Wound Healing in Butterfly Pupal Wing Tissues: Real-Time In Vivo Imaging of Long-Range Cell Migration, Cluster Formation, and Calcium Oscillations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Butterflies
2.2. Staining and Wounding Operations
2.3. Chemicals
2.4. Imaging
2.5. Statistical Analyses
3. Results
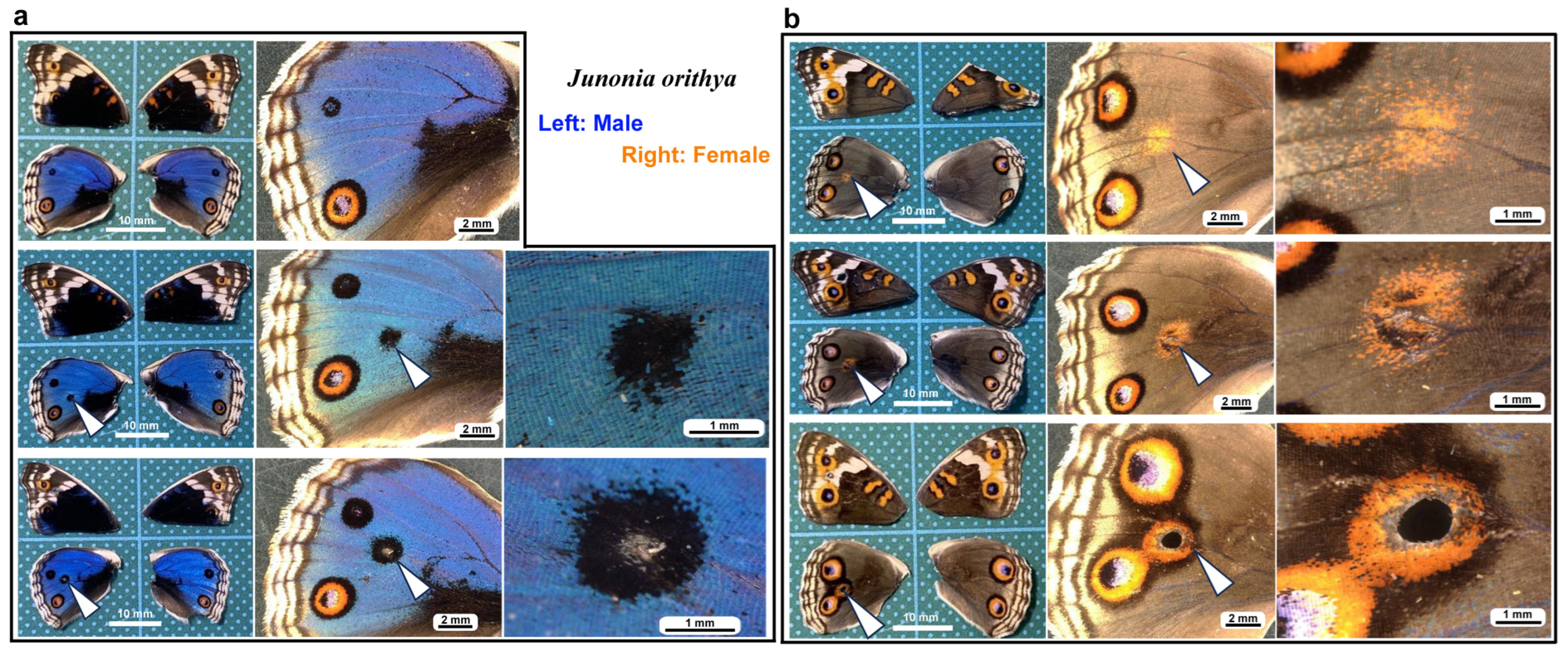
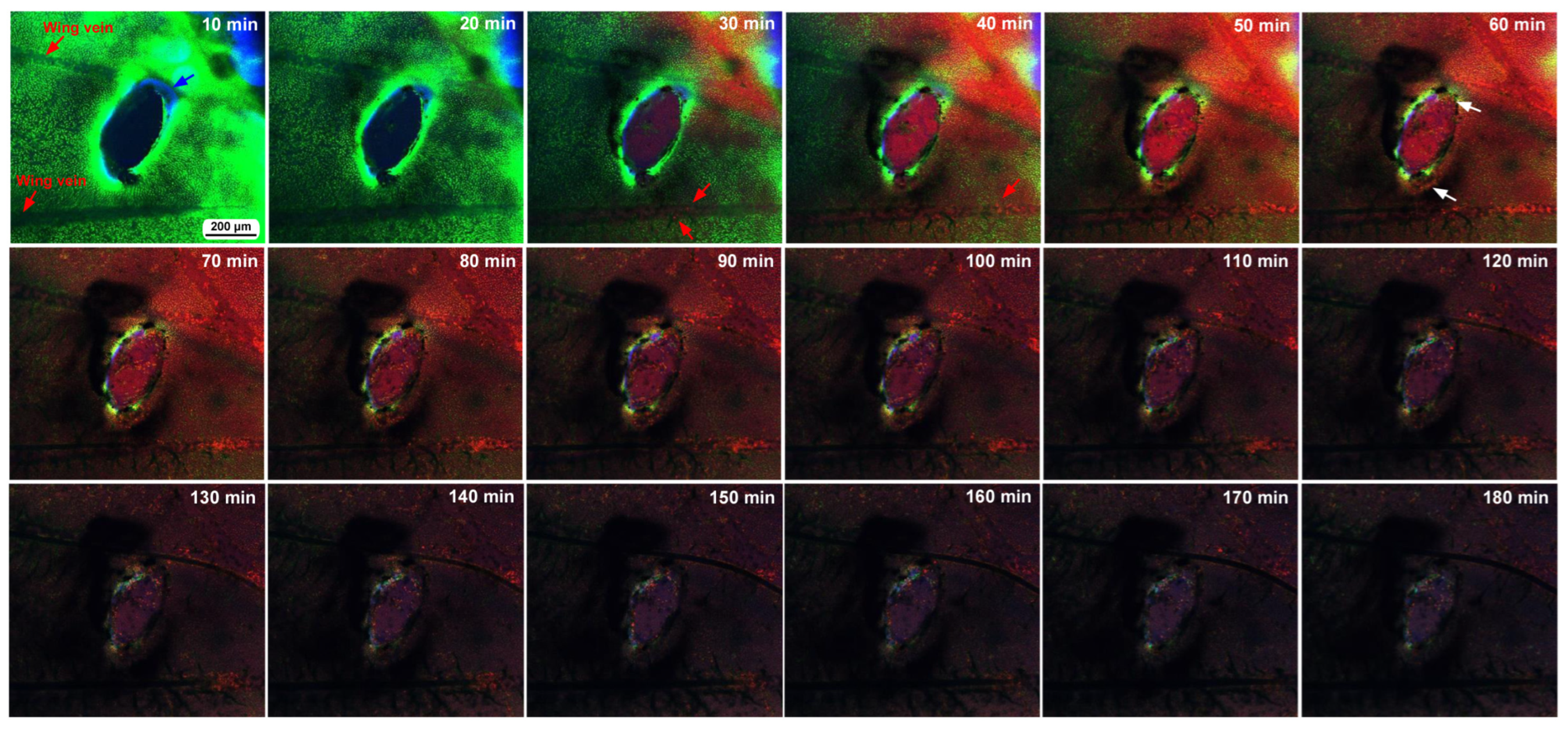
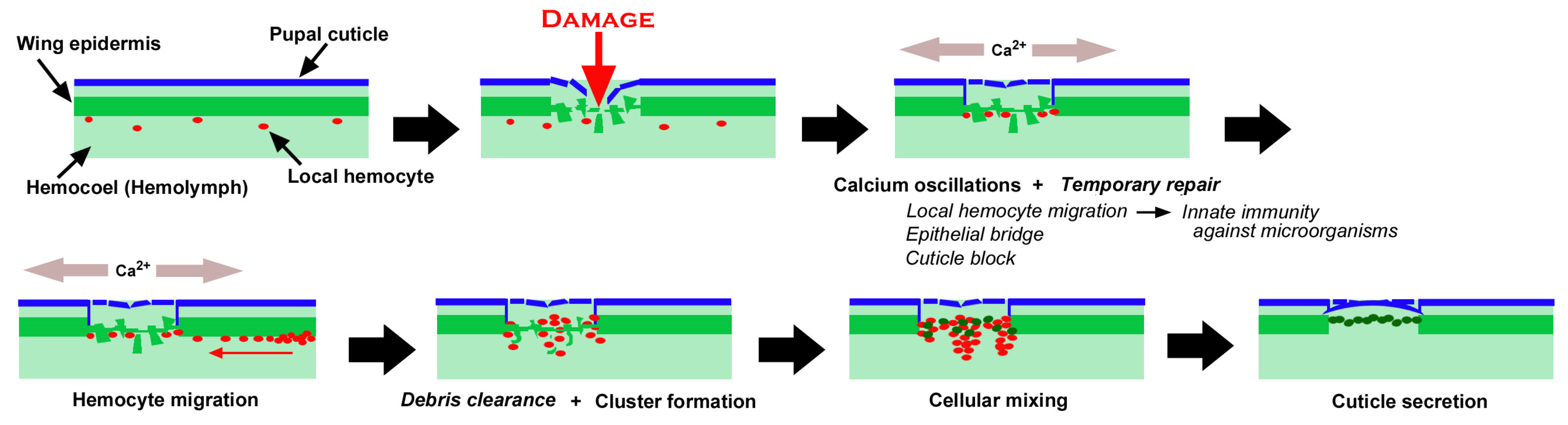
