Losses of Foliage to Defoliating Insects Increase with Leaf Damage Diversity Due to the Complementarity Effect
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Plants
2.2. Study Sites
2.3. Sampling
2.4. Measurement of Herbivory
2.5. Classification of Damage Types
2.6. Assessment of Leaf Damage Diversity
2.7. Data Analysis
3. Results
3.1. Classification and Occurrences of Leaf Damage Types
3.2. Differences Among Plant Species in Leaf Damage Types
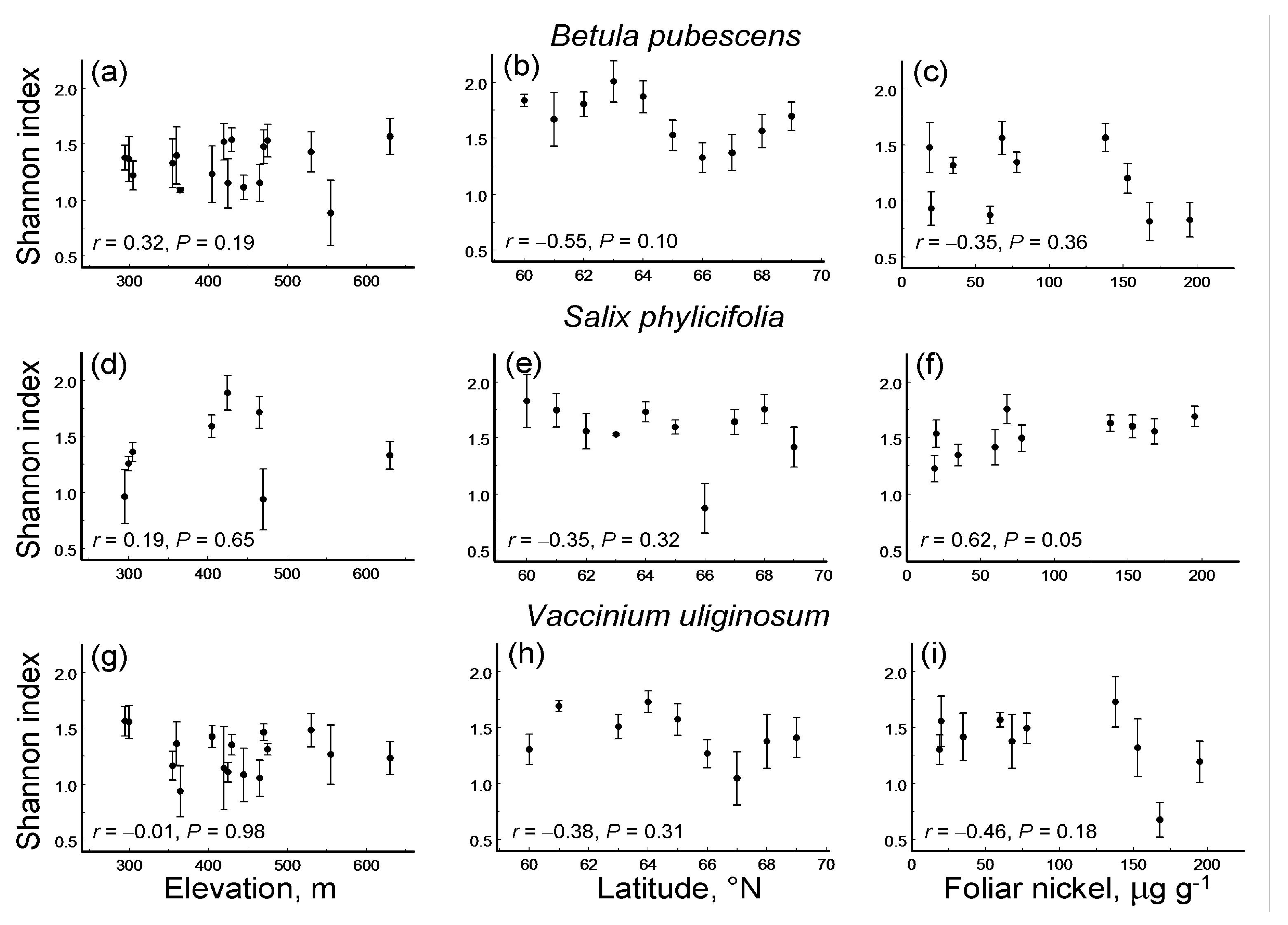
3.3. Leaf Damage Diversity Along Environmental Gradients
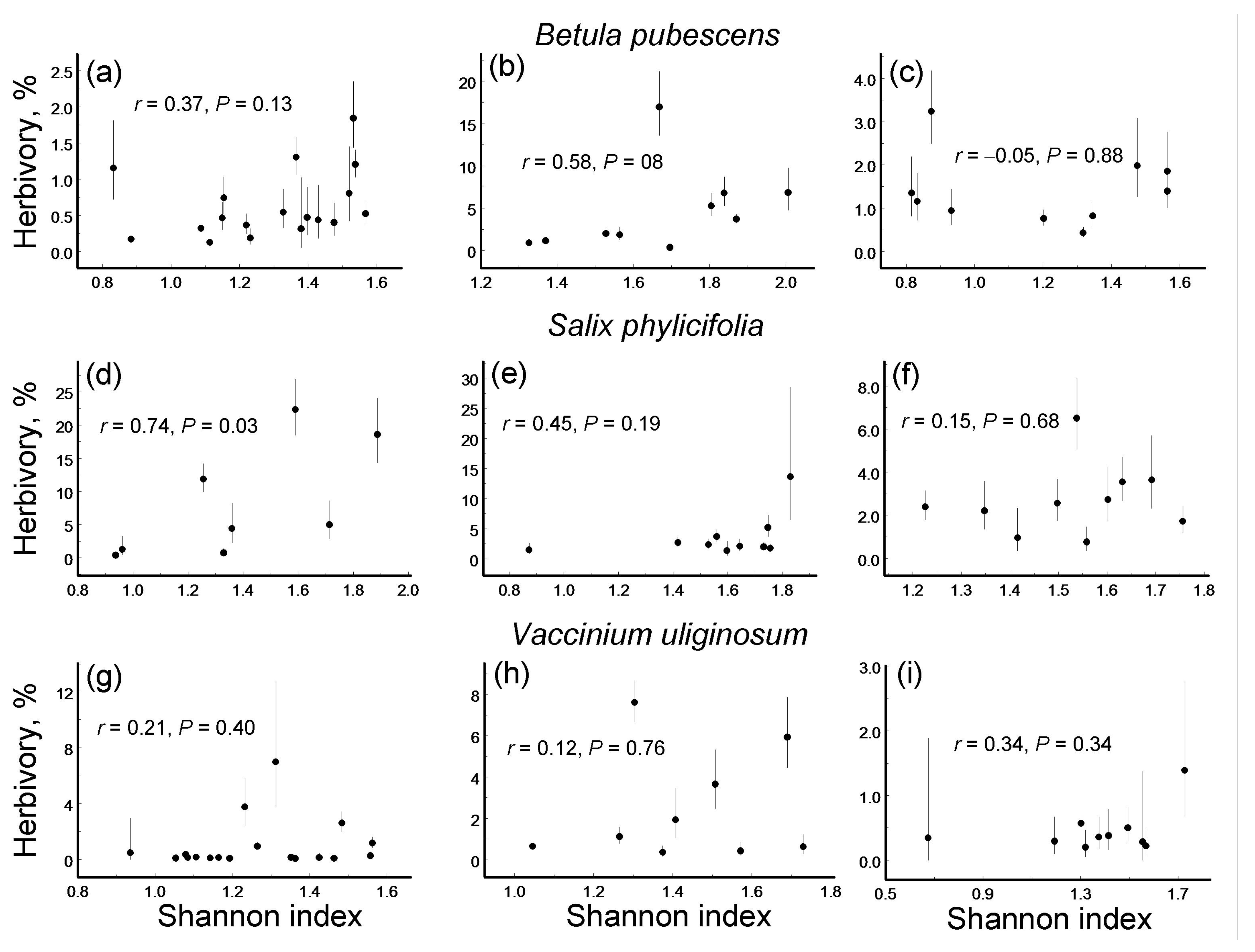
3.4. Leaf Damage Diversity and Herbivory

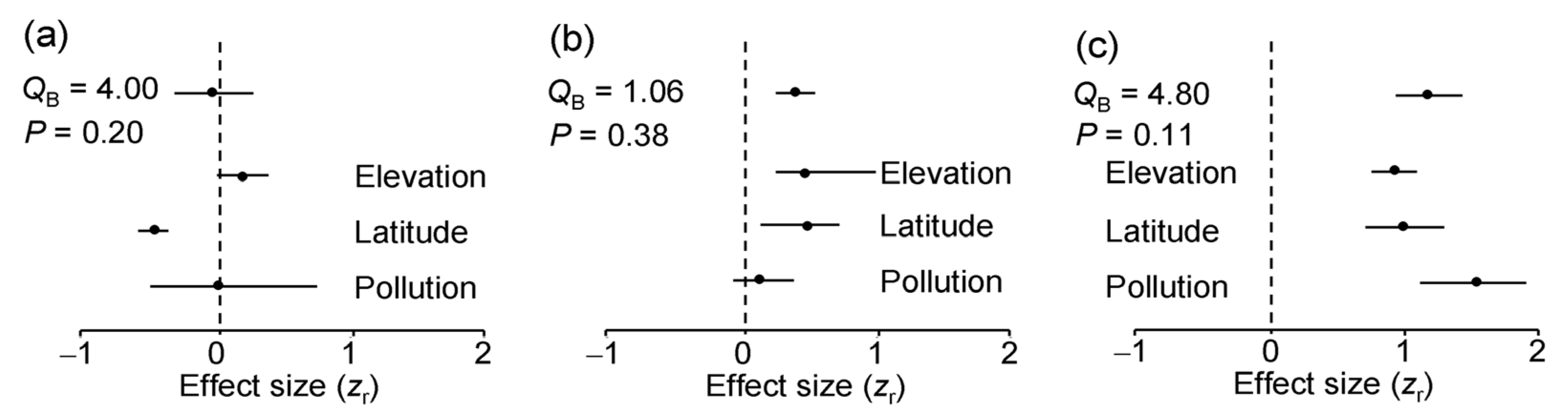
3.5. Number and Evenness of Leaf Damage Types
4. Discussion
4.1. Numbers of Leaf Damage Types and Insect Herbivore Species
4.2. Shelter Feeders: A Promising Group for Studying Leaf Damage Diversity
4.3. Leaf Damage Diversity and Herbivore Diversity Along Environmental Gradients
4.4. Leaf Damage Diversity and Herbivory: Patterns and Mechanisms
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Wu, D.; Xu, C.; Wang, S.; Zhang, L.; Kortsch, S. Why are biodiversity–ecosystem functioning relationships so elusive? Trophic interactions may amplify ecosystem function variability. J. Anim. Ecol. 2023, 92, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Castillo-Monroy, A.P.; Bowker, M.A.; Ochoa-Hueso, R. Species richness effects on ecosystem multifunctionality depend on evenness, composition and spatial pattern. J. Ecol. 2012, 100, 317–330. [Google Scholar] [CrossRef]
- Duffy, J.E.; Richardson, J.P.; Canuel, E.A. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecol. Lett. 2003, 6, 637–645. [Google Scholar] [CrossRef]
- Lawton, J.H.; McNeill, S. Between the devil and the deep blue sea: On the problem of being a herbivore. In Population Dynamics: Symposium of the British Ecological Society; Anderson, R.M., Turner, B.D., Taylor, L.R., Eds.; Blackwell: Oxford, UK, 1979; pp. 223–244. [Google Scholar]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Kambach, S.; Kühn, I.; Castagneyrol, B.; Bruelheide, H. The impact of tree diversity on different aspects of insect herbivory along a global temperature gradient—A meta-analysis. PLoS ONE 2016, 11, e0165815. