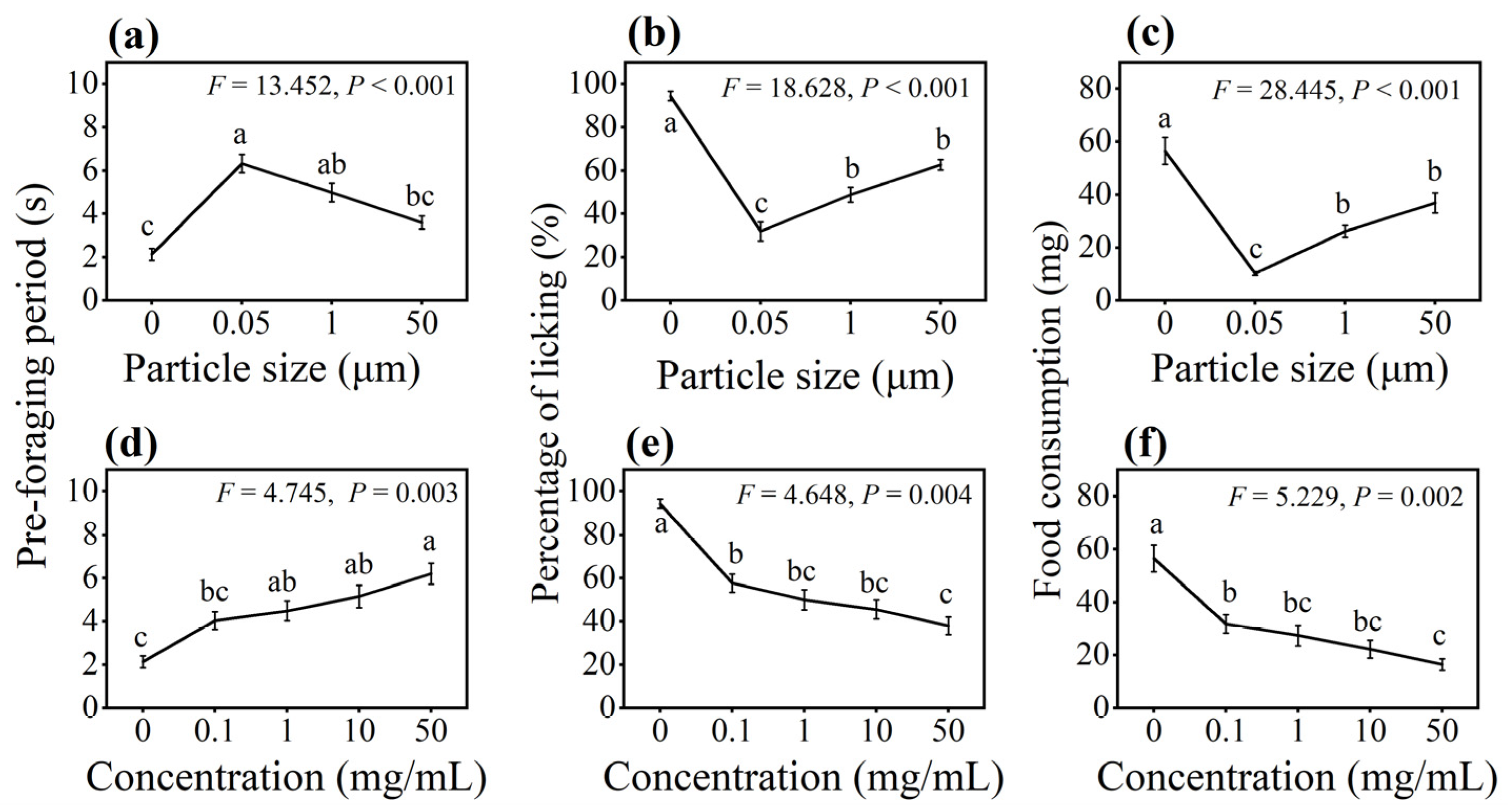
3.1. Effect of PS-M/NPs on Foraging Behavior in Multiple-Choice Trial
3.2. Effect of PS-M/NPs on Foraging Behavior in No-Choice Trial
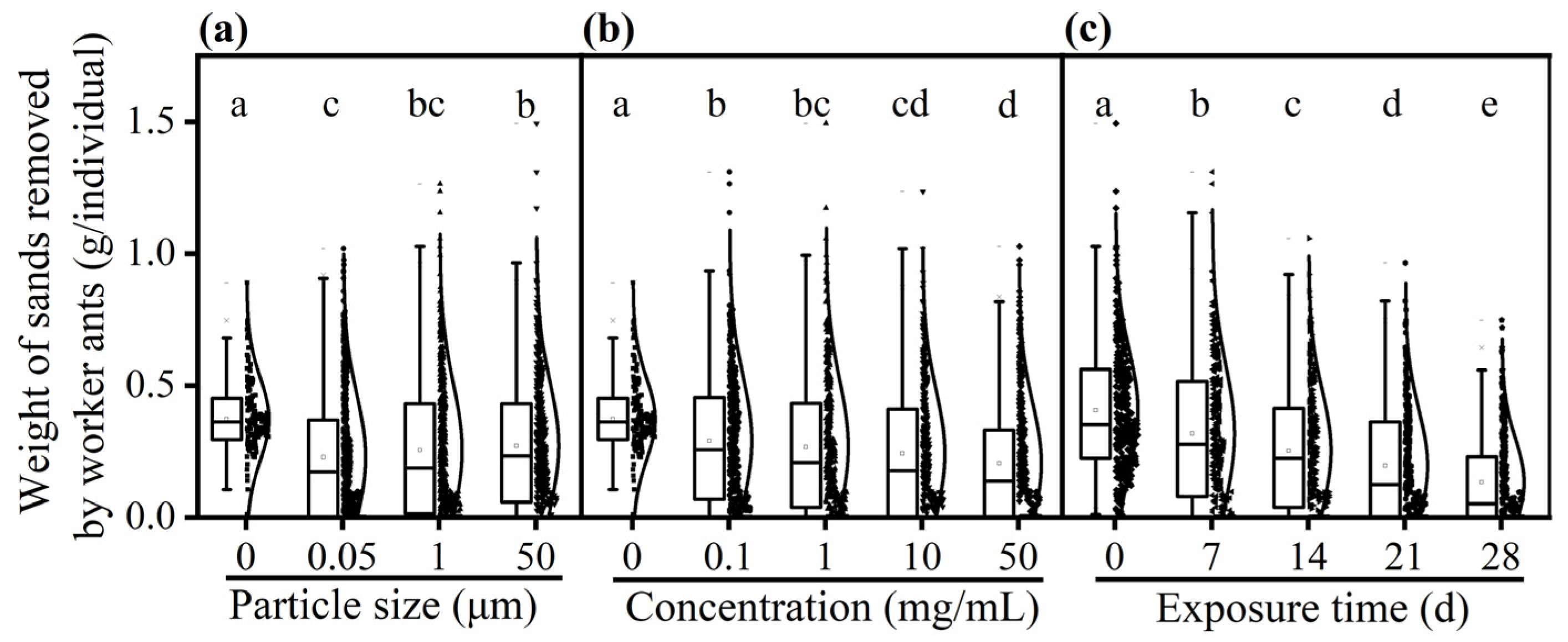
3.3. Effect of PS-M/NPs on Digging Ability of Worker Ants
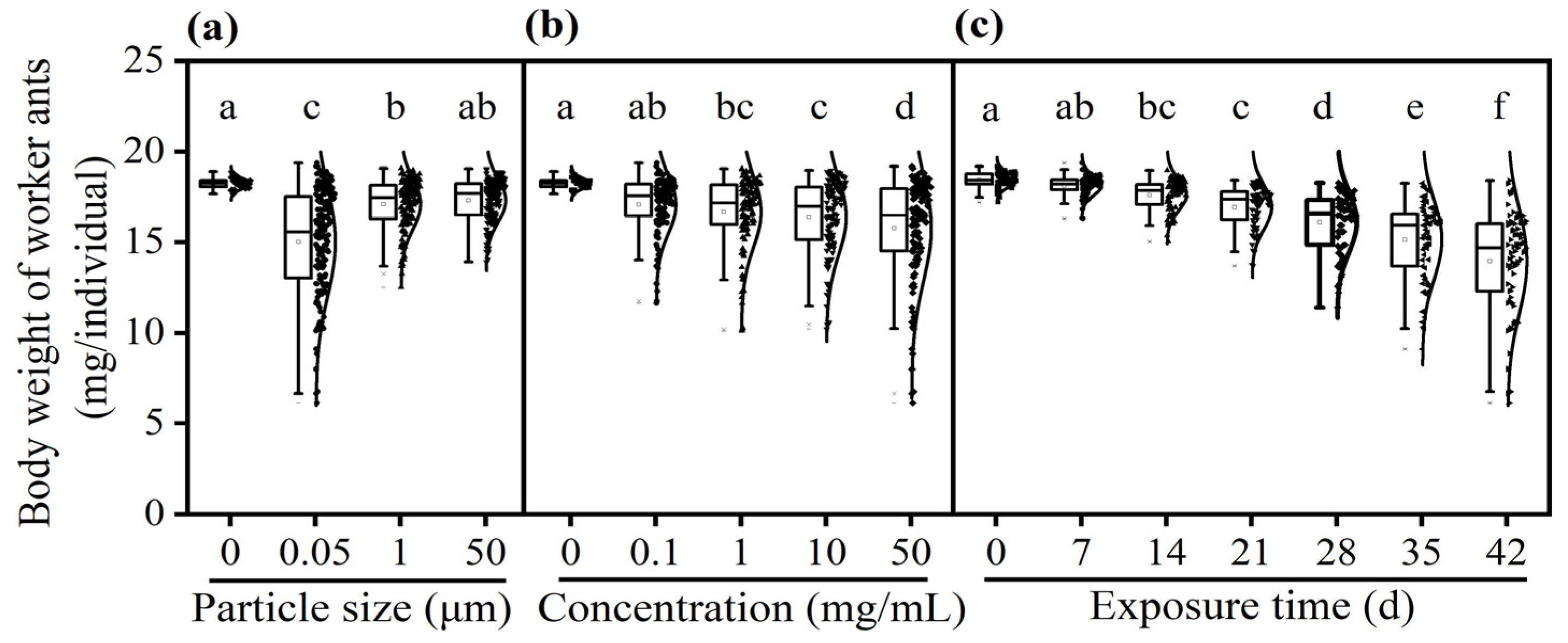
3.4. Effect of PS-M/NPs on Body Weight of Worker Ants
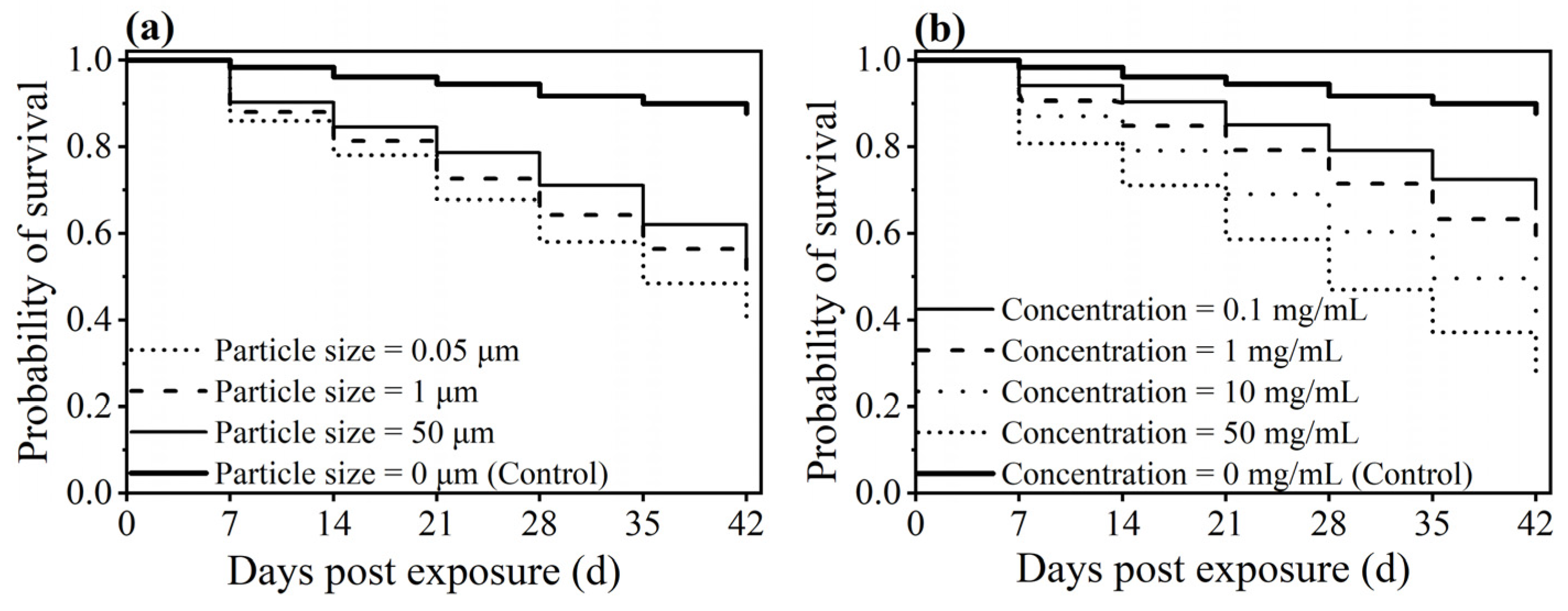
3.5. Effect of PS-M/NPs on the Survival of Worker Ants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Bayo, F.; Wyckhuys, K.A. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Eggleton, P. The state of the world’s insects. Annu. Rev. Environ. Resour. 2020, 45, 61–82. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.A.; Ansari, M.J.; Naggar, Y.A.; Hassan, S.; Nighat, S.; Zehra, S.B.; Rashid, R.; Hassan, M.; Hussain, B. Causes and Reasons of Insect Decline and the Way Forward. In Global Decline of Insects; El-Shafie, H.A.F., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef]
- Balzani, P.; Galeotti, G.; Scheggi, S.; Masoni, A.; Santini, G.; Baracchi, D. Acute and chronic ingestion of polyethylene (PE) microplastics has mild effects on honey bee health and cognition. Environ. Pollut. 2022, 305, 119318. [Google Scholar] [CrossRef]
- Buteler, M.; Alma, A.M.; Stadler, T.; Gingold, A.C.; Manattini, M.C.; Lozada, M. Acute toxicity of microplastic fibers to honeybees and effects on foraging behavior. Sci. Total Environ. 2022, 822, 153320. [Google Scholar] [CrossRef]
- Shen, J.; Liang, B.Y.; Jin, H. The impact of microplastics on insect physiology and the indication of hormesis. Trac Trend. Anal. Chem. 2023, 165, 117130. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, Y.; Craig, N.J.; He, W.; Su, L. Interactions between microplastics and insects in terrestrial ecosystems-A systematic review and meta-analysis. J. Hazard. Mater. 2024, 462, 132783. [Google Scholar] [CrossRef]
