Selection of Sclerodermus pupariae Reference Genes for Quantitative Real-Time PCR
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source
2.2. Sample Collection and Treatment
2.3. RNA Extraction and cDNA Synthesis
2.4. Primer Design and Quantitative Real-Time PCR
2.5. Reference Gene Screening
2.6. Validation of Reference Gene Stability
2.7. Statistical Analyses
3. Results
3.1. Validation of Primer Specificity for Candidate Reference Genes
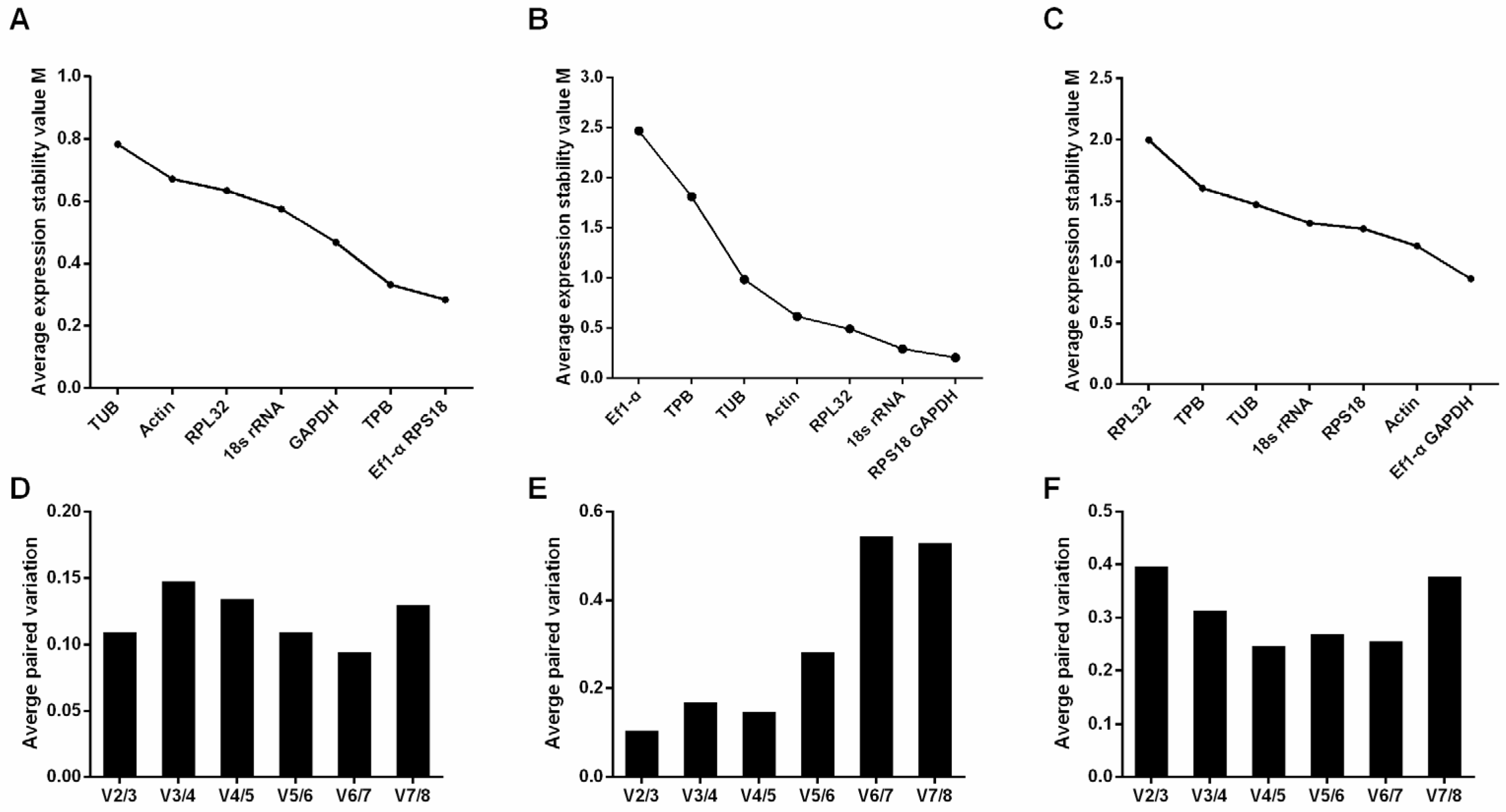
3.2. GeNorm Analysis
3.3. NormFinder Analysis
3.4. BestKeeper Analysis
3.5. Comprehensive Analysis and Ranking of Candidate Reference Genes
3.6. Validation of Reference Gene Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RPS18 | Ribosomal Protein S18 |
| qRT-PCR | Quantitative real-time PCR |
| TUB | Beta-tubulin |
| TPB | TATA-box binding protein |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| 18S rRNA | Ribosomal protein S18 |
| RPL32 | Ribosomal protein L32 |
| EF1-α | Elongation factor 1-α |
| Ct | Cycle threshold |
| InR | Insulin receptor |
| RPS5 | Ribosomal protein S5 |
| TPB-αf | TATA-box binding protein-αf |
| RPS3 | Ribosomal Protein S3 |
| RPL13α | Ribosomal protein L13α |
| Cq | Quantification cycle |
| RP49 | Ribosomal protein 49 |
| RPL13 | Ribosomal protein L32 |
| RPS11 | Ribosomal Protein S11 |
| RPS15 | Ribosomal Protein S15 |
References
- Fukaya, M.; Kiriyama, S.; Yasui, H. Mate-Location Flight of the Red-Necked Longicorn Beetle, Aromia bungii (Coleoptera: Cerambycidae): An Invasive Pest Lethal to Rosaceae Trees. Appl. Entomol. Zool. 2017, 52, 559–565. [Google Scholar] [CrossRef]
- Yu, Q.; Li, S.; Kong, Y.J.; Sun, Z.-X.; Cao, D.-D.; Wei, J.-R. Host Preference and Mortality Caused by the Parasitoid Sclerodermus guani on Different Cerambycid Species. BioControl 2024, 69, 611–621. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, X.Y.; Yao, Y.X.; Gould, J.R.; Cao, L.-M. A new species of Sclerodermus (Hymenoptera: Bethylidae) parasitizing Agrilus planipennis (Coleoptera: Buprestidae) from China, with a key to Chinese species in the genus. Ann. Entomol. Soc. Am. 2012, 105, 619–627. [Google Scholar] [CrossRef]
- Liu, X.; Tian, R.; Liu, L.; Ma, J. Studies on the control of Asias halodendri with Sclerodermus pupariae. Inner Mong. For. Sci. Tech. 2016, 42, 32–34. (In Chinese) [Google Scholar]
- Wu, H.; Wang, X.Y.; Li, M.L.; Yang, Z.Q.; Zeng, F.X.; Wang, H.Y.; Bai, L.; Liu, S.J.; Sun, J. Biological and ecological characteristics of the Sclerodermus pupariae and research on its breeding techniques. Acta Entomol. Sin. 2008, 51, 46–54. [Google Scholar] [CrossRef]
