Botanical Antifeedants: An Alternative Approach to Pest Control
Simple Summary
Abstract
1. Introduction
2. Antifeedance Phenomenon
- Neurons associated with antifeedant receptors, which inhibit insect feeding (feeding deterring effect) [36];
- Neurons causing the halting or slowing down of further feeding (feeding suppressing effect) [36];
- Blocking of function of receptors stimulating herbivore feeding or binding directly to their normal feeding stimuli, such as sugars and amino acids [36]. An example of this mechanism is azadirachtin, which reduces the sensitivity of cells sensitive to sugar in herbivorous insects, thus causing the insects to incorrectly assess the nutritional suitability of treated plants [37].
3. Antifeedant Efficacy Assessment Methods and Criteria
- A choice test that utilizes the principle of insects’ ability to naturally choose food with suitable nutritional potential and not burdened with hazardous compounds. To a certain extent, insects are able to enzymatically inactivate some indigestible or poisonous substances. However, this inactivation requires energy. Additionally, some substances may inhibit their food utilization ability, and thus plants without such substances are more convenient for the insects. Nevertheless, in the absence of choice, they are able to feed on food contaminated with growth-inhibiting substances. The choice test thus answers the question of whether a substance can discourage the individuals from food intake, and the results show whether the individuals class such substances as inappropriate for feeding or whether, on the contrary, food contaminated with these substances becomes more attractive for them.
- A non-choice test is more rigorous and provides more input for potential use in practice. If no food other than contaminated food is presented to the larvae, they are either able to feed with a time delay compared to untreated control or the treated food is unacceptable. Generally, it can be hypothesized that if the FDI is below 90%, the given substances will probably only reduce the rate of food intake, but pose no insurmountable barrier to food intake. However, an FDI value above 90% indicates that the tested compound may actually be a true antifeedant substance, as it can significantly inhibit the response of (1) olfactory receptor cells, (2) taste receptor cells, (3) oral mechanoreceptors, and/or (4) a post-ingestion response mechanism [36].
4. Available Literature Assessment Methods
5. Promising Plant Antifeedant Substances
6. Future Research Challenges
- Primary tests. The aim of primary tests is to select plants that contain substances with antifeedant potential. Within these tests, it is therefore necessary to unify the methods and use the generally accepted method of no-choice tests on leaf discs, because it most closely simulates the likely efficacy in agricultural practice [17,36,39]. For calculating the FDI and estimating effective concentrations or doses, it is important to use the above formula [40] to ensure better comparability of results. Only standardized methods and procedures that are statistically valid can provide results that can be compared between different laboratories.
- Expand knowledge about the efficacy of extracts. Another set of research goals is undoubtedly follow-up tests to the “Primary tests”, which should aim to find out other important information about the effectiveness of selected antifeedants. a) For extracts and EOs, it is important to find out which substances or their combinations cause the antifeedant effect, which is important information from the point of view of standardization of extracts as active substances of potential plant protection products. b) Study of the effect of extracts and active substances on non-target organisms, which is important information for estimating their environmental safety. c) Study of the persistence of the effect and synergetic relationships of the majority substances with regard to a possible increase in effectiveness or extension of the persistence period.
- Implementation of results, i.e., transfer of results from laboratory experiments to agricultural practice. It is important that scientific knowledge is put into practice in the form of plant protection preparations. a) It is therefore important to systematically investigate formulation methods that will extend the period of effectiveness (e.g., encapsulation methods, etc.). b) It is important to verify the effects under conditions simulating the real ones, that is, the translation of knowledge into practice by applying formulated products in container and field trials and comparing their efficacy with other insecticides, especially on the yield characteristics of treated crops.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K.; Seki, M. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 2003, 6, 410–417. [Google Scholar] [CrossRef]
- King, B.H.; Gunathunga, P.B. Gustation in insects: Taste qualities and types of evidence used to show taste function of specific body parts. J. Insect Sci. 2023, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M. Plant Resistance to Arthropods. Molecular and Conventional Approaches, 1st ed.; Springer: Dordrecht, The Netherlands, 2005; p. 423. [Google Scholar]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Pavela, R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015, 114, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Gensch, L.; Jantke, K.; Rasche, L.; Schneider, U.A. Pesticide risk assessment in European agriculture: Distribution patterns, ban-substitution effects and regulatory implications. Environ. Poll. 2024, 348, 123836. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.P.; Xu, L.; Wang, J.J. Mode of inheritance for pesticide resistance, importance and prevalence: A review. Pestic Biochem Physiol. 2024, 202, 105964. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Protect. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Reiter, S.; Campillo, R.C.; Sun, K.; Stopfer, M. Spatiotemporal coding of individual chemicals by the gustatory system. J. Neurosci. 2015, 35, 12309–12321. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Mehlhorn, H. The sensilla of Aedes and Anopheles mosquitoes and their importance in repellency. Parasitol. Res. 2006, 99, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Canale, A.; Mehlhorn, H.; Benelli, G. Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: A review. Res. Vet. Sci. 2016, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gahukar, R.T. Plant-derived products in crop protection: Effects of various application methods on pests and diseases. Phytoparasitica 2016, 44, 379–391. [Google Scholar] [CrossRef]
- Pavela, R.; Guedes, R.N.C.; Maggi, F.; Desneux, N.; Benelli, G. Essential oil antifeedants against armyworms: Promises and challenges. Entomol. Gen. 2023, 43, 689–704. [Google Scholar] [CrossRef]
- Schoonhoven, L.M. Biological aspects of antifeedants. Entomol. Exp. Appl. 1982, 31, 57–69. [Google Scholar] [CrossRef]
- Minnich, D.E. An experimental study of the tarsal chemoreceptors of two nymphalid butterflies. J. Exp. Zool. 1921, 33, 172–203. [Google Scholar] [CrossRef]
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral coding of taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- King, B.H.; Gunathunga, P.B. Gustation across the class Insecta: Body locations. Ann. Entomol. Soc. Am. 2023, 116, 76–82. [Google Scholar] [CrossRef]
- Popescu, A.; Couton, L.; Almaas, T.-J.; Rospars, J.-P.; Wright, G.A.; Marion-Poll, F.; Anton, S. Function and central projections of gustatory receptor neurons on the antenna of the noctuid moth Spodoptera littoralis. J. Comp. Physiol. A 2013, 199, 403–416. [Google Scholar] [CrossRef]