- Pasquini, E.; Ferrante, F.; Passaponti, L.; Pavone, F.S.; Costantini, I.; Baracchi, D. Microplastics reach the brain and interfere with honey bee cognition. Sci. Total Environ. 2024, 912, 169362. [Google Scholar] [CrossRef]
- Sheng, D.; Jing, S.; He, X.; Klein, A.M.; Köhler, H.R.; Wanger, T.C. Plastic pollution in agricultural landscapes: An overlooked threat to pollination, biocontrol and food security. Nat. Commun. 2024, 15, 8413. [Google Scholar] [CrossRef]
- Kumar, M.; Chen, H.Y.; Sarsaiya, S.; Qin, S.Y.; Liu, H.M.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.Q.; Bolan, N.S.; et al. Current research trends on micro-and nano-plastics as an emerging threat to global environment: A review. J. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef]
- Rose, P.K.; Yadav, S.; Kataria, N.; Khoo, K.S. Microplastics and nanoplastics in the terrestrial food chain: Uptake, translocation, trophic transfer, ecotoxicology, and human health risk. Trac Trend. Anal. Chem. 2023, 167, 117249. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, L.; Cao, T.; Chen, J.; Lv, M.; Wei, S.; Lu, S.; Tian, X. Microplastics are transferred by soil fauna and regulate soil function as material carriers. Sci. Total Environ. 2023, 857, 159690. [Google Scholar] [CrossRef] [PubMed]
- Torreggiani, G.; Manfrin, C.; Giglio, A.; Dissegna, A.; Chiandetti, C.; Giotta, P.; Renzi, M.; Anselmi, S.; Bentivoglio, T.; Babczyńska, A.; et al. The effect of tyre and road wear particles on the terrestrial Isopod Armadillidium pallasii. Biomolecules 2024, 14, 1640. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier-Hot, V.; Salin, K.; Devers, S.; Tasiemski, A.; Schaffner, P.; Boulay, R.; Billiard, S.; Lenoir, A. Impact of ecological doses of the most widespread phthalate on a terrestrial species, the ant Lasius niger. Environ. Res. 2014, 131, 104–110. [Google Scholar] [CrossRef]