- Gao, S.; Tang, Y.; Wei, K.; Wang, X.; Yang, Z.; Zhang, Y. Relationships between Body Size and Parasitic Fitness and Offspring Performance of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae). PLoS ONE 2016, 11, e0156831. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Z. Study on preservation conditions of artificial propagation of Sclerodermus pupariae. China Plant Prot. Guide 2018, 38, 10–13. (In Chinese) [Google Scholar]
- Singh, K.S.; Cordeiro, E.M.G.; Troczka, B.J.; Pym, A.; Mackisack, J.; Mathers, T.C.; Duarte, A.; Legeai, F.; Robin, S.; Bielza, P.; et al. Global Patterns in Genomic Diversity Underpinning the Evolution of Insecticide Resistance in the Aphid Crop Pest Myzus persicae. Commun. Biol. 2021, 4, 847. [Google Scholar] [CrossRef]
- Asgari, S.; Rivers, D.B. Venom Proteins from Endoparasitoid Wasps and Their Role in Host-Parasite Interactions. Annu. Rev. Entomol. 2011, 56, 313–335. [Google Scholar] [CrossRef]
- Li, B.; Duan, Y.; Du, Z.; Wang, X.; Liu, S.; Feng, Z.; Tian, L.; Song, F.; Yang, H.; Cai, W.; et al. Natural Selection and Genetic Diversity Maintenance in a Parasitic Wasp during Continuous Biological Control Application. Nat. Commun. 2024, 15, 1379. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, Z.; He, X.; Liang, G. Validation of Reference Genes in Solenopsis invicta in Different Developmental Stages, Castes and Tissues. PLoS ONE 2013, 8, e57718. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.N.; Zhao, R.N.; Fu, D.; Yu, C.; Pan, C.-N.; Zhou, W.; Chen, W.-L. Assessment of Suitable Reference Genes for qRT-PCR Normalization in Eocanthecona furcellata (Wolff). Insects 2022, 13, 773. [Google Scholar] [CrossRef]
- Freitas, F.C.P.; Depintor, T.S.; Agostini, L.T.; Luna-Lucena, D.; Nunes, F.M.F.; Bitondi, M.M.G.; Simões, Z.L.P.; Lourenço, A.P. Evaluation of Reference Genes for Gene Expression Analysis by Real-Time Quantitative PCR (qPCR) in Three Stingless Bee Species (Hymenoptera: Apidae: Meliponini). Sci. Rep. 2019, 9, 17692. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference Genes in Real-Time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, W.; Wang, S.; Wu, Q.; Yang, N.; Yang, X.; Pan, H.; Zhou, X.; Bai, L.; Xu, B.; et al. Reference Gene Selection for qRT-PCR Analysis in the Sweetpotato Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 2013, 8, e53006. [Google Scholar] [CrossRef]
- Jeon, J.H.; Moon, K.; Kim, Y.; Kim, Y.H. Reference Gene Selection for qRT-PCR Analysis of Season- and Tissue-Specific Gene Expression Profiles in the Honey Bee Apis mellifera. Sci. Rep. 2020, 10, 13935. [Google Scholar] [CrossRef]