- Tsuneto, K.; Takada, T.; Kasubuchi, M.; Yamagishi, T.; Adegawa, S.; Sato, R. BmGr10 is a putative functional gustatory receptor in the myo-inositol neuron in the epipharyngeal sensillum. J. Insect Biotechnol. Sericol. 2019, 88, 1_007–1_015. [Google Scholar] [CrossRef]
- Zhou, D.S.; Teng, T.; Liu, J.H.; Long, J.M. Cross-habituation to deterrents correlates with desensitisation of the corresponding deterrent neuron in the larva of the black cutworm, Agrotis ipsilon. Entomol. Exp. Appl. 2021, 169, 1039–1048. [Google Scholar] [CrossRef]
- King, B.H.; Taylor, E.E.; Burgess, E.R. Feeding response to select monosaccharides, sugar alcohols, and artificial sweeteners relative to sucrose in adult house flies, Musca domestica (Diptera: Muscidae). J. Med. Entomol. 2019, 57, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hostachy, C.; Couzi, P.; Hanafi-Portier, M.; Portemer, G.; Halleguen, A.; Murmu, M.; Deisig, N.; Dacher, M. Responsiveness to sugar solutions in the moth Agrotis ipsilon: Parameters affecting proboscis extension. Front. Physiol. 2019, 10, 1423. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Leonhardt, S.D.; Lunau, K.; Spaethe, J. Bumblebees are able to perceive amino acids via chemotactile antennal stimulation. J. Comp. Physiol. A 2019, 205, 321–331. [Google Scholar] [CrossRef]
- Ali, H.; Iqbal, J.; Raweh, H.S.; Alqarni, A.S. Proboscis behavioral response of four honey bee Apis species towards different concentrations of sucrose, glucose, and fructose. Saudi J. Biol. Sci. 2021, 28, 3275–3283. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, F.J.; d’Ettorre, P. Associative learning in ants: Conditioning of the maxilla-labium extension response in Camponotus aethiops. J. Insect Physiol. 2010, 56, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hou, W.; Zhang, J.; Dang, Y.; Yang, Q.; Zhao, X.; Ma, Y.; Tang, Q. Plant metabolites drive different responses in caterpillars of two closely related Helicoverpa species. Front Physiol. 2021, 12, 662978. [Google Scholar] [CrossRef]
- Devineni, A.V.; Sun, B.; Zhukovskaya, A.; Axel, R. Acetic acid activates distinct taste pathways in Drosophila to elicit opposing, state-dependent feeding responses. eLife 2019, 8, e47677. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2008, 54, 57–83. [Google Scholar] [CrossRef]
- Nataraj, N.; Hansson, B.S.; Knaden, M. Learning-based oviposition constancy in insects. Front. Ecol. Evol. 2024, 12, 1351400. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; Xie, F.; Ling, S.; Zeng, X. Development and evaluation of emulsifiable concentrate formulation containing Sophora alopecuroides L. extract for the novel management of Asian citrus psyllid. Environ. Sci. Pollut. Res. 2019, 26, 21871–21881. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Saber, M.; Bagheri, M.; Mahdavinia, G.R. Achillea millefolium essential oil and chitosan nanocapsules with enhanced activity against Tetranychus urticae. J. Pest Sci. 2018, 91, 837–848. [Google Scholar] [CrossRef]
- Koul, O. Insect Antifeedants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005; p. 1005. [Google Scholar]
- Carpinella, C.; Ferrayoli, C.; Valladares, G.; Defego, M.; Palacios, S. Potent limonoid insect antifeedant from Melia azedarach. Biosci. Biotechnol. Biochem. 2002, 66, 1731–1736. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology, 2nd ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Roessingh, P.; Xu, S.; Menken, S.B.J. Olfactory receptors on the maxillary palps of small ermine moth larvae: Evolutionary history of benzaldehyde sensitivity. J. Comp. Physiol. A 2007, 193, 635–647. [Google Scholar] [CrossRef]
- Isman, M.B.; Koul, O.; Luczynski, A.; Kaminski, A. Insecticidal and antifeedant bioactivity of neem oils and their relationship to azadirachtin content. J. Agric. Food Chem. 1990, 38, 1406–1411. [Google Scholar] [CrossRef]
- Nyirenda, J.; Kadango, Z.; Funjika, E.; Chipabika, G. Larvicidal, ovicidal and antifeedant activity of crude cashew nutshell liquid against fall armyworm, Spodoptera frugiperda (J.E. smith), (lepidoptera, Noctuidae). Crop Prot. 2024, 179, 106619. [Google Scholar] [CrossRef]
- Seffrin, R.C.; Shikano, I.; Akhtar, Y.; Isman, M.B. Effects of crude seed extracts of Annona atemoya and Annona squamosal L. against the cabbage looper, Trichoplusia ni in the laboratory and greenhouse. Crop Prot. 2010, 29, 20–24. [Google Scholar] [CrossRef]
- Arora, S.; Mogha, N.; Bhardwaj, T.; Srivastava, C. Antifeedant and insecticidal activity of plant extracts against Spodoptera litura (Fab.) and Lipaphis erysimi. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 1229–1236. [Google Scholar] [CrossRef]
- Pavela, R. Antifeedant activity of plant extracts on Leptinotarsa decemlineata Say. and Spodoptera littoralis Bois. larvae. Ind. Crops Prod. 2010, 32, 213–219. [Google Scholar] [CrossRef]
- Pavela, R.; Vrchotová, N. Insecticidal effect of furanocoumarins from fruits of Angelica archangelica L. against larvae Spodoptera littoralis Boisd. Ind. Crops Prod. 2013, 43, 33–39. [Google Scholar] [CrossRef]
- Kathuria, V.; Kaushik, N. Evaluation of bioactivity of some plant species against Spodoptera litura Fabricius (Noctuidae: Lepidoptera). Afr. Entomol. 2006, 14, 45–52. [Google Scholar]
- Yang, H.; Piao, X.; Zhang, L.; Song, S.; Xu, Y. Ginsenosides from the stems and leaves of Panax ginseng show antifeedant activity against Plutella xylostella (Linnaeus). Ind. Crops Prod. 2018, 124, 412–417. [Google Scholar] [CrossRef]
- Ruiz-Vásquez, L.; Reina, M.; Fajardo, V.; López, M.; González-Coloma, A. Insect antifeedant components of Senecio fistulosus var. fistulosus—Hualtata. Plants 2019, 8, 176. [Google Scholar] [CrossRef]
- Ruiz-Vásquez, L.; Reina, M.; López-Rodríguez, M.; Giménez, C.; Cabrera, R.; Cuadra, P.; Fajardo, V.; González-Coloma, A. Sesquiterpenes, flavonoids, shikimic acid derivatives and pyrrolizidine alkaloids from Senecio kingii Hook. Phytochemistry 2015, 117, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Santana, O.; Reina, M.; Fraga, B.M.; Sanz, J.; González-Coloma, A. Antifeedant activity of fatty acid esters and phytosterols from Echium wildpretii. Chem. Biodivers. 2012, 9, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Baskar, K.; Maheswaran, R.; Pavunraj, M.; Pacikam, S.M.; Ignacimuthu, S.; Duraipandiyan, V.; Benelli, G. Toxicity and antifeedant activity of Caesalpinia bonduc (L.) Roxb. (Caesalpiniaceae) extracts and fractions against the cotton bollworm Helicoverpa armigera Hub. (Lepidoptera: Noctuidae). Physiol. Mol. Plant Pathol. 2017, 101, 69–74. [Google Scholar] [CrossRef]
- Ningombam, A.; Ahluwalia, V.; Srivastava, C.; Walia, S. Antifeedant activity and phytochemical investigation of Millettia pachycarpa extracts against Tobacco Leaf Eating Caterpillar, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2017, 20, 381–385. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Andrés, M.F.; Contreras, R.; Zúñiga, G.E.; Díaz, C.E. Sustainable Production of Insecticidal Compounds from Persea indica. Plants 2022, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Y.; Isman, M.B. Comparative growth inhibitory and antifeedant effects of plant extracts and pure allelochemicals on four phytophagous insect species. J. Appl. Ent. 2004, 128, 32–38. [Google Scholar] [CrossRef]
- Pitan, O.O.R.; Ayelaagbe, O.O.; Wang, H.-L.; Wang, C.-Z. Identification, isolation and characterization of the antifeedant constituent of Clausena anisata against Helicoverpa armigera (Lepidoptera: Noctuidae). Insect Sci. 2009, 16, 247–253. [Google Scholar] [CrossRef]
- Baskar, K.; Ananthi, J.; Ignacimuthu, S. Toxic effects of Solanum xanthocarpum Sch &Wendle against Helicoverpa armigera (Hub.), Culex quinquefasciatus (Say.) and Eisenia fetida (Savigny, 1826). Environ. Sci. Pollut. Res. 2018, 25, 2774–2782. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Sánchez-Vioque, R.; Santana-Méridas, O.; Martín-Bejerano, M.; Alonso, G.L.; Salinas, M.R.; Zalacain, A. A potential use of vine-shoot wastes: The antioxidant, antifeedant andphytotoxic activities of their aqueous extracts. Ind. Crops Prod. 2017, 97, 120–127. [Google Scholar] [CrossRef]