3.1. Damage-Induced Wing Repair and Ectopic Color Patterns
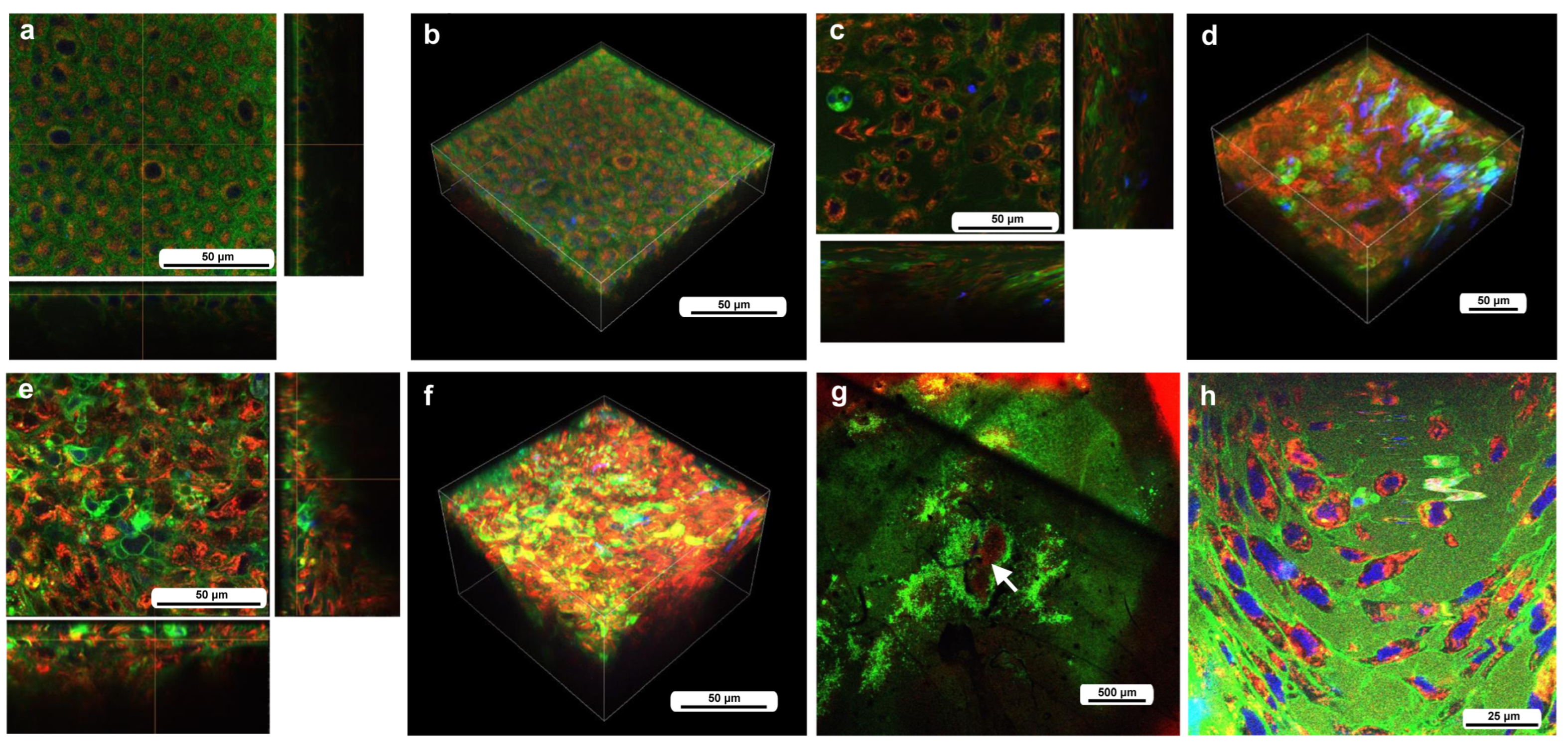
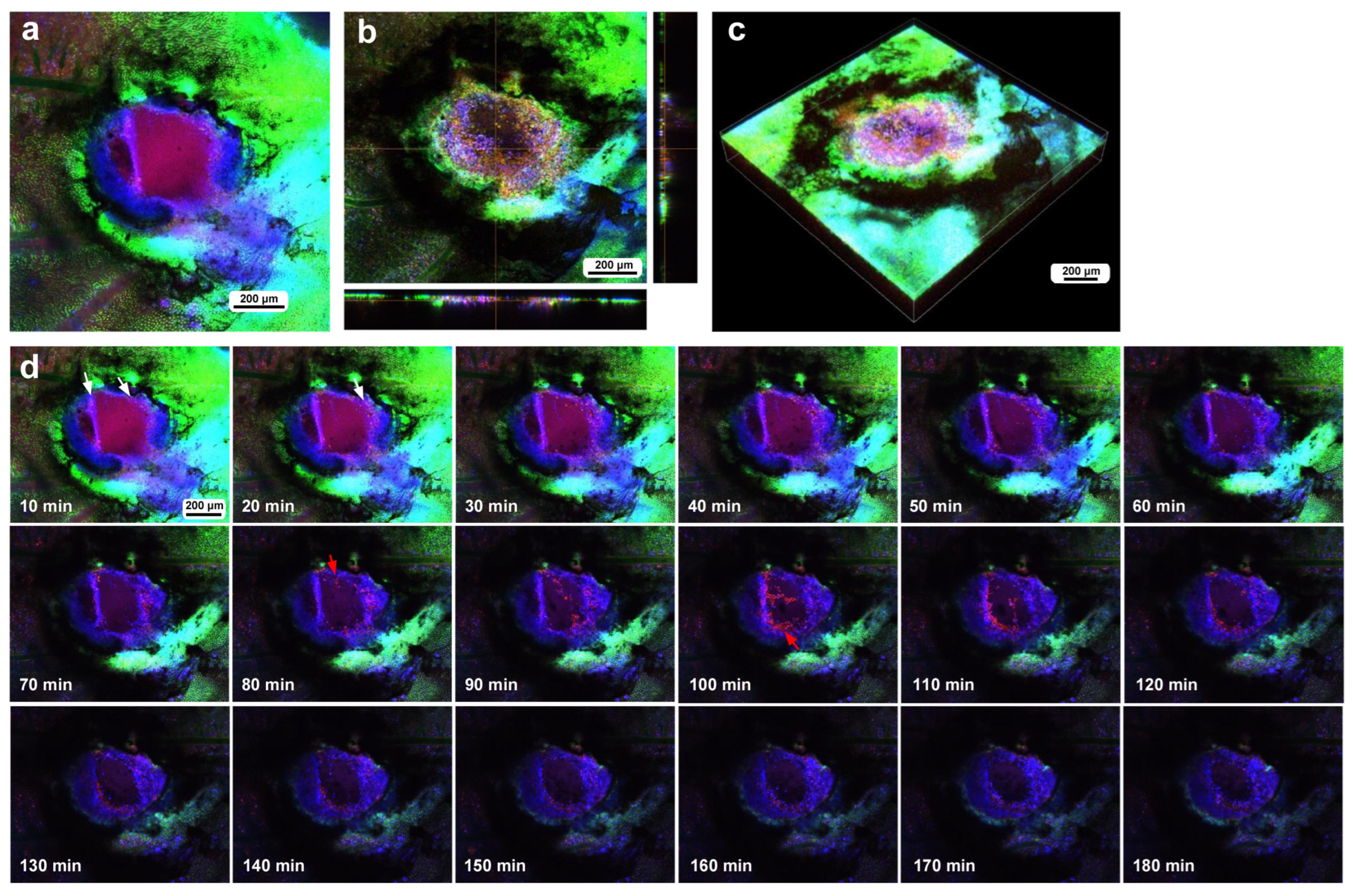
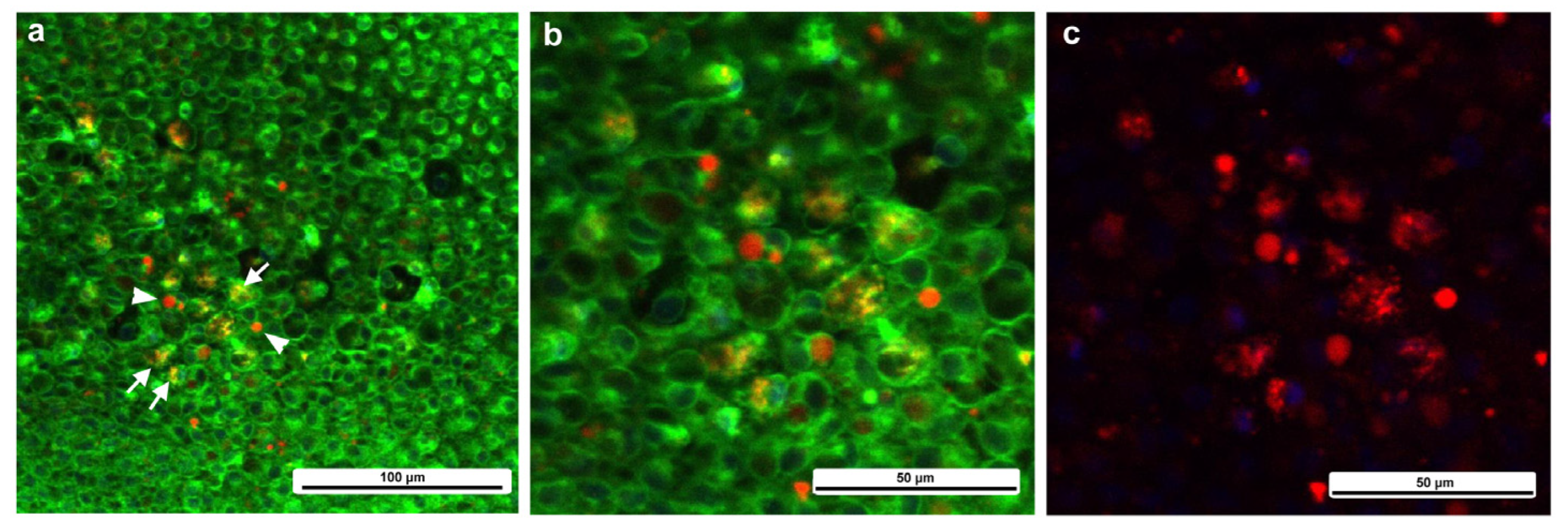