- Shakeel, M.; Rodriguez, A.; Tahir, U.B.; Jin, F. Gene expression studies of reference genes for quantitative real-time PCR: An overview in insects. Biotechnol. Lett. 2018, 40, 227–236. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A Web-Based Tool for Comprehensively Analyzing and Identifying Reference Genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef]
- Galiveti, C.R.; Rozhdestvensky, T.S.; Brosius, J.; Lehrach, H.; Konthur, Z. Application of Housekeeping npcRNAs for Quantitative Expression Analysis of Human Transcriptome by Real-Time PCR. RNA 2010, 16, 267–273. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper—Excel-Based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, H.J.; Yang, J.P.; Zhang, J.-L.; Shen, Z.-C.; Xu, H.-J.; Ye, Y.-X. Chromosome-level genome assembly of the bethylid ectoparasitoid wasp Sclerodermus sp. ‘alternatusi’. Sci. Data 2024, 11, 438. [Google Scholar] [CrossRef]
- Kang, K.; Zhang, M.; Yue, L.; Chen, W.; Dai, Y.; Lin, K.; Liu, K.; Lv, J.; Guan, Z.; Xiao, X.; et al. Oxalic Acid Binds to Gustatory Receptor Gr23a and Inhibits Feeding in the Brown Planthopper. Cells 2023, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of Housekeeping Genes for Gene Expression Studies in Human Reticulocytes Using Real-Time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Valasek, M.A.; Repa, J.J. The Power of Real-Time PCR. Adv. Physiol. Educ. 2005, 29, 151–159. [Google Scholar] [CrossRef]
- Feuer, R.; Vlaic, S.; Arlt, J.; Sawodny, O.; Dahmen, U.; Zanger, U.M.; Thomas, M. LEMming: A Linear Error Model to Normalize Parallel Quantitative Real-Time PCR (qPCR) Data as an Alternative to Reference Gene-Based Methods. PLoS ONE 2015, 10, e0135852. [Google Scholar] [CrossRef]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-Time RT-PCR Normalization: Strategies and Considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef]
- Bustin, S.A. Absolute Quantification of mRNA Using Real-Time Reverse Transcription Polymerase Chain Reaction Assays. J. Mol. Endocrinol. 2000, 25, 169–193. [Google Scholar] [CrossRef]
- Reim, T.; Thamm, M.; Rolke, D.; Blenau, W.; Scheiner, R. Suitability of Three Common Reference Genes for Quantitative Real-Time PCR in Honey Bees. Apidologie 2013, 44, 342–350. [Google Scholar] [CrossRef]
- Scharlaken, B.; De Graaf, D.C.; Goossens, K.; Brunain, M.; Peelman, L.J.; Jacobs, F.J. Reference Gene Selection for Insect Expression Studies Using Quantitative Real-Time PCR: The Head of the Honeybee, Apis mellifera, After a Bacterial Challenge. J. Insect Sci. 2008, 8, 33. [Google Scholar] [CrossRef]