- Richard, C.M.; Dejoie, E.; Wiegand, C.; Gouesbet, G.; Colinet, H.; Balzani, P.; Siaussat, D.; Renault, D. Plastic pollution in terrestrial ecosystems: Current knowledge on impacts of micro and nano fragments on invertebrates. J. Hazard. Mater. 2024, 477, 135299. [Google Scholar] [CrossRef]
- Al-Jaibachi, R.; Cuthbert, R.N.; Callaghan, A. Examining effects of ontogenic microplastic transference on Culex mosquito mortality and adult weight. Sci. Total Environ. 2019, 651, 871–876. [Google Scholar] [CrossRef]
- El Kholy, S.; Al Naggar, Y. Exposure to polystyrene microplastic beads causes sex-specific toxic effects in the model insect Drosophila melanogaster. Sci. Rep. 2023, 13, 204. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Bakr, N.R.; Abouzid, M.; Shedid, E.S.; Giesy, J.P.; Khalifa, S.A.; El-Seedi, H.R.; El Wakil, A.; Al Naggar, Y. Nanoparticles-mediated entomotoxicology: Lessons from biologica. Ecotoxicology 2024, 33, 305–324. [Google Scholar] [CrossRef]
- Kolenda, K.; Kuśmierek, N.; Kujawa, K.; Smolis, A.; Wiśniewski, K.; Salata, S.; Maltz, T.K.; Stachowiak, M.; Kadej, M. Bottled & canned–Anthropogenic debris as an understudied ecological trap for small animals. Sci. Total Environ. 2022, 837, 155616. [Google Scholar]
- Luna, Á.; Rausell-Moreno, A.; Vidal-Cordero, J.M. Plastics and insects: Records of ants entangled in synthetic fibres. Ecol. Entomol. 2023, 49, 145–148. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, D.; Chae, Y.; An, Y.J. Dietary uptake, biodistribution, and depuration of microplastics in the freshwater diving beetle Cybister japonicus: Effects on predacious behavior. Environ. Pollut. 2018, 242, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Thormeyer, M.; Tseng, M. No effect of realistic microplastic exposure on growth and development of wild-caught Culex (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 2023, 60, 604–607. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, P.; Zhao, N.; Jiang, S.; Zhu, H.; Jin, H. Microplastics in terrestrial insects, long-horned beetles (Coleoptera: Cerambycidae), from China. Sci. Total Environ. 2023, 888, 164197. [Google Scholar] [CrossRef]