- Koul, O.; Smirle, M.J.; Isman, M.B. Asarones from Acorus calamus L. oil. Their effect on feeding behavior and dietary utilization Peridroma saucia. J. Chem. Ecol. 1990, 16, 1911–1920. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef]
- Julio, L.F.; Martín, L.; Muñoz, R.; Mainar, A.M.; Urieta, J.S.; Sanz, J.; Burillo, J.; González-Coloma, A. Comparative chemistry and insect antifeedant effects of conventional (Clevenger and Soxhlet) and supercritical extracts (CO2) of two Lavandula luisieri populations. Ind. Crops Prod. 2014, 58, 25–30. [Google Scholar] [CrossRef]
- Santana, O.; Andrés, M.F.; Sanz, J.; Errahmani, N.; Abdeslam, L.; González-Coloma, A. Valorization of essential oils from Moroccan aromatic plants. Nat. Prod. Commun. 2014, 9, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Andrés, M.F.; Rossa, G.E.; Cassel, E.; Vargas, R.M.F.; Santana, O.; Díaz, C.E. Biocidal effects of Piper hispidinervum (Piperaceae) essential oil and synergism among its main components. Food Chem. Toxicol. 2017, 109, 1086–1092. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Pino-Benitez, N.; González-Coloma, A. Volatile composition and biocidal (antifeedant and phytotoxic) activity of the essential oils of four Piperaceae species from Choco-Colombia. Ind. Crops Prod. 2019, 138, 111463. [Google Scholar] [CrossRef]
- de Melo, J.P.R.; da Câmara, C.A.G.; de Moraes, M.M. Management of the diamondback moth via citrus oil. Biocatal. Agric. Biotechnol. 2023, 51, 102775. [Google Scholar] [CrossRef]
- Wu, H.; Wu, H.; Wang, W.; Liu, T.; Qi, M.; Feng, J.; Li, X.; Liu, Y. Insecticidal activity of sesquiterpene lactones and monoterpenoidfrom the fruits of Carpesium abrotanoides. Ind. Crops Prod. 2016, 92, 77–83. [Google Scholar] [CrossRef]
- Shi, W.; Shihong, L.; Shenghong, L. Defensive sesquiterpenoids from leaves of Eupatorium adenophorum. Chin. J. Chem. 2012, 30, 1331–1334. [Google Scholar] [CrossRef]
- Díaz Napal, G.N.D.; Defagó, M.T.; Valladares, G.R.; Palacios, S.M. Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010, 36, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.E.; Fraga, B.M.; Portero, A.G.; Brito, I.; López-Balboa, C.; Ruiz-Vásquez, L.; González-Coloma, A. Insect antifeedant benzofurans from Pericallis species. Molecules 2023, 28, 975. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vásquez, L.; Olmeda, A.S.; Zúñiga, G.; Villarroel, L.; Echeverri, L.F.; González-Coloma, A.; Reina, M. Insect antifeedant and ixodicidal compounds from Senecio adenotrichius. Chem. Biodiversity 2017, 14, e1600155. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; Gutiérrez, C.; Cabrera, R.; Reina, M. silphinene derivatives: Their effects and modes of action on Colorado potato beetle. J. Agric. Food Chem. 1997, 45, 946–950. [Google Scholar] [CrossRef]
- Tsunaki, K.; Morimoto, M. Chemical defense of yacón (Smallanthus sonchifolius) leaves against phytophagous insects: Insect antifeedants from yacón leaf trichomes. Plants 2020, 9, 848. [Google Scholar] [CrossRef]
- Nebapure, S.M.; Srivastava, C.; Walia, S. Antifeedant activity of Gloriosa superba Linn. tuber extracts against Spodoptera litura (Fabricius). Natl. Acad. Sci. Lett. 2016, 39, 333–336. [Google Scholar] [CrossRef]
- Ponsankar, A.; Sahayaraj, K.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Thanigaivel, A.; Petchidurai, G.; Madasamy, M.; Hunter, W.B. Toxicity and developmental effect of cucurbitacin E from Citrullus colocynthis L. (Cucurbitales: Cucurbitaceae) against Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Environ. Sci. Pollut. Res. 2020, 27, 23390–23401. [Google Scholar] [CrossRef]
- Gao, R.; Gao, C.; Tian, X.; Yu, X.; Di, X.; Xiao, H.; Zhang, X. Insecticidal activity of deoxypodophyllotoxin, isolated from Juniperus sabina L., and related lignans against larvae of Pieris rapae L. Pest Manag. Sci. 2004, 60, 1131–1136. [Google Scholar] [CrossRef]
- Li, C.H.; Luo, S.H.; Li, S.H.; Gao, J.M. New antifeedant grayanane diterpenoids from the flowers of Pieris formosa. Molecules 2017, 22, 1431. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Li, X.N.; Gao, L.H.; Li, H.Z.; Wu, G.X.; Li, R.T. Neopierisoids A and B, two new chlorinated 3,4-seco-grayanane diterpenoids with antifeedant activity from flowers of Pieris japonica. J. Agric. Food Chem. 2013, 61, 7219–7224. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Huang, S.; Shan, L.; Gao, F.; Zhou, X. Diterpenoid alkaloids from Aconitum leucostomum and their antifeedant activity. Chin. J. Org. Chem. 2017, 37, 1839–1843. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, J.; Weng, Q.; Hu, M.; Luo, J. Laboratory and field evaluations of rhodojaponin-III against the imported cabbage worm Pieris rapae (L.) (Lepidoptera: Pieridae). Pest Manag. Sci. 2006, 62, 976–981. [Google Scholar] [CrossRef]
- Nihei, K.I.; Asaka, Y.; Mine, Y.; Ito, C.; Furukawa, H.; Ju-Ichi, M.; Kubo, I. Insect antifeedants from tropical plants: Structures of dumnin and dumsenin. J. Agric. Food Chem. 2004, 52, 3325–3328. [Google Scholar] [CrossRef]
- Nihei, K.I.; Asaka, Y.; Mine, Y.; Yamada, Y.; Iigo, M.; Yanagisawa, T.; Kubo, I. Musidunin and musiduol, insect antifeedants from Croton jatrophoides. J. Nat. Prod. 2006, 69, 975–977. [Google Scholar] [CrossRef]
- González-Coloma, A.; López-Balboa, C.; Santana, O.; Reina, M.; Fraga, B.M. Triterpene-based plant defenses. Phytochem. Rev. 2011, 10, 245–260. [Google Scholar] [CrossRef]
- Morimoto, M.; Fukumoto, H.; Hiratani, M.; Chavasiri, W.; Komai, K. Insect antifeedants, pterocarpans and pterocarpol, in heartwood of Pterocarpus macrocarpus Kruz. Biosci. Biotech. Bioch. 2006, 70, 1864–1868. [Google Scholar] [CrossRef]
- Abbaszadeh, G.; Srivastava, C.; Walia, S. Insecticidal and antifeedant activities of clerodane diterpenoids isolated from the Indian bhant tree, Clerodendron infortunatum, against the cotton bollworm, Helicoverpa armigera. J. Insect Sci. 2014, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M.; Díaz, C.E.; Bailén, M.; González-Coloma, A. Sesquiterpene lactones from Artemisia absinthium. Biotransformation and rearrangement of the insect antifeedant 3α-hydroxypelenolide. Plants 2021, 10, 891. [Google Scholar] [CrossRef]
- González-Coloma, A.; Gutiérrez, C.; Hübner, H.; Achenbach, H.; Terrero, D.; Fraga, B.M. Selective insect antifeedant and toxic action of ryanoid diterpenes. J. Agric. Food Chem. 1999, 47, 4419–4424. [Google Scholar] [CrossRef] [PubMed]
- Satasook, C.; Isman, M.B.; Wiriyachitra, P. Activity of rocaglamide, an insecticidal natural product, against the variegated cutworm, Peridroma saucia (lepidoptera: Noctuidae). Pestic. Sci. 1992, 36, 53–58. [Google Scholar] [CrossRef]
- Arnason, J.T.; Philogène, B.J.R.; Donskov, N.; Hudon, M.; McDougall, C.; Fortier, G.; Morand, P.; Gardner, D.; Lambert, J.; Morris, C.; et al. Antifeedant and insecticidal properties of azadirachtin to the European Corn Borer, Ostrinia nubilalis. Entomol. Exp. Appl. 1985, 38, 29–34. [Google Scholar] [CrossRef]
- Rajab, M.S.; Bentley, M.D.; Alford, A.R.; Mendel, M.J. A new limonoid insect antifeedant from the fruit of Melia volkensii. J. Nat. Prod. 1988, 51, 168–171. [Google Scholar] [CrossRef]
- Chen, W.; Isman, M.B.; Chiu, S.-F. Antifeedant and growth inhibitory effects of the limonoid toosendanin and Melia toosendan extracts on the variegated cutworm, Peridroma saucia (Lep., Noctuidae). J. Appl. Entomol. 1995, 119, 367–370. [Google Scholar] [CrossRef]
- Kitayama, T.; Yasuda, K.; Kihara, T.; Ito, M.; Fukumoto, H.; Morimoto, M. Piperine analogs in a hydrophobic fraction from Piper ribersoides (Piperaceae) and its insect antifeedant activity. Appl. Entomol. Zool. 2013, 48, 455–459. [Google Scholar] [CrossRef]