- Lord, J.C.; Hartzer, K.; Toutges, M.; Oppert, B. Evaluation of Quantitative PCR Reference Genes for Gene Expression Studies in Tribolium castaneum After Fungal Challenge. J. Microbiol. Methods 2010, 80, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Cappelle, K.; de Miranda, J.R.; Smagghe, G.; Meeus, I. Analysis of Reference Gene Stability After Israeli Acute Paralysis Virus Infection in Bumblebees Bombus terrestris. J. Invertebr. Pathol. 2014, 115, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Horgan, F.G.; Arida, A.; Ardestani, G.; Almazan, M.L.P. Positive and Negative Interspecific Interactions Between Coexisting Rice Planthoppers Neutralise the Effects of Elevated Temperatures. Funct. Ecol. 2021, 35, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, H.; Xu, X.; Zhou, S.; Zhou, B.; Li, X.; Xu, H.; Tian, Y.; Wang, Y.; Chu, Y.; et al. The Mushroom Body Development and Learning Ability of Adult Honeybees Are Influenced by Cold Exposure During Their Early Pupal Stage. Front. Physiol. 2023, 14, 1173808. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, Z.; Wang, F.; Zhou, J.; Zhang, Z. Selection of Reliable Reference Genes for Gene Expression Studies in Peach Using Real-Time PCR. BMC Mol. Biol. 2009, 10, 71. [Google Scholar] [CrossRef]
- Daude, M.M.; Ságio, S.A.; Rodrigues, J.N.; Lima, N.M.P.; Lima, A.A.; Sarmento, M.I.; Sarmento, R.A.; Barreto, H.G. Reference Genes for Eucalyptus spp. Under Beauveria bassiana Inoculation and Subsequently Infestation by the Galling Wasp Leptocybe invasa. Sci. Rep. 2024, 14, 2556. [Google Scholar] [CrossRef]
- Cusson, M. The Molecular Biology Toolbox and Its Use in Basic and Applied Insect Science. Bioscience 2008, 58, 691–700. [Google Scholar] [CrossRef]
- De Brito, M.W.D.; De Carvalho, S.S.; Mota, M.B.; Mesquita, R.D. RNA-Seq Validation: Software for Selection of Reference and Variable Candidate Genes for RT-qPCR. BMC Genom. 2024, 25, 697. [Google Scholar] [CrossRef]
- Qin, H.; Dong, K.; Huang, J.; He, S.; Wu, J. Identification of Reference Genes for Gene Expression Analysis at Different Developmental Stages of the Bumblebee Bombus terrestris (Hymenoptera: Apidae). Apidologie 2021, 52, 825–836. [Google Scholar] [CrossRef]
- Matouskova, P. Bombus Terrestris Partial mRNA for Ribosomal Protein L13 (RPL13 Gene). 2009. Available online: https://www.ncbi.nlm.nih.gov/nuccore/FM179871 (accessed on 6 September 2024).