- Tu, Q.H.; Deng, J.H.; Di, M.M.; Lin, X.R.; Chen, Z.Z.; Li, B.; Tian, L.; Zhang, Y.Y. Reproductive toxicity of polystyrene nanoplastics in Drosophila melanogaster under multi-generational exposure. Chemosphere 2023, 330, 138724. [Google Scholar] [CrossRef]
- Urbisz, A.Z.; Małota, K.; Chajec, Ł.; Sawadro, M.K. Size-dependent and sex-specific negative effects of micro-and nano-sized polystyrene particles in the terrestrial invertebrate model Drosophila melanogaster. Micron 2024, 176, 103560. [Google Scholar] [CrossRef]
- Deng, Y.C.; Jiang, X.J.; Zhao, H.X.; Yang, S.; Gao, J.; Wu, Y.Y.; Diao, Q.Y.; Hou, C.S. Microplastic polystyrene ingestion promotes the susceptibility of honeybee to viral infection. Environ. Sci. Technol. 2021, 55, 11680–11692. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Sayes, C.M.; Collom, C.; Ayorinde, T.; Qi, S.; El-Seedi, H.R.; Paxton, R.J.; Wang, K. Chronic exposure to polystyrene microplastic fragments has no effect on honey bee survival, but reduces feeding rate and body weight. Toxics 2023, 11, 100. [Google Scholar] [CrossRef]
- Muhammad, A.; Zhou, X.; He, J.; Zhang, N.; Shen, X.; Sun, C.; Yan, B.; Shao, Y. Toxic effects of acute exposure to polystyrene microplastics and nanoplastics on the model insect, silkworm Bombyx mori. Environ. Pollut. 2021, 285, 117255. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Huang, Z.C.; Zhou, N.H.; Xie, Y.L.; Tang, Y.; Hu, F.L.; Liu, G.X.; Zheng, H.Q. Complete digestion/biodegradation of polystyrene microplastics by greater wax moth (Galleria mellonella) larvae: Direct in vivo evidence, gut microbiota independence, and potential metabolic pathways. J. Hazard. Mater. 2022, 423, 127213. [Google Scholar] [CrossRef]