- Gonzalez-Coloma, A.; Gutierrez, C.; Miguel del Corral, J.M.; Gordaliza, M.; de la Puente, M.L.; San Feliciano, A. Structure- and species-dependent insecticidal effects of neo-Clerodane diterpenes. J. Agric. Food Chem. 2000, 48, 3677–3681. [Google Scholar] [CrossRef] [PubMed]
- de Melo, J.P.R.; da Câmara, C.A.G.; de Moraes, M.M. Bioactivity of formulas containing essential oils from the family Myrtaceae for the management of deltamethrin-resistant Plutella xylostella (L.) (Lepidoptera: Plutellidae). Phytoparasitica 2023, 51, 305–321. [Google Scholar] [CrossRef]
- Shao, X.; Lai, D.; Xiao, W.; Yang, W.; Yan, Y.; Kuang, S. The botanical eurycomanone is a potent growth regulator of the diamondback moth. Ecotoxicol. Environ. Saf. 2021, 208, 111647. [Google Scholar] [CrossRef]
- Montenegro, I.J.; del Corral, S.; Diaz Napal, G.N.; Carpinella, M.C.; Mellado, M.; Madrid, A.M.; Villena, J.; Palacios, S.M.; Cuellar, M.A. Antifeedant effect of polygodial and drimenol derivatives against Spodoptera frugiperda and Epilachna paenulata and quantitative structure-activity analysis. Pest Manag. Sci. 2018, 74, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U.; Devanand, P. Bioactivities of caffeic acid methyl ester (methyl-(E)-3-(3,4-dihydroxyphenyl)prop-2-enoate): A hydroxycinnamic acid derivative from Solanum melongena L. fruits. J. Pest Sci. 2013, 86, 579–589. [Google Scholar] [CrossRef]
- Mullin, C.A.; Gonzalez-Coloma, A.; Gutierrez, C.; Reina, M.; Eichenseer, H.; Hollister, B.; Chyb, S. Antifeedant effects of some novel terpenoids on chrysomelidae beetles: Comparisons with alkaloids on an alkaloid-adapted and nonadapted species. J. Chem. Ecol. 1997, 23, 1851–1866. [Google Scholar] [CrossRef]
- da Camara, C.A.G.; Doboszewski, B.; de Melo, J.P.R.; Nazarenko, A.Y.; dos Santos, R.B.; Moraes, M.M. Novel insecticides from alkylated and acylated derivatives of thymol and eugenol for the control of Plutella xylostella (Lepidoptera: Plutellidae). J. Braz. Chem. Soc. 2022, 33, 196–204. [Google Scholar] [CrossRef]
- Navarro-Rocha, J.; Barrero, A.F.; Burillo, J.; Olmeda, A.S.; González-Coloma, A. Valorization of essential oils from two populations (wild and commercial) of Geranium macrorrhizum L. Ind. Crops Prod. 2018, 116, 41–45. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; González-Coloma, A.; Andrés, M.F.; Vidali, V.P.; Polissiou, M.G.; Santana-Méridas, O. Biocidal compounds from Mentha sp. essential oils and their structure–activity relationships. Chem. Biodivers. 2017, 14, e1600270. [Google Scholar] [CrossRef] [PubMed]
- Rodilla, J.M.; Tinoco, M.T.; Morais, J.C.; Gimenez, C.; Cabrera, R.; Martín-Benito, D.; Castillo, L.; Gonzalez-Coloma, A. Laurus novocanariensis essential oil: Seasonal variation and valorization. Biochem. Syst. Ecol. 2008, 36, 167–176. [Google Scholar] [CrossRef]
- Pavela, R.; Žabka, M.; Bednář, J.; Tříska, J.; Vrchotová, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Nguemtchouin, M.; Ngassoum, M.; Ngamo, L.; Mapongmetsem, P.; Sieliechi, J.; Malaisse, F.; Lognay, G.C.; Haubruge, E.; Hance, T. Adsorption of essential oil components of Xylopia aethiopica (Annonaceae) by kaolin from Wak, Adamawa province (Cameroon). Appl. Clay Sci. 2009, 44, 1–6. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Pavela, R.; Vrchotová, N.; Šerá, B. Growth inhibitory effect of extracts from Reynoutria sp plants against Spodoptera littoralis larvae. Agrociencia 2008, 42, 573–584. [Google Scholar]
- Sadek, M.M. Antifeedant and toxic activity of Adhatoda vasica leaf extract against Spodoptera littoralis (Lep., Noctuidae). J. Appl. Entomol. 2003, 127, 396–404. [Google Scholar] [CrossRef]
- Pavunraj, M.; Muthu, C.; Ignacimuthu, S.; Janarthanan, S.; Duraipandiyan, V.; Raja, N.; Vimalraj, S. Antifeedant activity of a novel 6-(4,7-hydroxy-heptyl) quinone® from the leaves of the milkweed Pergularia daemia on the cotton bollworm Helicoverpa armigera (Hub.) and the tobacco armyworm Spodoptera litura (Fab.). Phytoparasitica 2011, 39, 145–150. [Google Scholar] [CrossRef]
- Mayanglambam, S.; Raghavendra, A.; Rajashekar, Y. Use of Ageratina adenophora (Spreng.) essential oil as insecticidal and antifeedant agents against diamondback moth, Plutella xylostella (L.). J. Plant Dis. Protect. 2022, 129, 439–448. [Google Scholar] [CrossRef]
- Vats, T.K.; Rawal, V.; Mullick, S.; Devi, M.R.; Singh, P.; Singh, A.K. Bioactivity of Ageratum conyzoides (L.) (Asteraceae) on feeding and oviposition behaviour of diamondback moth Plutella xylostella (L.) (Lepidoptera: Plutellidae). Int. J. Trop. Insect Sc. 2019, 39, 311–318. [Google Scholar] [CrossRef]
- Yano, K.; Tanaka, N. Antifeedant activity toward larvae of Pieris rapae crucivora of aromatic carbonyl compounds related to capillin isolated from Artemisia capillaris. Biosci. Biotech. Bioch. 1995, 59, 1130–1132. [Google Scholar] [CrossRef]
- Yano, K.; Kamimura, H. Antifeedant activity toward larvae of Pieris rapae crucivora of phenolethers related to methyleugenol isolated from Artemisia capillaris. Biosci. Biotech. Bioch. 1993, 57, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.S.; Fernández, E.N.; Campos-Soldini, M.P. Antifeedant and contact repellent activity of thyme, tarragon, marigold and lavender crude extracts against Epicauta atomaria (Coleoptera: Meloidae). Rev. Soc. Entomol. Arg. 2023, 82, 56–64. [Google Scholar] [CrossRef]
- Haouas, D.; Flamini, G.; ben Halima-Kamel, M.; ben Hamouda, M.H. Feeding perturbation and toxic activity of five Chrysanthemum species crude extracts against Spodoptera littoralis (Boisduval) (Lepidoptera; Noctuidae). Crop Prot. 2010, 29, 992–997. [Google Scholar] [CrossRef]
- Henagamage, A.P.; Ranaweera, M.N.; Peries, C.M.; Premetilake, M.M.S.N. Repellent, antifeedant and toxic effects of plants-extracts against Spodoptera frugiperda larvae (fall armyworm). Biocatal. Agric. Biotechnol. 2023, 48, 102636. [Google Scholar] [CrossRef]
- da Costa Inácio, G.; Alves, J.V.B.; Santos, M.F.C.; Vacari, A.M.; Figueiredo, G.P.; Bernardes, W.A.; Veneziani, R.C.S.; Ambrósio, S.R. Feeding deterrence towards Helicoverpa armigera by Tithonia diversifolia tagitinin C-enriched extract. Arab. J. Chem. 2020, 13, 5292–5298. [Google Scholar] [CrossRef]
- Jayasinghe, U.L.B.; Kumarihamy, B.M.M.; Bandara, A.G.D.; Waiblinger, J.; Kraus, W. Antifeedant activity of some Sri Lankan plants. Nat. Prod. Res. 2003, 17, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, S.E.; Leksono, A.S.; Gama, Z.P.; Tarno, H. The active compounds composition and antifeedant activity of leaf extract of two cultivar Carica papaya L. on Spodoptera litura F. larvae. AIP Conf. Proc. 2020, 2231, 040085. [Google Scholar] [CrossRef]
- Hidayati, D.; Darmanto, Y.; Nurhidayati, T.; Abdulgani, N. Short Communication: Larvicidal and antifeedant activities of Kalanchoe daigremontiana against Plutella xylostella larvae. Nusant. Bioscie. 2016, 8, 312–315. [Google Scholar] [CrossRef]
- Koul, O.; Shankar, J.S.; Mehta, N.; Taneja, S.C.; Tripathi, A.K.; Dhar, K.L. Bioefficacy of crude extracts of Aglaia species (Meliaceae) and some active fractions against lepidopteran larvae. J. Appl. Entomol. 1997, 121, 245–248. [Google Scholar] [CrossRef]
- Smirle, M.J.; Wei, S.G. Effects of neem oil on feeding behaviour and development of the pear sawfly, Caliroa cerasi. Entomol. Exp. Appl. 1996, 80, 403–407. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Isman, M.B. Effect of Trichilia americana extract on feeding behavior of asian armyworm, Spodoptera litura. J. Chem. Ecol. 2000, 26, 2791–2800. [Google Scholar] [CrossRef]
- Mehboob, A.; Zaka, S.M.; Sarmad, M.; Bajwa, M. Feeding and oviposition deterrence of Murraya paniculata, Piper nigrum and Moringa oleifera extracts against Spodoptera litura (F). Pak. J. Zool. 2019, 51, 567–574. [Google Scholar] [CrossRef]