- Lourenço, A.P.; Mackert, A.; Cristino, A.D.S.; Simões, Z.L.P. Validation of Reference Genes for Gene Expression Studies in the Honey Bee, Apis mellifera, by Quantitative Real-Time RT-PCR. Apidologie 2008, 39, 372–385. [Google Scholar] [CrossRef]
- Augustine, N.; Selvapandian, U.; Venkatesan, T.; Srinivasa, N.; Rao, A.M.; Saraswathy, B.P.; Mohan, M. Evaluation of Reference Genes for Expression Studies in the Broad Mite, Polyphagotarsonemus latus (Acari: Tarsonemidae). Appl. Entomol. Zool. 2024, 59, 31–40. [Google Scholar] [CrossRef]
- Sun, W.; Jin, Y.; He, L.; Lu, W.-C.; Li, M. Suitable Reference Gene Selection for Different Strains and Developmental Stages of the Carmine Spider Mite, Tetranychus cinnabarinus, Using Quantitative Real-Time PCR. J. Insect Sci. 2010, 10, 20801. [Google Scholar] [CrossRef]
- Yuan, M.; Lu, Y.; Zhu, X.; Wan, H.; Shakeel, M.; Zhan, S.; Jin, B.-R.; Li, J. Selection and Evaluation of Potential Reference Genes for Gene Expression Analysis in the Brown Planthopper, Nilaparvata lugens (Hemiptera: Delphacidae), Using Reverse-Transcription Quantitative PCR. PLoS ONE 2014, 9, e86503. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pan, H.; Noland, J.; Zhang, D.; Zhang, Z.; Liu, Y.; Zhou, X. Selection of Reference Genes for RT-qPCR Analysis in a Predatory Biological Control Agent, Coleomegilla maculata (Coleoptera: Coccinellidae). Sci. Rep. 2015, 5, 18201. [Google Scholar] [CrossRef]
- Babitzke, P.; Baker, C.S.; Romeo, T. Regulation of translation initiation by RNA binding proteins. Annu. Rev. Microbiol. 2009, 63, 27–44. [Google Scholar] [CrossRef]
- Rugjee, K.N.; Chaudhury, S.R.; Al-Jubran, K.; Ramanathan, P.; Matina, T.; Wen, J.; Brogna, S. Fluorescent protein tagging confirms the presence of ribosomal proteins at Drosophila polytene chromosomes. PeerJ 2013, 1, e15. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Ren, Y.; Gong, C.; Chen, Y.; Ge, X.; Kong, J.; Sun, W.; Du, X. 40S ribosomal protein S18 is a novel maternal peptidoglycan-binding protein that protects embryos of zebrafish from bacterial infections. Dev. Comp. Immunol. 2021, 125, 104212. [Google Scholar] [CrossRef]
- Lü, J.; Chen, S.; Guo, M.; Ye, C.; Qiu, B.; Wu, J.; Yang, C.; Pan, H. Selection and validation of reference genes for RT-qPCR analysis of the ladybird beetle Henosepilachna vigintioctomaculata. Front. Physiol. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Gao, X.K.; Zhang, S.; Luo, J.Y.; Wang, C.Y.; Lü, L.M.; Zhang, L.J.; Zhu, X.Z.; Wang, L.; Cui, J.J. Identification and validation of reference genes for gene expression analysis in Aphidius gifuensis (Hymenoptera: Aphidiidae). PLoS ONE 2017, 12, e0188477. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Kim, Y.H. Evaluation of quantitative real-time PCR reference genes for the investigation of gene expression profiles in honeybee developmental stages. J. Apic. 2021, 36, 251–259. [Google Scholar] [CrossRef]