- Fudlosid, S.; Ritchie, M.W.; Muzzatti, M.J.; Allison, J.E.; Provencher, J.; MacMillan, H.A. Ingestion of microplastic fibers, but not microplastic beads, impacts growth rates in the tropical house cricket Gryllodes sigillatus. Front. Physiol. 2022, 13, 871149. [Google Scholar] [CrossRef] [PubMed]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Offenberg, J. Ants as tools in sustainable agriculture. J. Appl. Ecol. 2015, 52, 1197–1205. [Google Scholar] [CrossRef]
- Natsume, K.; Hayashi, S.; Miyashita, T. Ants are effective pollinators of common buckwheat Fagopyrum esculentum. Agric. Forest Entomol. 2022, 24, 446–452. [Google Scholar] [CrossRef]
- Rillig, M.C.; Bonkowski, M. Microplastic and soil protists: A call for research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ameixa, O.M.; Soares, A.M. Are ecosystem services provided by insects “bugged” by micro (nano) plastics? Trac Trend. Anal. Chem. 2019, 113, 317–320. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.J.; Zhang, L.Y.; Zhu, Y. The transport of microplastics by ants cannot be neglected in the soil ecosystem. Environ. Pollut. 2023, 317, 120796. [Google Scholar] [CrossRef]
- Seidenath, D.; Holzinger, A.; Kemnitz, K.; Langhof, N.; Lücker, D.; Opel, T.; Otti, O.; Feldhaar, H. Individual vs. com-bined short-term effects of soil pollutants on colony founding in a common ant species. Front. Insect Sci. 2021, 1, 761881. [Google Scholar] [CrossRef]
- Le Hen, G.; Masoni, A.; Manuelli, M.; Falsini, S.; Corti, E.; Balzani, P.; Renault, D.; Papini, A.; Santini, G. Ants avoid food contaminated with micro- and nanoplastics. Environ. Pollut. 2024, 360, 124625. [Google Scholar] [CrossRef]
- Shen, S.; Li, W. Phylogenetic relationship and characterization of the complete mitochondrial genome of Camponotus japonicus (Hymenoptera: Formicoidea: Formicidae). Mitochondrial DNA B 2022, 7, 686–688. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Billen, J.; He, H. Morphology and ultrastructure of Dufour’s and venom glands in the ant Camponotus japonicus Mayr (Hymenoptera: Formicidae). Micron 2018, 104, 72–79. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Billen, J. Morphology of the exocrine glands associated with the maxillolabial complex in the ant Camponotus japonicus Mayr, 1866 (Hymenoptera: Formicidae). Insect. Soc. 2021, 68, 59–67. [Google Scholar] [CrossRef]