- Martínez, M.L.; von Poser, G.; Henriques, A.; Gattuso, M.; Rossini, C. Simaroubaceae and Picramniaceae as potential sources of botanical pesticides. Ind. Crops Prod. 2013, 44, 600–602. [Google Scholar] [CrossRef]
- Pengsook, A.; Bullangpoti, V.; Koul, O.; Nobsathian, S.; Saiyaitong, C.; Yooboon, T.; Phankaen, P.; Pluempanupat, W.; Kumrungsee, N. Antifeedant activity and biochemical responses in Spodoptera exigua Hübner (Lepidoptera: Noctuidae) infesting broccoli, Brassica oleracea var. alboglabra exposed to Piper ribesioides Wall extracts and allelochemicals. Chem. Biol. Technol. Agric. 2022, 9, 17. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Z.; Chen, X.; Zheng, R.; Lu, S.; Seyab, M.; Yang, F.; Li, Q.; Tang, Q. Insecticidal potential of a Consolida ajacis extract and its major compound (ethyl linoleate) against the diamondback moth, Plutella xylostella. Pestic. Biochem. Phys. 2023, 195, 105557. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.D.; Li, Y.J.; Ge, L.; Qin, Z.Z. Isolation of triterpenoids from Catunaregam spinosa. Adv. Mater. Res. 2011, 236–238, 1731–1737. [Google Scholar] [CrossRef]
- Baskar, K.; Kingsley, S.; Vendan, S.E.; Paulraj, M.G.; Duraipandiyan, V.; Ignacimuthu, S. Antifeedant, larvicidal and pupicidal activities of Atalantia monophylla (L.) Correa against Helicoverpa armigera Hubner (Lepidoptera: Noctuidae). Chemosphere 2009, 75, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Melanie, M.; Hermawan, W.; Kasmara, H.; Kholifa, A.H.; Rustama, M.M.; Panatarani, C. Antifeedant properties of fractionation Lantana camara leaf extract on cabbage caterpillars (Crocidolomia pavonana Fabricius) larvae. IOP Conf. Ser. Earth Environ. Sci. 2020, 457, 012047. [Google Scholar] [CrossRef]
- Biswas, S.; Kundu, A.; Suby, S.B.; Kushwah, A.S.; Patanjali, N.; Shasany, A.K.; Verma, R.; Saha, S.; Mandal, A.; Banerjee, T.; et al. Lippia alba—A potential bioresource for the management of Spodoptera frugiperda (Lepidoptera: Noctuidae). Front. Plant Sci. 2024, 15, 1422578. [Google Scholar] [CrossRef] [PubMed]
- Zapata, N.; Budia, F.; Viñuela, E.; Medina, P. Antifeedant and growth inhibitory effects of extracts and drimanes of Drimys winteri stem bark against Spodoptera littoralis (Lep., Noctuidae). Ind. Crops Prod. 2009, 30, 119–125. [Google Scholar] [CrossRef]
- Song, C.; Zhao, J.; Zheng, R.; Hao, C.; Yan, X. Chemical composition and bioactivities of thirteen non-host plant essential oils against Plutella xylostella L. (Lepidoptera: Plutellidae). J. Asia-Pac. Entomol. 2022, 25, 101881. [Google Scholar] [CrossRef]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crops Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef]
- Malhotra, A.; Rawat, A.; Prakash, O.; Kumar, R.; Srivastava, R.M.; Kumar, S. Chemical composition and pesticide activity of essential oils from Artemisia annua L. harvested in the rainy and winter seasons. Biochem. Syst. Ecol. 2023, 107, 104601. [Google Scholar] [CrossRef]
- Sainz, P.; Fe Andrés, M.; Martínez-Díaz, R.A.; Bailén, M.; Navarro-Rocha, J.; Díaz, C.E.; González-Coloma, A. Chemical composition and biological activities of Artemisia pedemontana subsp. assoana essential oils and hydrolate. Biomolecules 2019, 9, 558. [Google Scholar] [CrossRef]
- Devrani, A.; Kumar, R.; Bargali, P.; Karakoti, H.; Mahawer, S.K.; Prakash, O.; Kumar, S.; Rawat, D.S.; Srivastava, R.M. Nematicidal and insecticidal activity of essential oils from Artemisia scoparia and Centratherum punctatum and their mixtures. Biochem. Syst. Ecol. 2024, 116, 104859. [Google Scholar] [CrossRef]
- Thapa, P.; Prakash, O.; Rawat, A.; Kumar, R.; Srivastava, R.M.; Rawat, D.S.; Pant, A.K. Essential oil composition, antioxidant, anti-inflammatory, insect antifeedant and sprout suppressant activity in essential oil from aerial parts of Cotinus coggygria Scop. J. Essent. Oil Bear. Plants 2020, 23, 65–76. [Google Scholar] [CrossRef]
- Ortiz de Elguea-Culebras, G.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Peñalver, D.; González-Coloma, A.; Andrés, M.F.; Santana-Méridas, O. Biocidal Potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula × intermedia var. Super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, e1700313. [Google Scholar] [CrossRef] [PubMed]
- González-Coloma, A.; Delgado, F.; Rodilla, J.M.; Silva, L.; Sanz, J.; Burillo, J. Chemical and biological profiles of Lavandula luisieri essential oils from western Iberia Peninsula populations. Biochem. Syst. Ecol. 2011, 39, 1–8. [Google Scholar] [CrossRef]
- Akhtar, Y.; Pages, E.; Stevens, A.; Bradbury, R.; da Camara, C.A.G.; Isman, M.B. Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol. Entomol. 2012, 37, 81–91. [Google Scholar] [CrossRef]
- Karakoti, H.; Kabdal, T.; Kumar, R.; Prakash, O.; Rawat, D.S.; Srivastava, R.M.; Santana de Oliveira, M. Chemical composition, biological activities and in silico evaluation of essential oils from the aerial, and root parts of Nepeta hindostana (B. Heyne ex Roth)-Haines grown in North India. Biochem. Syst. Ecol. 2022, 105, 104512. [Google Scholar] [CrossRef]
- Kostić, M.; Popović, Z.; Brkić, D.; Milanović, S.; Sivčev, I.; Stanković, S. Larvicidal and antifeedant activity of some plant-derived compounds to Lymantria dispar L. (Lepidoptera: Limantriidae). Bioresour. Technol. 2008, 99, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- Manjesh, K.; Kundu, A.; Dutta, A.; Saha, S.; Neelakanthaiah, B.S. Bio-insecticidal nanoemulsions of essential oil and lipid-soluble fractions of Pogostemon cablin. Front. Plant Sci. 2022, 13, 874221. [Google Scholar] [CrossRef]
- Pavela, R.; Sajfrtová, M.; Sovová, H.; Karban, J.; Bárnet, M. The effects of extracts obtained by supercritical fluid extraction and traditional extraction techniques on larvae Leptinotarsa decemlineata SAY. J. Essent. Oil Res. 2009, 21, 367–373. [Google Scholar] [CrossRef]
- Valcárcel, F.; Olmeda, A.S.; González, M.G.; Andrés, M.F.; Navarro-Rocha, J.; González-Coloma, A. Acaricidal and insect antifeedant effects of essential oils from selected aromatic plants and their main components. Front. Agron. 2021, 3, 662802. [Google Scholar] [CrossRef]
- Kabdal, T.; Himani; Kumar, R.; Prakash, O.; Nagarkoti, K.; Rawat, D.S.; Srivastava, R.M.; Kumar, S.; Dubey, S.K. Seasonal variation in the essential oil composition and biological activities of Thymus linearis Benth. collected from the Kumaun region of Uttarakhand, India. Biochem. Syst. Ecol. 2022, 103, 104449. [Google Scholar] [CrossRef]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Yao, X.; Tang, F.; Hua, R.M.; Wu, X.W.; Cao, H.Q. Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. J. Appl. Entomol. 2017, 141, 721–728. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.; Julio-Torres, J.; Duarte-Restrepo, E.; Gonzalez-Coloma, A.; Julio-Torres, L.F. Comparative study of volatile composition and biological activities of essential oil from Colombian Piper marginatum Jacq. Bol. Latinoam. Caribe Plantas Med. Aromat. 2015, 14, 343–354. [Google Scholar]
- Jeon, H.; Tak, J.H. Gustatory habituation to essential oil induces reduced feeding deterrence and neuronal desensitization in Spodoptera litura. J. Pest Sci. 2024, 97, 1–16. [Google Scholar] [CrossRef]
- Sharaby, A. Anti-insect properties of the essential oil of lemon grass, Cymbopogen citratus against the lesser cotton leafworm Spodoptera exigua (Hbn). Int. J. Trop. Insect Sci. 1988, 9, 77–80. [Google Scholar] [CrossRef]
- Javier, A.M.J.; Ocampo, V.R.; Ceballo, F.A.; Javier, P.A. Insecticidal activities of essential oils from different plants against the cabbage worm, Crocidolomia pavonana Fabricius (Lepidoptera: Crambidae). Philipp. Agric. Sci. 2018, 101, 158–166. [Google Scholar]