| Gene | R2 | Efficiency (%) |
|---|---|---|
| EF1-α | 0.9842 | 93 |
| RPS18 | 0.9859 | 101 |
| RPL32 | 0.992 | 91 |
| GAPDH | 0.9985 | 92 |
| 18s rRNA | 0.9922 | 106 |
| TUB | 0.9985 | 93 |
| TPB | 0.9989 | 100 |
| Actin | 0.9829 | 91 |
| Gene | Larva | Pupa | Adult | |||
|---|---|---|---|---|---|---|
| Stability Value | Stability Rank | Stability Value | Stability Rank | Stability Value | Stability Rank | |
| EF1-α | 0.236 | 3 | 2.904 | 7 | 0.299 | 1 |
| RPS18 | 0.214 | 2 | 0.070 | 1 | 0.767 | 3 |
| RPL32 | 0.387 | 6 | 0.611 | 4 | 2.067 | 8 |
| GAPDH | 0.492 | 7 | 0.070 | 1 | 0.698 | 2 |
| 18s rRNA | 0.379 | 5 | 0.113 | 2 | 0.773 | 4 |
| TUB | 0.706 | 8 | 1.189 | 5 | 0.850 | 5 |
| TPB | 0.194 | 1 | 2.669 | 6 | 1.311 | 7 |
| Actin | 0.377 | 4 | 0.263 | 3 | 0.863 | 6 |
| Gene | Larva | Pupa | Adult | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SD | CV | Stability Rank | SD | CV | Stability Rank | SD | CV | Stability Rank | |
| EF1-α | 0.77 | 5.12 | 4 | 2.41 | 13.45 | 8 | 1.43 | 4.95 | 3 |
| RPS18 | 0.72 | 4.51 | 3 | 0.49 | 2.84 | 4 | 1.87 | 7.59 | 5 |
| RPL32 | 0.85 | 3.10 | 5 | 0.35 | 1.30 | 1 | 0.96 | 2.91 | 1 |
| GAPDH | 0.56 | 3.27 | 2 | 0.37 | 2.09 | 2 | 2.11 | 7.39 | 7 |
| 18s rRNA | 0.43 | 3.60 | 1 | 0.42 | 3.47 | 3 | 1.41 | 7.06 | 2 |
| TUB | 0.99 | 5.08 | 8 | 1.76 | 7.99 | 6 | 1.63 | 5.08 | 4 |
| TPB | 0.88 | 3.79 | 6 | 2.36 | 9.73 | 7 | 1.99 | 6.33 | 6 |
| Actin | 0.96 | 3.13 | 7 | 0.82 | 2.71 | 5 | 2.12 | 7.08 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Feng, H.; Zhang, J.; Tang, Y.; Dong, X.; Kang, K. Selection of Sclerodermus pupariae Reference Genes for Quantitative Real-Time PCR. Insects 2025, 16, 268. https://doi.org/10.3390/insects16030268
Zhou T, Feng H, Zhang J, Tang Y, Dong X, Kang K. Selection of Sclerodermus pupariae Reference Genes for Quantitative Real-Time PCR. Insects. 2025; 16(3):268. https://doi.org/10.3390/insects16030268
Chicago/Turabian StyleZhou, Ting, Huahua Feng, Jie Zhang, Yanlong Tang, Xiaoling Dong, and Kui Kang. 2025. "Selection of Sclerodermus pupariae Reference Genes for Quantitative Real-Time PCR" Insects 16, no. 3: 268. https://doi.org/10.3390/insects16030268
APA StyleZhou, T., Feng, H., Zhang, J., Tang, Y., Dong, X., & Kang, K. (2025). Selection of Sclerodermus pupariae Reference Genes for Quantitative Real-Time PCR. Insects, 16(3), 268. https://doi.org/10.3390/insects16030268