3.2. Cellular Characterization at the Damage Site
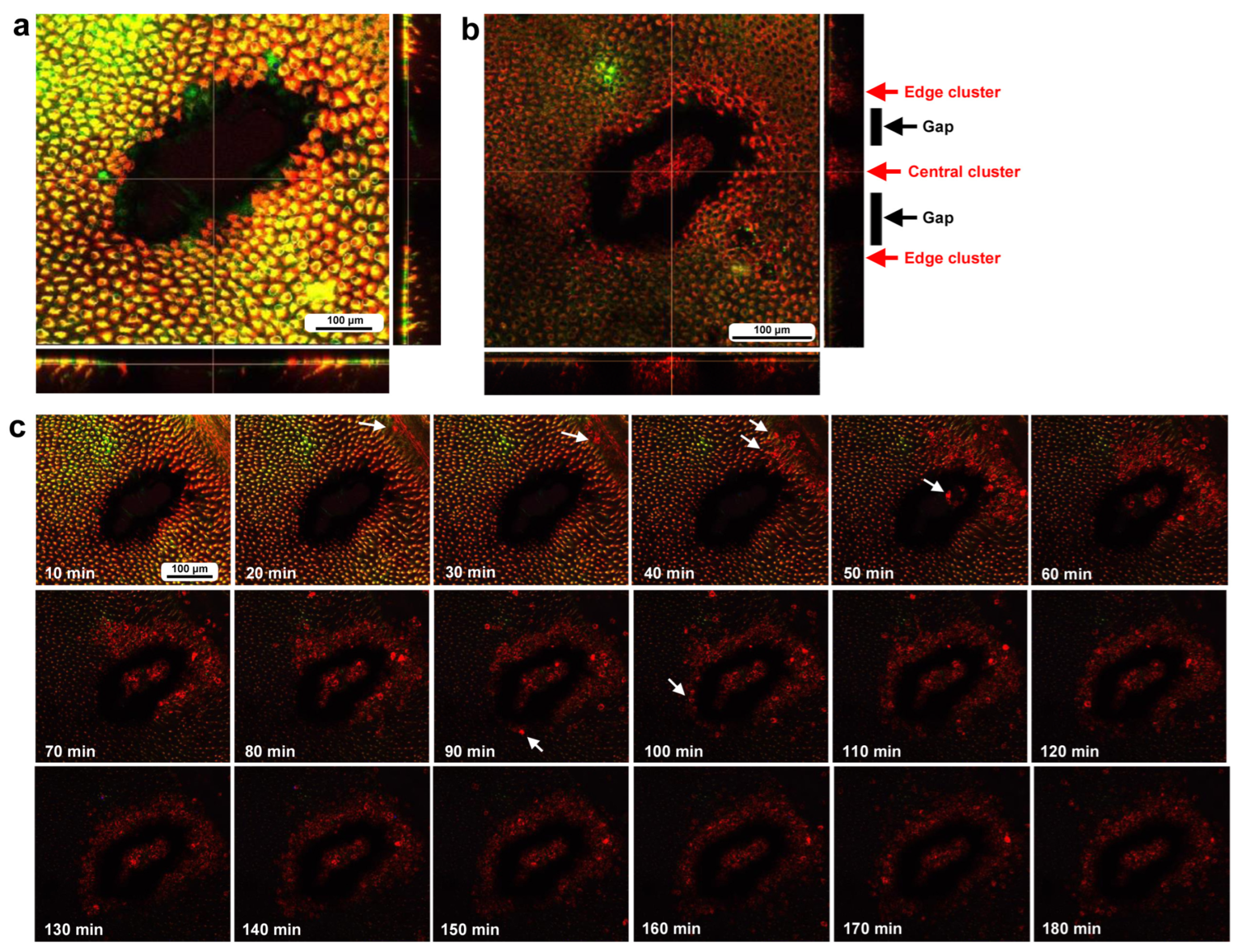
3.3. Damage-Induced Cell Migration: First Three Individuals
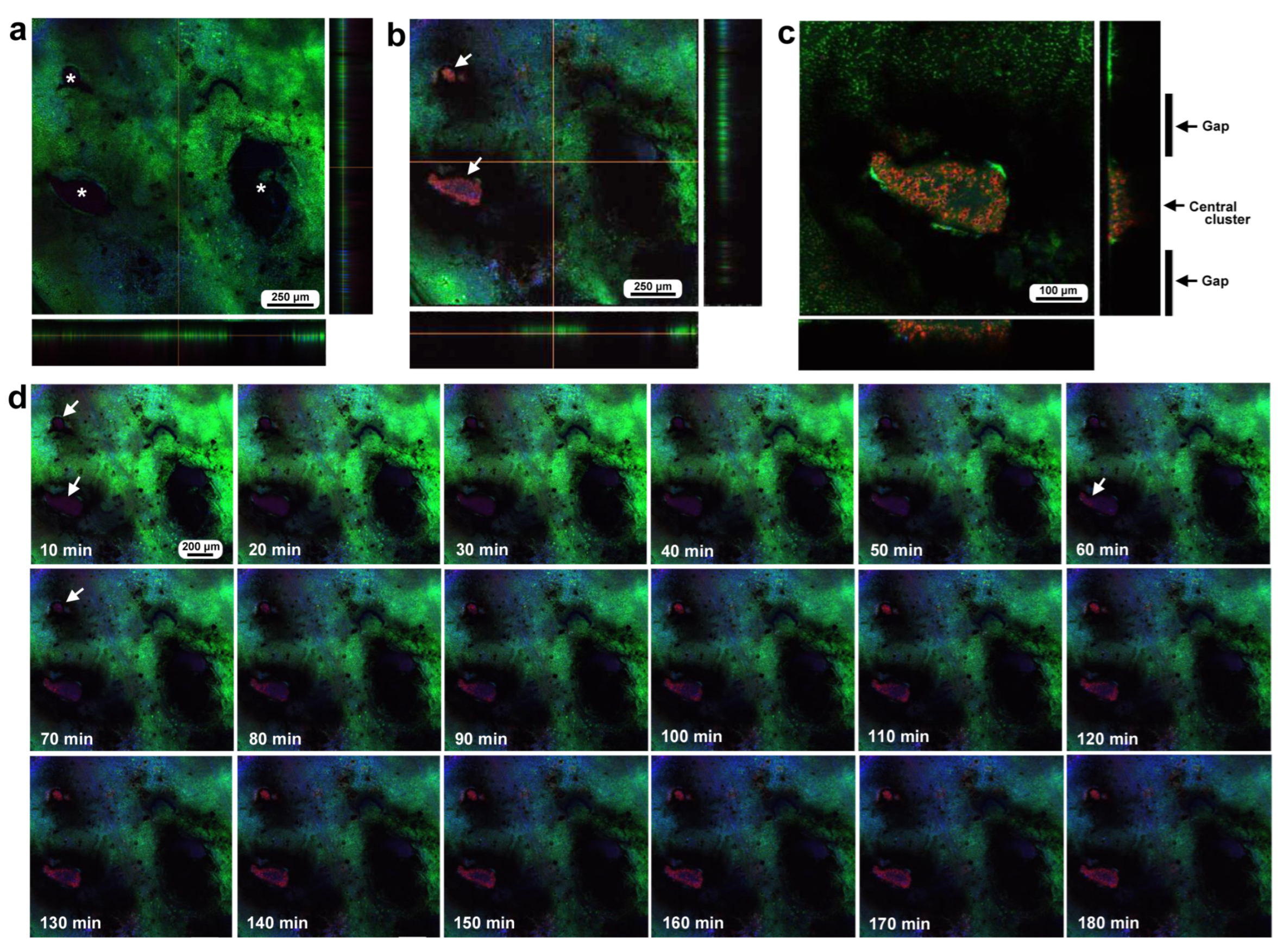
3.4. Damage-Induced Cell Migration: Two Additional Individuals with FB28
3.5. Damage Repair and Chitin Dynamics: Three Additional Individuals with FB28
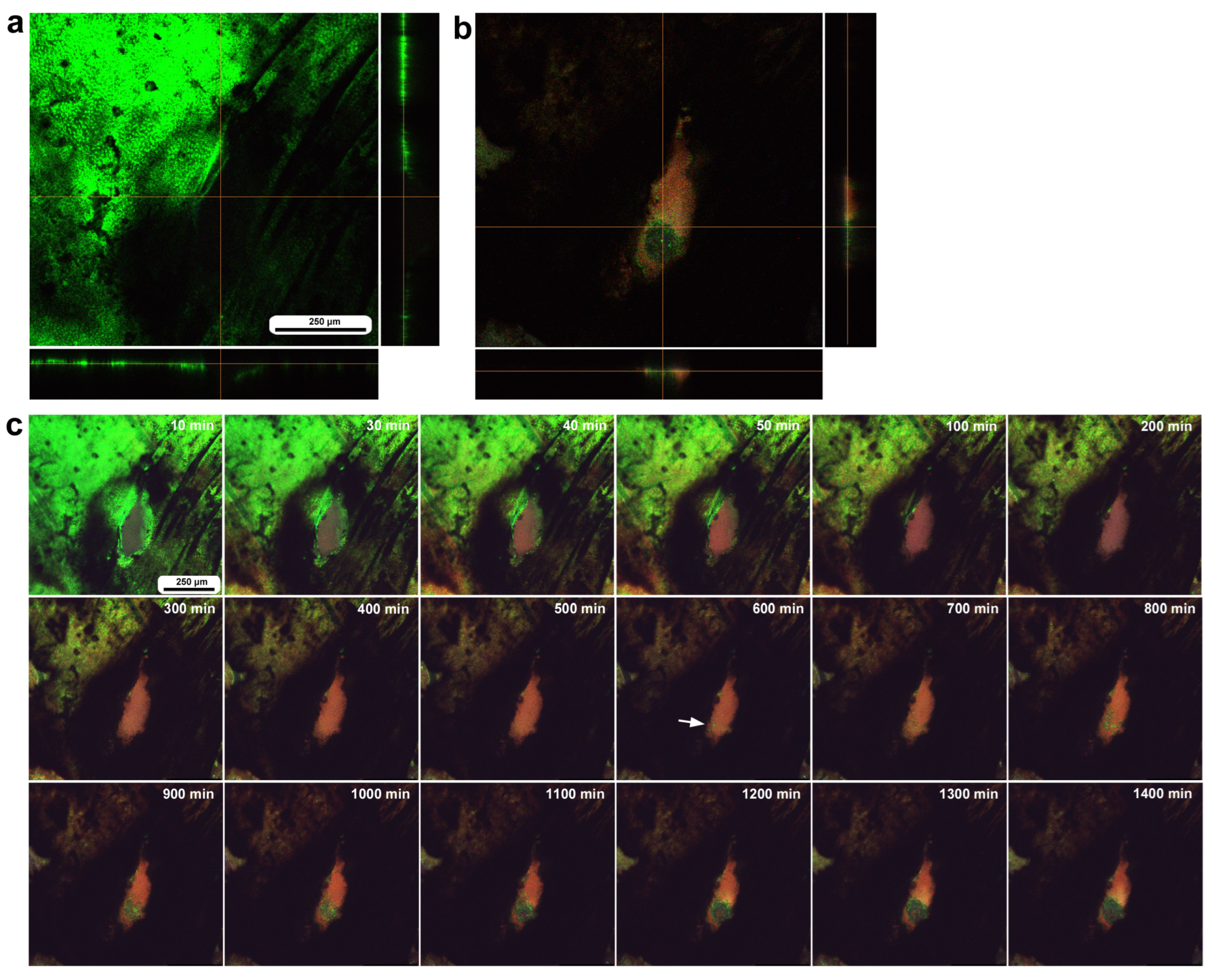
3.6. Long-Term Image Recording: Migration of BODIPY-Positive Cells