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Feng, L.X.; Frew, A.; Lu, A.Q.; Yu, Z.P.; Huang, Z.Q. Neighbourhood diversity effects on insect herbivory: Plant leaf traits mediate associational resistance. J. Ecol. 2024, 112, 2613–2623. [Google Scholar] [CrossRef]
- Barbero-Palacios, L.; Barrio, I.C.; Criado, M.G.; Kater, I.; Bon, M.P.; Kolari, T.H.M.; Bjorkas, R.; Trepel, J.; Lundgren, E.; Bjornsdottir, K.; et al. Herbivore diversity effects on Arctic tundra ecosystems: A systematic review. Environ. Evid. 2024, 13, 6. [Google Scholar] [CrossRef]
- Bachelot, B.; Kobe, R.K. Rare species advantage? Richness of damage types due to natural enemies increases with species abundance in a wet tropical forest. J. Ecol. 2013, 101, 846–856. [Google Scholar] [CrossRef]
- Milosavljević, I.; Esser, A.D.; Bosque-Pérez, N.A.; Crowder, D.W. The identity of belowground herbivores, not herbivore diversity, mediates impacts on plant productivity. Sci. Rep. 2016, 6, 39629. [Google Scholar] [CrossRef]
- Berryman, A.A. The theory and classification of outbreaks. In Insect Outbreaks; Barbosa, P., Schultz, J.C., Eds.; Academic Press: New York, NY, USA, 1987; pp. 269–286. [Google Scholar]
- Yang, L.H. The ecological consequences of insect outbreaks. In Insect Outbreaks Revisited; Barbosa, P., Letourneau, D.K., Agrawal, A.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 197–218. [Google Scholar]
- Alliende, M.C. Demographic studies of a dioecious tree. 2. The distribution of leaf predation within and between trees. J. Ecol. 1989, 77, 1048–1058. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. Background insect herbivory: Impacts, patterns and methodology. In Progress in Botany 79; Cánovas, F., Lüttge, U., Matyssek, R., Eds.; Springer: Cham, Switzerland, 2017; pp. 315–355. [Google Scholar]