- Agarwal, M.; Walia, S.; Dhingra, S.; Khambay, B.P.S. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/ derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag. Sci. 2001, 57, 289–300. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Z.; Lei, F.; Fu, J.; Zhang, X.; Ma, W.; Zhang, L. Antifeedant and oviposition-deterring activity of total ginsenosides against Pieris rapae. Saudi J. Biol. Sci. 2017, 24, 1751–1753. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Xu, Y.; Zhang, R.; Xiao, S.; Wang, Y.; Zhang, L. Antifeedant and ovicidal activities of ginsenosides against Asian corn borer, Ostrinia furnacalis (Guenee). PLoS ONE 2019, 14, e0211905. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Wang, H.T.; Shi, S.; Meng, Y.; Feng, J.C.; Wu, H.B. Sesquiterpenoids from Artemisia vestita and their antifeedant and antifungal activities. Molecules 2019, 24, 3671. [Google Scholar] [CrossRef]
- Sachdev-Gupta, K.; Radke, C.D.; Alan, J.; Renwick, A. Antifeedant activity of cucurbitacins from Iberis amara against larvae of Pieris rapae. Phytochemistry 1993, 33, 1385–1388. [Google Scholar] [CrossRef]
- Morimoto, M.; Fukumoto, H.; Nozoe, T.; Hagiwara, A.; Komai, K. Synthesis and insect antifeedant activity of aurones against Spodoptera litura larvae. J. Agric. Food Chem. 2007, 55, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Dohmura, A.; Takada, N.; Ueda, M. Cytotoxic antifeedant from Dionaea muscipula Ellis: A defensive mechanism of carnivorous plants against predators. Bull. Chem. Soc. Jpn. 2004, 77, 537–541. [Google Scholar] [CrossRef]
- Deng, Y.-Y.; Qu, B.; Zhan, Z.-L.; Wang, A.-Q.; Zhou, W.; Jia, M.-Y.; Hua, J.; Luo, S.-H. Bioactive tigliane diterpenoids from the latex of Euphorbia fischeriana. Nat. Prod. Res. 2019, 35, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Ignacimuthu, S.; Gabriel Paulraj, M. Antifeedant and larvicidal activities of Rhein isolated from the flowers of Cassia fistula L. Saudi J. Biol. Sci. 2011, 18, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.C.; Kogan, M.; Paxton, J. Effect of Glyceollin, a soybean phytoalexin, on feeding by three phytophagous beetles (Coleoptera: Coccinellidae and Chrysomelidae): Dose versus response. Environ. Entomol. 1990, 19, 1278–1282. [Google Scholar] [CrossRef]
- Wu, H.-B.; Liu, T.-T.; Lian, Y.-X.; Chen, X.; Wang, W.-S. Antifeedant activities of secondary metabolites from Hyssopus cuspidatus against Plutella xylostella. Chem. Nat. Compnd. 2018, 54, 1088–1090. [Google Scholar] [CrossRef]
- Luo, S.H.; Luo, Q.; Niu, X.M.; Xie, M.J.; Zhao, X.; Schneider, B.; Gershenzon, J.; Li, S.H. Glandular trichomes of Leucosceptrum canum harbor defensive sesterterpenoids. Angew. Chem. Int. Ed. 2010, 49, 4471–4475. [Google Scholar] [CrossRef]
- Morimoto, M.; Tanimoto, K.; Nakano, S.; Ozaki, T.; Nakano, A.; Komai, K. Insect antifeedant activity of flavones and chromones against Spodoptera litura. J. Agric. Food Chem. 2003, 51, 389–393. [Google Scholar] [CrossRef]
- Bruno, M.; Piozzi, F.; Maggio, A.M.; Rosselli, S.; Simmonds, J.; Servettaz, O. Antifeedant activity of neo-clerodane diterpenoids from Teucrium arduini. Biochem. Syst. Ecol. 2002, 30, 595–599. [Google Scholar] [CrossRef]
- Bruno, M.; Piozzi, F.; Roselli, S. Natural and hemisynthetic neoclerodane diterpenoids from Scutellaria and their antifeedant activity. Nat. Prod. Rep. 2002, 19, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Caballero, C.; Castan, P.; Ortego, F.; Fontana, G.; Pierro, P.; Savona, G.; Rodríguez, B. Effects of ajugarins and related neoclerodane diterpenoids on feeding behaviour of Leptinotarsa decemlineata and Spodoptera exigua larvae. Phytochemistry 2001, 58, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.Y.; Chen, D.Z.; Luo, S.H.; Kong, N.C.; Zhang, Y.; Di, Y.T.; Zhang, Q.; Hua, J.; Jing, S.X.; Li, S.L.; et al. Limonoids from Aphanamixis polystachya and their antifeedant activity. J. Nat. Prod. 2014, 77, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.S.; Kalaivani, K.; Murugan, K.; Chung, P.G. Efficacy of neem limonoids on Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae) the rice leaffolder. Crop Prot. 2005, 24, 760–763. [Google Scholar] [CrossRef]
- Lin-er, L.; van Loon, J.J.A.; Schoonhoven, L.M. Behavioural and sensory responses to some neem compounds by Pieris brassicae larvae. Physiol. Entomol. 1995, 20, 134–140. [Google Scholar] [CrossRef]
- Koul, O.; Isman, M.B. Toxicity of the limonoid allelochemical cedrelone to noctuid larvae. Entomol. Exp. Appl. 1992, 64, 281–287. [Google Scholar] [CrossRef]
- Mootoo, B.S.; Ali, A.; Motilal, R.; Pingal, R.; Ramlal, A.; Khan, A.; Reynolds, W.F.; McLean, S. Limonoids from Swietenia macrophylla and S. aubrevilleana. J. Nat. Prod. 1999, 62, 1514–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, J.-B.; Yang, H.B.; Gao, F.; Zhou, X.L.; Chen, L.; Huang, S. Diterpenoid alkaloids from Aconitum leucostomum and their antifeedant activity. Heterocycles 2021, 102, 1579–1587. [Google Scholar] [CrossRef]
- Huang, S.; Feng, Y.; Ren, J.; Yang, C.; Chen, L.; Zhou, X. Diterpenoid alkaloids from the roots of Aconitum rockii and their antifeedant activity. Chin. J. Org. Chem. 2022, 42, 18561862. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, L.; Huang, S.; Shan, L.H.; Gao, F.; Zhou, X.L. Diterpenoid alkaloids from two Aconitum species with antifeedant activity against Spodoptera exigua. J. Nat. Prod. 2017, 80, 3136–3142. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Hao, N.; Su, X.; Du, J.; Hu, J.; Tian, X. The antifeedant, insecticidal and insect growth inhibitory activities of triterpenoid saponins from Clematis aethusifolia Turcz against Plutella xylostella (L.). Pest Manag. Sci. 2021, 77, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Park, I.K.; Lee, H.S.; Lee, S.G.; Park, J.D.; Ahn, Y.J. Antifeeding activity of isoquinoline alkaloids identified in Coptis japonica roots against Hyphantria cunea (Lepidoptera: Arctiidae) and Agelastica coerulea (Coleoptera: Galerucinae). J. Econ. Entomol. 2000, 93, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Chen, L.; Gao, F.; Zhou, X. Diterpenoid alkaloids from Delphinium naviculare var. lasiocarpum with their antifeedant activity on Spodoptera exigua. Nat. Prod. Res. 2019, 33, 3254–3259. [Google Scholar] [CrossRef]
- Gao, G.; Lu, Z.; Tao, S.; Zhang, S.; Wang, F. Triterpenoid saponins with antifeedant activities from stem bark of Catunaregam spinosa (Rubiaceae) against Plutella xylostella (Plutellidae). Carbohydr. Res. 2011, 346, 2200–2205. [Google Scholar] [CrossRef]
- Morimoto, M.; Tanimoto, K.; Sakatani, A.; Komai, K. Antifeedant activity of an anthraquinone aldehyde in Galium aparine L. against Spodoptera litura F. Phytochemistry 2002, 60, 163–166. [Google Scholar] [CrossRef]
- Baskar, K.; Duraipandiyan, V.; Ignacimuthu, S. Bioefficacy of the triterpenoid friedelin against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2014, 70, 1877–1883. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Wu, G.; Tang, Q.; Duan, X.; Liu, Q.; Lan, M.; Zhao, Y.; Hao, X.; Qin, X.; et al. Antifeedant mechanism of Dodonaea viscosa saponin A isolated from the seeds of Dodonaea viscosa. Molecules 2022, 27, 4464. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kumar, J.; Dhingra, S.; Parmar, B.S. Screening for feeding deterrent and insect growth regulatory activity of triterpenic saponins from Diploknema butyracea and Sapindus mukorossi. J. Agric. Food Chem. 2010, 58, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.N.; Rani, A.; Tripathi, A.K.; Sharma, S. Antifeedant activity of ursolic acid isolated from Duboisia myoporoides. Phytother. Res. 1996, 10, 359–360. [Google Scholar] [CrossRef]
- Gabaston, J.; el Khawand, T.; Waffo-Teguo, P.; Decendit, A.; Richard, T.; Mérillon, J.M.; Pavela, R. Stilbenes from grapevine root: A promising natural insecticide against Leptinotarsa decemlineata. J. Pest Sci. 2018, 91, 897–906. [Google Scholar] [CrossRef]