- Wang, C.; Billen, J.; Wei, C.; He, H. Morphology and ultrastructure of the infrabuccal pocket in Camponotus japonicus Mayr (Hymenoptera: Formicidae). Insect. Soc. 2019, 66, 637–646. [Google Scholar] [CrossRef]
- Goko, H.; Yamanaka, O.; Shiraishi, M.; Nishimori, H. Characteristics of daily foraging activity of Camponotus japonicus via time series analysis. PLoS ONE 2023, 18, e0293455. [Google Scholar] [CrossRef]
- Tang, H.; Zhong, L.; Xu, Y.; Jin, Z.; Pan, Z.; Shen, J. Polypropylene microplastics affect the physiology in Drosophila model. B. Entomol. Res. 2023, 113, 355–360. [Google Scholar] [CrossRef]
- Chen, J. Digging behavior of Solenopsis invicta workers when exposed to contact insecticides. J. Econ. Entomol. 2006, 99, 634–640. [Google Scholar] [CrossRef]
- Parenti, C.C.; Binelli, A.; Caccia, S.; Della Torre, C.; Magni, S.; Pirovano, G.; Casartelli, M. Ingestion and effects of polystyrene nanoparticles in the silkworm Bombyx mori. Chemosphere 2020, 257, 127203. [Google Scholar] [CrossRef]
- Viana, T.A.; Botina, L.L.; Bernardes, R.C.; Barbosa, W.F.; Xavier, T.K.; Lima, M.A.; dos Santos Araújo, R.; Martins, G.F. Ingesting microplastics or nanometals during development harms the tropical pollinator Partamona helleri (Apinae: Meliponini). Sci. Total Environ. 2023, 893, 164790. [Google Scholar] [CrossRef]
- Rondoni, G.; Chierici, E.; Agnelli, A.; Conti, E. Microplastics alter behavioural responses of an insect herbivore to a plant-soil system. Sci. Total Environ. 2021, 787, 147716. [Google Scholar] [CrossRef]
- Cuthbert, R.N.; Al-Jaibachi, R.; Dalu, T.; Dick, J.T.A.; Callaghan, A. The influence of microplastics on trophic interaction strengths and oviposition preferences of dipterans. Sci. Total Environ. 2019, 651, 2420–2423. [Google Scholar] [CrossRef]