- Andrew, N.R.; Hughes, L. Diversity and assemblage structure of phytophagous Hemiptera along a latitudinal gradient: Predicting the potential impacts of climate change. Glob. Ecol. Biogeogr. 2005, 14, 249–262. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Stekolshchikov, A.V.; Soderman, G.; Labina, E.S.; Zverev, V.; Zvereva, E.L. Sap-feeding insects on forest trees along latitudinal gradients in northern Europe: A climate-driven patterns. Glob. Change Biol. 2015, 21, 106–116. [Google Scholar] [CrossRef]
- Lewinsohn, T.M.; Novotny, V.; Basset, Y. Insects on plants: Diversity of herbivore assemblages revisited. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 597–620. [Google Scholar] [CrossRef]
- López-Carretero, A.; Díaz-Castelazo, C.; Boege, K.; Rico-Gray, V. Temporal variation in structural properties of tropical plant-herbivore networks: The role of climatic factors. Acta Oecol. 2018, 92, 59–66. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Wilf, P.; Barrios, H.; Windsor, D.M.; Currano, E.D.; Labandeira, C.C.; Jaramillo, C.A. Insect leaf-chewing damage tracks herbivore richness in modern and ancient forests. PLoS ONE 2014, 9, e94950. [Google Scholar] [CrossRef]
- Wilf, P.; Labandeira, C.C.; Johnson, K.R.; Rube, N. Richness of plant–insect associations in Eocene Patagonia: A legacy for South American biodiversity. Proc. Natl. Acad. Sci. USA 2005, 102, 8944–8948. [Google Scholar] [CrossRef]
- Currano, E.D.; Wilf, P.; Wing, S.L.; Labandeira, C.C.; Lovelock, E.C.; Royer, D.L. Sharply increased insect herbivory during the Paleocene-Eocene thermal maximum. Proc. Natl. Acad. Sci. USA 2008, 105, 1960–1964. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Wappler, T. Arthropod and pathogen damage on fossil and modern plants: Exploring the origins and evolution of herbivory on land. Annu. Rev. Entomol. 2023, 68, 341–361. [Google Scholar] [CrossRef]
- Adams, J.M.; Ahn, S.; Ainuddin, N.; Lee, M.L. A further test of a Palaeoecological thermometer: Tropical rainforests have more herbivore damage diversity than temperate forests. Rev. Palaeobot. Palynol. 2011, 164, 60–66. [Google Scholar] [CrossRef]