- Rodilla, J.M.; Silva, L.A.; Martinez, N.; Lorenzo, D.; Davyt, D.; Castillo, L.; Giménez, C.; Cabrera, R.; González-Coloma, A.; Zrostíková, J.; et al. Advances in the identification and agrochemical importance of sesquiterpenoids from Bulnesia sarmientoi essential oil. Ind. Crops Prod. 2011, 33, 497–503. [Google Scholar] [CrossRef]
- Pavela, R. Antifeedant and Larvicidal Effects of Some phenolic components of essential oils lasp lines of introduction against Spodoptera littoralis (Boisd.). J. Essent. Oil Bear. Plants 2011, 14, 266–273. [Google Scholar] [CrossRef]
- Gols, G.J.Z.; van Loon, J.J.A.; Messchendorp, L. Antifeedant and toxic effects of drimanes on Colorado potato beetle larvae. Entomol. Exp. Appl. 1996, 79, 69–76. [Google Scholar] [CrossRef]
- Eichenseer, H.; Mullin, C.A. Antifeedant comparisons of gaba/ glycinergic antagonists for diabroticite leaf beetles (coleoptera: Chrysomelidae). J. Chem. Ecol. 1997, 23, 71–82. [Google Scholar] [CrossRef]
- Kiran, S.R.; Reddy, A.S.; Devi, P.S.; Reddy, K.J. Insecticidal, antifeedant and oviposition deterrent effects of the essential oil and individual compounds from leaves of Chloroxylon swietenia DC. Pest Manag. Sci. 2006, 62, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Diaz Napal, G.N.; Palacios, S.M. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Abbassy, M.A.; Belal, A.S.H.; Abdel Rasoul, M.A.A. Bioactivity of two major constituents isolated from the essential oil of Artemisia judaica L. Bioresour. Technol. 2008, 99, 5947–5950. [Google Scholar] [CrossRef]
- Sreelatha, T.; Hymavathi, A.; Babu, K.S.; Murthy, J.M.; Pathipati, U.R.; Rao, J.M. Synthesis and insect antifeedant activity of plumbagin derivatives with the amino acid moiety. J. Agric. Food Chem. 2009, 57, 6090–6094. [Google Scholar] [CrossRef]
- Vargas-Méndez, L.Y.; Sanabria-Flórez, P.L.; Saavedra-Reyes, L.M.; Merchan-Arenas, D.R.; Kouznetsov, V.V. Bioactivity of semisynthetic eugenol derivatives against Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae infesting maize in Colombia. Saudi J. Biol. Sci. 2019, 26, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Pour, S.A.; Shahriari, M.; Zibaee, A.; Mojarab-Mahboubkar, M.; Sahebzadeh, N.; Hoda, H. Toxicity, antifeedant and physiological effects of trans-anethole against Hyphantria cunea Drury (Lep: Arctiidae). Pestic. Biochem. Physiol. 2022, 185, 105135. [Google Scholar] [CrossRef]
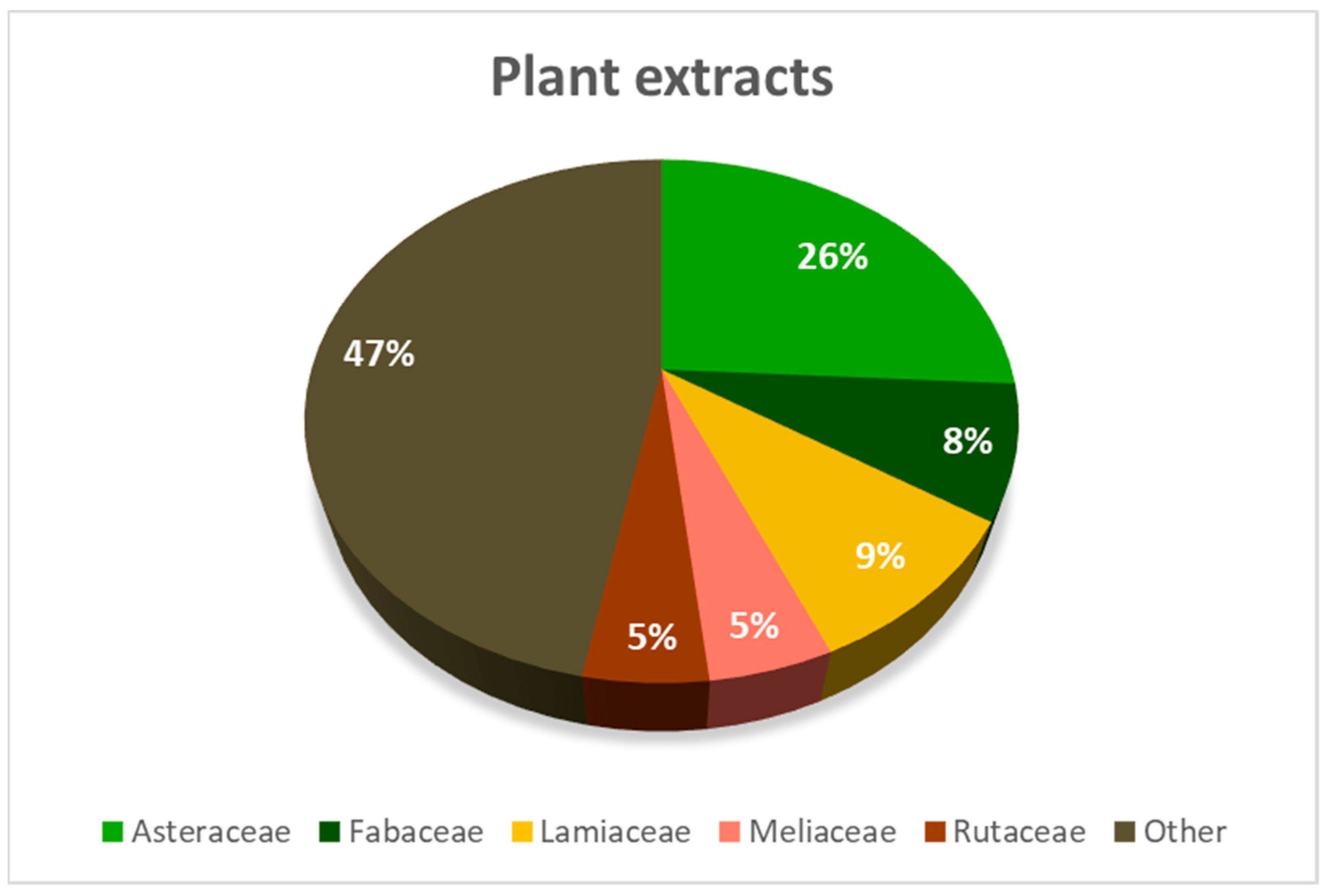
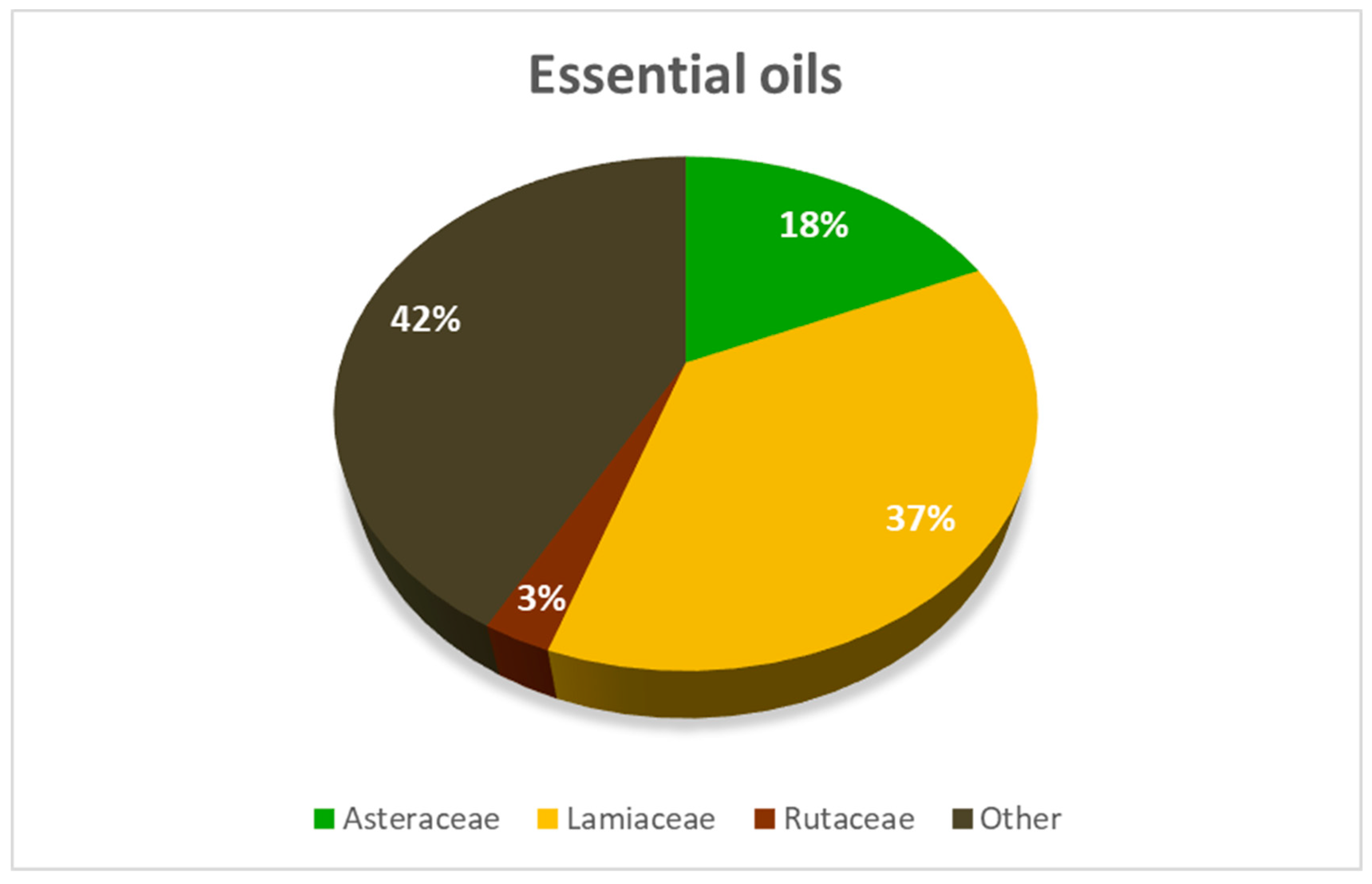
| Family/Plant | Type | Majority Compounds with Antifeedant Effect | Insect (Instar) | Test | EC50 (μg mL−1) | ED50 (μg cm−2) | References |
|---|---|---|---|---|---|---|---|
| Anacardiaceae | |||||||
| Anacardium occidentale L. | Cashew nutshell liquid | Phenolic compounds | Spodoptera frugiperda—L4 | no choice | 3400 | [41] | |
| Annonaceae | |||||||
| Annona squamosa L. | Crude seed methanol extracts | Sesquiterpenes, monoterpenes | Trichoplusia ni—L3 | choice | 2300 | [42] | |
| Polyalthia longifolia (Sonn.) Thwaites | Methanol extract | Terpenes | Spodoptera litura—L3 | no choice | 1080 | [43] | |
| Apiaceae | |||||||
| Angelica archangelica L. | Methanol extracts | Unspecified | Leptinotarsa decemlineata—L4 | no choice | 0.6 | [44] | |
| Angelica archangelica L. | Seeds benzene extract | Bergapten, imperatorin, phellopterin | Spodoptera littoralis—L3 | no choice | 0.31 | [45] | |
| Angelica archangelica L. | Seeds acetone extract | Bergapten, imperatorin, phellopterin | Spodoptera littoralis—L3 | no choice | 0.65 | [45] | |
| Angelica archangelica L. | Seeds methanol extract | Bergapten, imperatorin, phellopterin | Spodoptera littoralis—L3 | no choice | 0.54 | [45] | |
| Apocynaceae | |||||||
| Tylophora indica (Burm. f.) Merr. | Ethanolic extract | Unspecified | Spodoptera litura—L4 | 8300 | [46] | ||
| Araliaceae | |||||||
| Panax ginseng C. A. Meyer | Stems and leaves extract—ginsenosides | Ginsenosides | Plutella xylostella—L2 | choice | 2740 | [47] | |
| Asteraceae | |||||||
| Grindelia camporum Hook. & Arn. | Methanol extracts | Unspecified | Leptinotarsa decemlineata—L4 | no choice | 0.2 | [44] | |
| Inula auriculata Boiss. & Balansa | Methanol extracts | Unspecified | Leptinotarsa decemlineata—L4 | no choice | 0.2 | [44] | |
| Pyrethrum corymbosum (L.) Scop. | Methanol extracts | Unspecified | Leptinotarsa decemlineata—L4 | no choice | 3 | [44] | |
| Senecio fistulosus Poepp. ex DC | Furanoeremophilane | Eremophilane-typesesquiterpenes of the furanoeremophilane and eremophilanolidesesquiterpenes | Spodoptera littoralis—L6 | 0.64 | [48] | ||
| Senecio kingii Hook.f. | Aerial parts alkaloidal extracts | Eremophilanolidess, shikimic acid derivatives, flavonoids | Spodoptera littoralis—L6 | choice | 0.09 | [49] | |
| Xeranthemum cylindraceum Sibth. & Sm. | Methanol extracts | Unspecified | Leptinotarsa decemlineata—L4 | no choice | 8 | [44] | |
| Boraginaceae | |||||||
| Echium wildpretii H. Pearson ex Hook. f. subsp. wildpretii | Fraction 2 from ethanol extract: hexane/ethyl acetate, 90: 10 v/v); steroidal fraction—compound 3 | Fatty acid esters, phytosterols | Leptinotarsa decemlineata (unspecified stadium) | choice | 0.4 | [50] | |
| Fabaceae | |||||||
| Caesalpinia bonduc (L.) Roxb. | Chloroform extract—fraction 3 | Coumarins, flavonoids, terpenoids, phenols, quinones | Helicoverpa armigera—L3 | no choice | 357.13 | [51] | |
| Millettia pachycarpa (Benth.) | Fresh leaves methanol extract -> dichlormethane -> fraction 2 | Triterpenoid (lupeol) | Spodoptera litura—L3 | no choice | 227.13 | [52] | |
| Lamiaceae | |||||||
| Teucrium hircanicum L. | Methanol extracts | Unspecified | Leptinotarsa decemlineata—L4 | no choice | 6 | [44] | |
| Lauraceae | |||||||
| Persea indica (L.) Spreng. | Stem extract | Ryanoids | Spodoptera littoralis—L6 | no choice | 8.5 | [53] | |
| Meliaceae | |||||||
| Melia volkensii Gürke | Refined seed extract | Terpenoids | Epilachna varivestis—A | choice | 2.3 | [54] | |
| Rutaceae | |||||||
| Clausena anisata (Willd.) Hook.f. ex Benth. | Chloroform root extracts | Osthol (coumarin derivate) | Helicoverpa armigera—L5 | choice | 140 | [55] | |
| Clausena anisata (Willd.) Hook.f. ex Benth. | Petroleum ether root extracts | Osthol (coumarin derivate) | Helicoverpa armigera—L5 | choice | 160 | [55] | |
| Solanaceae | |||||||
| Solanum xanthocarpum Schrad. & Wendl. | Chloroform extract, fraction 4 | Terpenoids, flavonoid, quinone | Helicoverpa armigera—L3 | no choice | 378.3 | [56] | |
| Vitaceae | |||||||
| Vitis vinifera L. | Vine-shoot wastes: Conventional Solid–Liquid Extraction 60 min | Flavanols | Leptinotarsa decemlineata—A | choice | 0.08 | [57] |
| Family/Plant | Parts | Majority Compounds with Antifeedant Effect | Insect (Instar) | Test | EC50 (μg mL−1) | ED50 (μg cm−2) | References |
|---|---|---|---|---|---|---|---|
| Acoraceae | |||||||
| Acorus calamus L. | Rhizomes—cis-asarone | Cis-asarone, trans-asarone | Peridroma saucia—L4 | choice | 2.5 | [58] | |
| Apiaceae | |||||||
| Angelica archangelica L. | Seeds | β-Phellandrene, sabinene, α-pinene, α-phellandrene | Spodoptera littoralis—L3 | no choice | 7.12 | [45] | |
| Asteraceae | |||||||
| Artemisia nakaii Pamp. | Aerial parts | Feropodin, (+)-camphor, 1,8-cineole, rishitin | Spodoptera litura—L3 | choice | 3.76 ± 0.73 | [59] | |
| Lamiaceae | |||||||
| Lavandula luisieri (Rozeira)—cultivated pop. | Flowering parts | 3-Oxo-cadinol, 2,3,4,4-Tetramethyl-5-methylidenecyclopent-2-en-1-one, Hydroxymethyl-2,3,4,4-tetramethylcyclopent-2-en-1-one | Spodoptera littoralis—L6 | 10.23 | [60] | ||
| Mentha pulegium Mill. | Leaves and flowers | Pulegone, 1,3,4-trimethyl-3-cyclohexene-1-carboxaldehyde, piperitenone | Spodoptera littoralis—L6 | choice | 1.3 (0.4, 4.1) | [61] | |
| Piperaceae | |||||||
| Piper hispidinervum C.DC. | Fresh leaves and twigs | Safrole, terpinolene | Leptinotarsa decemlineata | choice | 0.4 | [62] | |