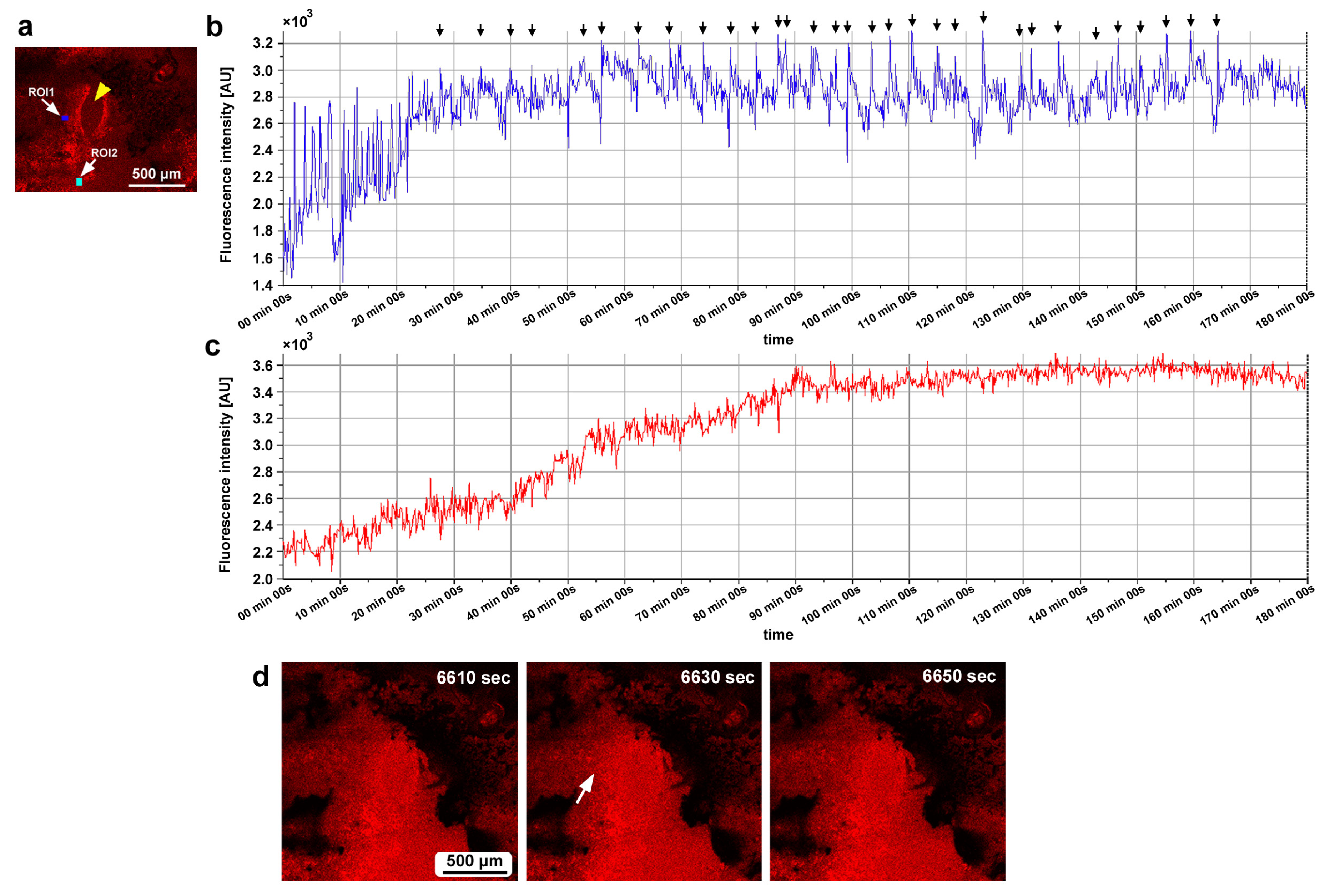
3.7. Damage-Induced Calcium Oscillations
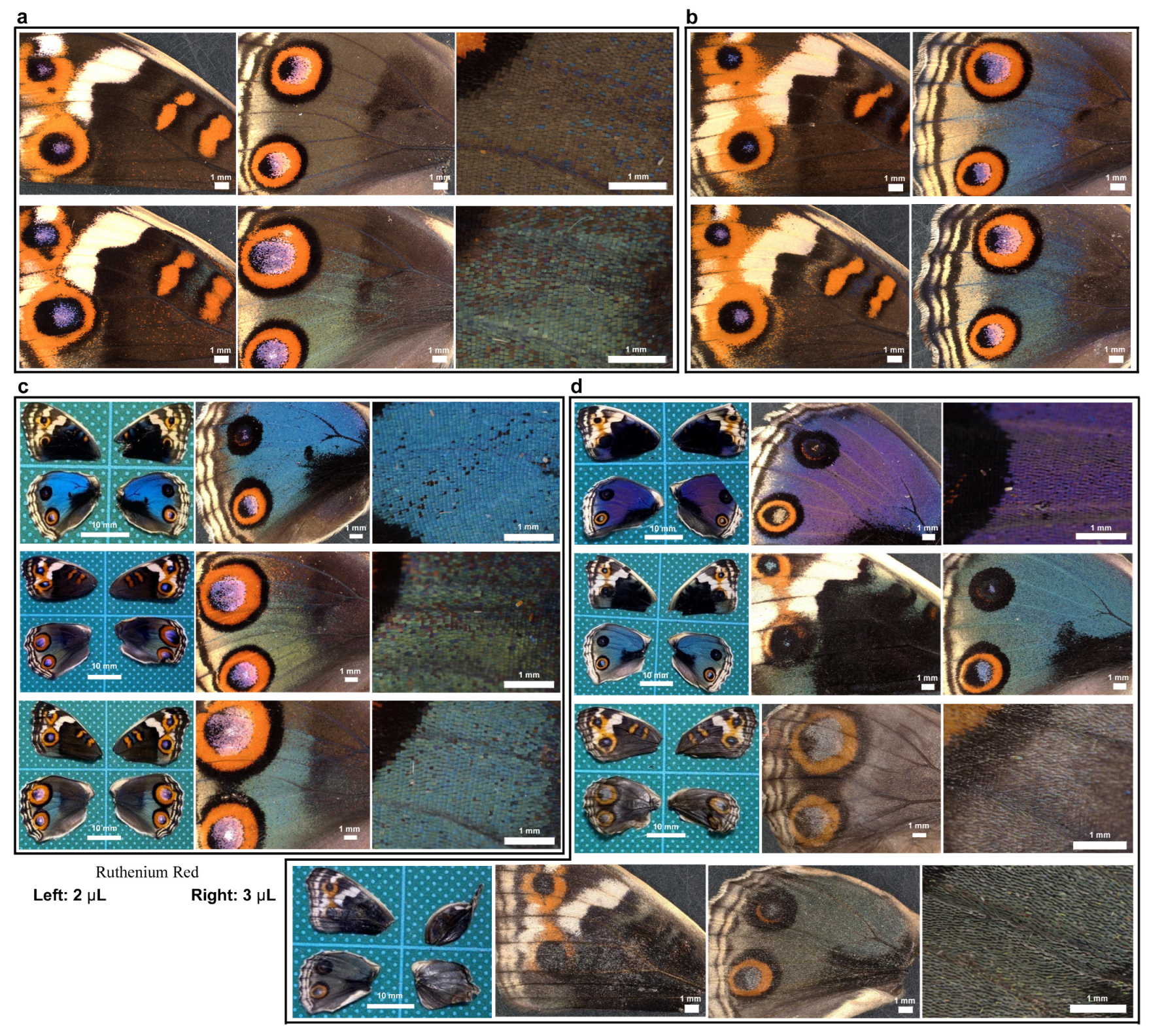
3.8. Ruthenium Red Affected Damage-Induced Calcium Oscillations