- Schueller, S.K.; Paul, S.; Payer, N.; Schultze, R.; Vikas, M. Urbanization decreases the extent and variety of leaf herbivory for native canopy tree species Quercus rubra, Quercus alba, and Acer saccharum. Urban Ecosyst. 2019, 5, 907–916. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.D. Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescens Ehrh. J. Ecol. 1992, 80, 837–870. [Google Scholar] [CrossRef]
- Jacquemart, A.-L. Vaccinium uliginosum L. J. Ecol. 1996, 84, 771–785. [Google Scholar] [CrossRef]
- Bieńkowski, A.O. Leaf Beetles of European Russia; Lambert Academic Publishing: Saarbrücken, Germany, 2011. (In Russian) [Google Scholar]
- Robinson, G.S.; Ackery, P.R.; Kitching, I.; Beccaloni, J.W.; Hernández, L.M. HOSTS—A Database of the World’s Lepidopteran Hostplants [Data Set Resource]; Natural History Museum: London, UK, 2023; Available online: https://data.nhm.ac.uk/dataset/hosts (accessed on 28 January 2025).
- Zvereva, E.L.; Zverev, V.; Kozlov, M.V. Insect herbivory increases from forest to alpine tundra in Arctic mountains. Ecol. Evol. 2022, 12, e8537. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L.; Zverev, V. Impacts of Point Polluters on Terrestrial Biota: Comparative Analysis of 18 Contaminated Areas; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Combined effects of environmental disturbance and climate warming on insect herbivory in mountain birch in subarctic forests: Results of 26-year monitoring. Sci. Tot. Environ. 2017, 601–602, 802–811. [Google Scholar] [CrossRef]
- Kozlov, M.V.; van Nieukerken, E.J.; Zverev, V.; Zvereva, E.L. Abundance and diversity of birch-feeding leafminers along latitudinal gradients in Northern Europe. Ecography 2013, 36, 1138–1149. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Lanta, V.; Zverev, V.; Zvereva, E.L. Global patterns in background losses of woody plant foliage to insects. Glob. Ecol. Biogeogr. 2015, 24, 1126–1135. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Wilf, P.; Johnson, K.R.; Marsh, F. Guide to Insect (and Other) Damage Types on Compressed Plant Fossils, Version 3.01; Smithsonian Institution: Washington, DC, USA, 2007. [Google Scholar]