| Piper hispidinervum C.DC. | Fresh leaves and twigs | Safrole | Spodoptera littoralis—L | choice | 3.1 | [62] | |
| Piper sanctifelicis Trel. | Leaves | δ-3-carene, limonene, p-cymene, β-pinene, nerolidol | Spodoptera littoralis—L6 | choice | 4.5 (4.3–4.7) | [63] | |
| Rutaceae | |||||||
| Citrus aurantifolia (L.) Swingle | Commercial EOs and limonene | γ-Muurolene, o-cymene, bornyl acetate, α-bisabolol | Plutella xylostella—L3—deltamethrin susceptible strain | choice | 68.93 | [64] |
| Plant | Type | Insect (Instar) | Test | EC50 (μg mL−1) | ED50 (μg cm−2) | References |
|---|---|---|---|---|---|---|
| Asteraceae | ||||||
| Carpesium abrotanoides L. | Air-dried fruits—compound 1 | Plutella xylostella—L3 | choice | 19.8 | [65] | |
| Eupatorium adenophorum Spreng | Sesquiterpenoids, compound 2 and 3 | Helicoverpa armigera—L2 | choice | 2.5 and 3.0 | [66] | |
| Flourensia oolepis S.F. Blake | Aerial parts—flavonoid pinocembrin | Epilachna paenulata—L3 | choice | 10 | [67] | |
| Pericallis spp. | 3-ethoxy-hydroxy-tremetone; (-)-eupachinin A | Spodoptera litoralis—L6 | choice | 130 | [68] | |
| Senecio adenotrichius DC. | Compound 1—dehydrofukinone | Spodoptera litoralis—L6 | unspecified | 1.6 | [69] | |
| Senecio palmensis C. Sm. | 11β, 5α-dihydroxysilphinen-3-one | Leptinotarsa decemlineata—L4 | no choice | 11.3 | [70] | |
| Smallanthus sonchifolius (Poepp. & Endl.) H. Rob | Uvedalin | Spodoptera litura—L3 | choice | 8 | [71] | |
| Colchicaceae | ||||||
| Gloriosa superba L. | Chloroform tuber extract—GST4 | Spodoptera litura—L3 | no choice | 26 | [72] | |
| Cucurbitaceae | ||||||
| Citrullus colocynthis (L.) Schrad. | Cucurbitacin E—fruits (fraction III) | Spodoptera litura—L5 | choice | 24.1 | [73] | |
| Cupressaceae | ||||||
| Juniperus sabina L. | Petroleum ether extract—deoxypodophyllotoxin (1) | Pieris rapae—L5 | 60 (48 h) | [74] | ||
| Ericaceae | ||||||
| Pieris formosa (Wallich) D. Don | Grayanane diterpenoids—10 | Spodoptera exigua | choice modified | 6.58 | [75] | |
| Pieris japonica (Thunb.) D. Don ex G. Don | Neopierisoid B—isolated from flowers | Pieris brassicae—L3 | choice | 5.33 | [76] | |
| Pieris japonica (Thunb.) D. Don ex G. Don | Flower diterpenoids—C10 | Pieris brassicae—L3 | choice | 0.03 | [77] | |
| Rhododendron molle (Blume) G. Don | Rhodojaponin III—grayanoid diterpene from flowers | Pieris rapae—L3 | no choice | 1.16 | [78] | |
| Rhododendron molle (Blume) G. Don | Rhodojaponin III—grayanoid diterpene from flowers | Pieris rapae—L5 | no choice | 15.85 | [78] | |
| Euphorbiaceae | ||||||
| Croton jatrophoides Pax. | Limonoids dumnin, dumsenin | Pectinophora gossypiella—L2 Spodoptera frugiperda—L2 | choice | ≤2 | [79] | |
| Croton jatrophoides Pax. | Limonoids from methanol extract—Musidunin | Pectinophora gossypiella—L2 | choice | 3 | [80] | |
| Croton jatrophoides Pax. | Limonoids from methanol extract—Musiduol | Pectinophora gossypiella—L2 | choice | 4 | [80] | |
| Croton jatrophoides Pax. | Limonoids from methanol extract—Musiduol | Spodoptera frugiperda—L2 | choice | 2 | [80] | |
| Euphorbia paralias L. | Ursane type triterpenoid—compound 21 (uvaol) | Leptinotarsa decemlineata—A | choice | 0.2 | [81] | |
| Euphorbia paralias L. | Ursane type triterpenoid—compound 21 (uvaol) | Spodoptera litoralis—L6 | choice | 3.3 | [81] | |
| Fabaceae | ||||||
| Caesalpinia bonduc (L.) Roxb. | Chloroform extract | Helicoverpa armigera—L3 | no choice | 1.08 | [56] | |
| Pterocarpus macrocarpus Kurz | Homopterocarpin | Spodoptera litura—L3 | choice | 0.04 | [82] | |
| Lamiaceae | ||||||
| Clerodendrum infortunatum L. | Clerodane diterpenoids—compound 1 | Helicoverpa armigera—L3 | choice | 6 | [83] | |
| Lauraceae | ||||||
| Persea indica (L.) Spreng. | Anhydrocinnzeylanine | Spodoptera littoralis—L5 | choice | 0.09 | [84] | |
| Persea indica (L.) Spreng. | Anhydrocinnzeylanine | Leptinotarsa decemlineata—A | choice | 0.94 | [84] | |
| Persea indica (L.) Spreng. | Cinnzeylanine | Spodoptera litoralis—L5 | choice | 0.004 | [85] | |
| Persea indica (L.) Spreng. | Leptinotarsa decemlineata—A | choice | 0.08 | [85] | ||
| Cinnzeylanone | ||||||
| Meliaceae | ||||||
| Aglaia odorata Lour. | Rocaglamide from dried twigs | Peridroma saucia—L4 | choice | 3.45 | [86] | |
| Azadirachta indica A. Juss. | Azadirachtin | Ostrinia nubilalis—L1 and L3 | no choice | 3.5 and 24 | [87] | |
| Melia volkensii Gürke | Volkensin | Spodoptera frugiperda—L3 | choice | 3.5 | [88] | |
| Melia volkensii Gürke | Xanthotoxin 99% | Trichopulsia ni—L3 | choice | 0.9 | [54] | |
| Melia toosendan Siebold & Zucc. | Toosedanin—limonoid isolated from bark | Peridroma saucia—L4 | choice | 8.04 | [89] | |
| Piperaceae | ||||||
| Piper ribesioides Wall. | Piperine | Spodoptera litura—L3 | choice | 3.1 | [90] | |
| Plantaginaceae | ||||||
| Linaria saxatilis (L.) Chaz. | Neo-clerodane diterpenoids, compound 2 | Leptinotarsa decemlineata—A | choice/no choice | 10.5/8.5 | [91] | |
| Linaria saxatilis (L.) Chaz. | Neo-clerodane diterpenoids, compound 6 | Leptinotarsa decemlineata—A | choice/no choice | 12.8/7.7 | [91] | |
| Linaria saxatilis (L.) Chaz. | Neo-clerodane diterpenoids, compound 8 | Leptinotarsa decemlineata—A | choice | 6.4 | [91] | |
| Rutaceae | ||||||
| Citrus aurantiifolia (Christm.) Swingle | Limonene | Plutella xylostella—L3—deltamethrin susceptible strain | choice | 4.44 | [92] | |
| Citrus aurantiifolia (Christm.) Swingle | Limonene | Plutella xylostella—L3—deltamethrin resistant strain | choice | 17.83 | [92] | |
| Simaroubaceae | ||||||
| Eurycoma longifolia Jack | Eurycomanone | Plutella xylostella—L3 | choice | 14.2 | [93] | |
| Winteraceae | ||||||
| Drimys winteri J.R. Forster et G. Forster | Polygodial | Spodoptera frugiperda—L3 | choice | 5.59 | [94] | |
| Unspecified | ||||||
| α-Pinene | Spodoptera litura—L3 | no choice | 1.13 uL cm−2 | [95] | ||
| Aconitine | Diabrotica virgifera—A | choice | 0.27 | [96] | ||
| Dehydrofukinone (SO) | Spodoptera littoralis—L6 | choice | 1.68 | [69] | ||
| Derivatives of eugenol and thymol (6) | Plutella xylostella—L3 | choice | 4.29 | [97] | ||
| Derivatives of eugenol and thymol (8) | Plutella xylostella—L3 | choice | 3.3 | [97] | ||
| Derivatives of eugenol and thymol (10) | Plutella xylostella—L3 | choice | 6.52 | [97] | ||
| Derivatives of eugenol and thymol (thymol) | Plutella xylostella—L3 | choice | 6.38 | [97] | ||
| Germacrone (SO) | Spodoptera littoralis—L6 | choice | 1.9 (0.1–3.6) | [98] | ||
| Piperitenone epoxide | Spodoptera littoralis—L6 | choice | 0.18 (0.01, 3.0) | [99] | ||
| Pulegone | Spodoptera littoralis—L6 | choice | 0.2 | [61] | ||
| Pulegone | Spodoptera littoralis—L6 | choice | 0.25 | [99] | ||
| Silphinene | Leptinotarsa decemlineata—A | choice | 0.15 | [96] | ||
| Thujone | Spodoptera littoralis—L6 | choice | 0.2 | [61] | ||
| 11α-Epoxy-eremophil-9-en-8-one (ligudicin A)1 | Spodoptera littoralis—L6 | choice | 0.08 (0.04–0.18) | [69] | ||
| (E)-β-Ocimene | Spodoptera littoralis—L6 | choice | 10.6 (7.1, 15.9) | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavela, R.; Kovaříková, K.; Novák, M. Botanical Antifeedants: An Alternative Approach to Pest Control. Insects 2025, 16, 136. https://doi.org/10.3390/insects16020136
Pavela R, Kovaříková K, Novák M. Botanical Antifeedants: An Alternative Approach to Pest Control. Insects. 2025; 16(2):136. https://doi.org/10.3390/insects16020136
Chicago/Turabian StylePavela, Roman, Kateřina Kovaříková, and Matěj Novák. 2025. "Botanical Antifeedants: An Alternative Approach to Pest Control" Insects 16, no. 2: 136. https://doi.org/10.3390/insects16020136
APA StylePavela, R., Kovaříková, K., & Novák, M. (2025). Botanical Antifeedants: An Alternative Approach to Pest Control. Insects, 16(2), 136. https://doi.org/10.3390/insects16020136