- Richter, A.; Economo, E.P. The feeding apparatus of ants: An overview of structure and function. Philos. Trans. R. Soc. B 2023, 378, 20220556. [Google Scholar] [CrossRef]
- Chen, J. Evidence of social facilitation and inhibition in digging behavior of red imported fire ants, Solenopsis invicta. Insect Sci. 2021, 28, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, C.H.; Kim, M.J.; Chung, H. Effects of microplastics and salinity on food waste processing by black soldier fly (Hermetia illucens) larvae. J. Ecol. Environ. 2020, 44, 7. [Google Scholar] [CrossRef]
- Ribeiro-Brasil, D.R.G.; Brasil, L.S.; Veloso, G.K.O.; de Matos, T.P.; de Lima, E.S.; Dias-Silva, K. The impacts of plastics on aquatic insects. Sci. Total Environ. 2022, 813, 152436. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.-F.; Liu, X.-Y.; Feng, H.-S.; Zhang, J.-T.; Liu, X.-P. Impact of Polystyrene Micro- and Nanoplastics on the Biological Traits of the Japanese Carpenter Ant, Camponotus japonicus Mayr (Hymenoptera: Formicidae). Insects 2025, 16, 292. https://doi.org/10.3390/insects16030292
Wei L-F, Liu X-Y, Feng H-S, Zhang J-T, Liu X-P. Impact of Polystyrene Micro- and Nanoplastics on the Biological Traits of the Japanese Carpenter Ant, Camponotus japonicus Mayr (Hymenoptera: Formicidae). Insects. 2025; 16(3):292. https://doi.org/10.3390/insects16030292
Chicago/Turabian StyleWei, Li-Feng, Xin-Ying Liu, Han-Song Feng, Jiang-Tao Zhang, and Xing-Ping Liu. 2025. "Impact of Polystyrene Micro- and Nanoplastics on the Biological Traits of the Japanese Carpenter Ant, Camponotus japonicus Mayr (Hymenoptera: Formicidae)" Insects 16, no. 3: 292. https://doi.org/10.3390/insects16030292
APA StyleWei, L.-F., Liu, X.-Y., Feng, H.-S., Zhang, J.-T., & Liu, X.-P. (2025). Impact of Polystyrene Micro- and Nanoplastics on the Biological Traits of the Japanese Carpenter Ant, Camponotus japonicus Mayr (Hymenoptera: Formicidae). Insects, 16(3), 292. https://doi.org/10.3390/insects16030292