- SAS Institute. SAS/STAT. User’s Guide, Version 9.2; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Rosenberg, M.S.; Adams, D.C.; Gurevich, J. MetaWin: Statistical Software for Meta-Analysis, Version 2.0; Sinauer: Sunderland, MA, USA, 2000. [Google Scholar]
- Schachat, S.R.; Payne, J.L.; Boyce, C.R.; Labandeira, C.C. Generating and testing hypotheses about the fossil record of insect herbivory with a theoretical ecospace. Rev. Palaeobot. Palynol. 2022, 297, 104564. [Google Scholar] [CrossRef]
- Lill, J.T.; Marquis, R.J. Microhabitat manipulation: Ecosystem engineering by shelter-building insects. In Ecosystem Engineers: Plants to Protists; Cuddington, K., Byers, J.E., Wilson, W.G., Hastings, A., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 107–138. [Google Scholar]
- Labandeira, C.C. Paleobiology of middle Eocene plant-insect associations of the Pacific Northwest: A preliminary report. Rocky Mount. Geol. 2002, 37, 31–59. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Shelters of leaf-tying herbivores decompose faster than untied leaves damaged by free-living insects. Sci. Tot. Environ. 2016, 568, 946–951. [Google Scholar] [CrossRef] [PubMed]
- DeVries, P. The Butterflies of Costa Rica; Princeton University Press: Princeton, NJ, USA, 1987. [Google Scholar]
- Kozlov, M.V. Defensive behaviour of the lepidopteran caterpillars. Uspekhi Sovrem. Biol. Adv. Mod. Biol. 1987, 104, 297–310. (In Russian) [Google Scholar]
- Stehr, F. Immature Insects; Kendall/Hunt Publishing: Dubuque, IA, USA, 1987. [Google Scholar]
- Scoble, M. The Lepidoptera; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef]
- Kinlock, N.L.; Prowant, L.; Herstoff, E.M.; Foley, C.M.; Akin-Fajiye, M.; Bender, N.; Umarani, M.; Ryu, H.Y.; Şen, B.; Gurevitch, J. Explaining global variation in the latitudinal diversity gradient: Meta-analysis confirms known patterns and uncovers new ones. Glob. Ecol. Biogeogr. 2018, 27, 125–141. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Responses of terrestrial arthropods to air pollution: A meta-analysis. Environ. Sci. Pollut. Res. 2010, 17, 297–311. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Castagneyrol, B.; Zverev, V.; Zvereva, E.L. Recovery of moth and butterfly (Lepidoptera) communities in a polluted region following emission decline. Sci. Total Environ. 2022, 838, 155800. [Google Scholar] [CrossRef]
- Corcos, D.; Cerretti, P.; Mei, M.; Vigna Taglianti, A.; Paniccia, D.; Santoiemma, G.; De Biase, A.; Marini, L. Predator and parasitoid insects along elevational gradients: Role of temperature and habitat diversity. Oecologia 2018, 188, 193–202. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Rossetti, M.R.; Tscharntke, T.; Aguilar, R.; Batáry, P. Responses of insect herbivores and herbivory to habitat fragmentation: A hierarchical meta-analysis. Ecol. Lett. 2017, 20, 264–272. [Google Scholar] [CrossRef]
- Salazar, D.; Marquis, R.J. Herbivore pressure increases toward the equator. Proc. Natl. Acad. Sci. USA 2012, 109, 12616–12620. [Google Scholar] [CrossRef]
- Deraison, H.; Badenhausser, I.; Loeuille, N.; Scherber, C.; Gross, N. Functional trait diversity across trophic levels determines herbivore impact on plant community biomass. Ecol. Lett. 2015, 18, 1346–1355. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, M.V.; Zverev, V. Losses of Foliage to Defoliating Insects Increase with Leaf Damage Diversity Due to the Complementarity Effect. Insects 2025, 16, 139. https://doi.org/10.3390/insects16020139
Kozlov MV, Zverev V. Losses of Foliage to Defoliating Insects Increase with Leaf Damage Diversity Due to the Complementarity Effect. Insects. 2025; 16(2):139. https://doi.org/10.3390/insects16020139
Chicago/Turabian StyleKozlov, Mikhail V., and Vitali Zverev. 2025. "Losses of Foliage to Defoliating Insects Increase with Leaf Damage Diversity Due to the Complementarity Effect" Insects 16, no. 2: 139. https://doi.org/10.3390/insects16020139
APA StyleKozlov, M. V., & Zverev, V. (2025). Losses of Foliage to Defoliating Insects Increase with Leaf Damage Diversity Due to the Complementarity Effect. Insects, 16(2), 139. https://doi.org/10.3390/insects16020139