3.9. Ruthenium Red Affected Color and Scale Development
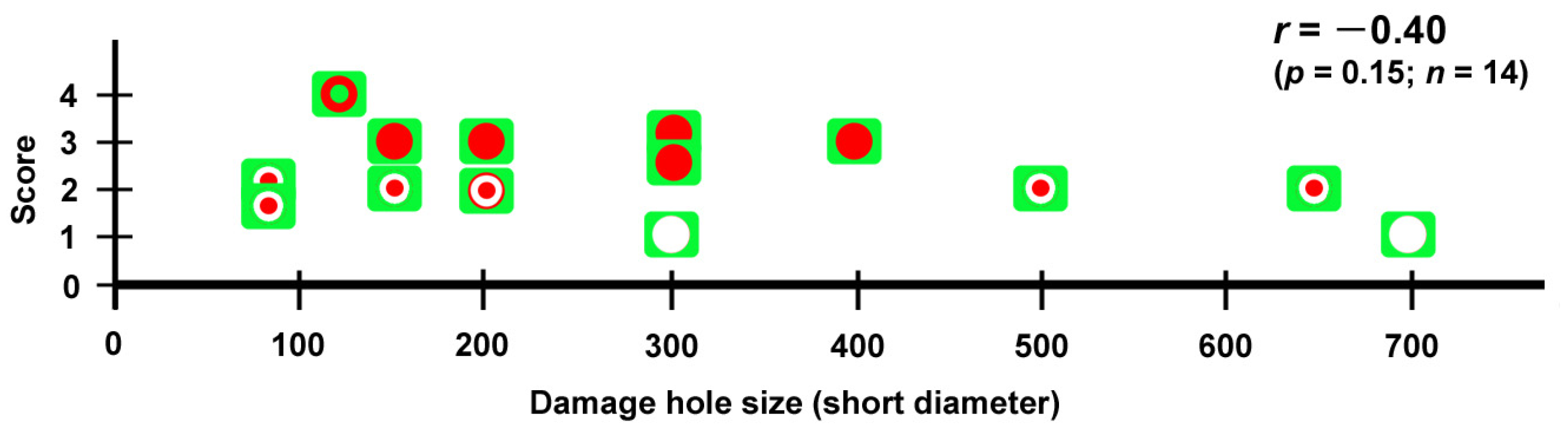
3.10. Repair Was Not Sensitive to Ruthenium Red Injection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brockes, J.P.; Kumar, A. Comparative aspects of animal regeneration. Annu. Rev. Cell Dev. Biol. 2008, 24, 525–549. [Google Scholar] [CrossRef]
- Das, S. Morphological, molecular, and hormonal basis of limb regeneration across Pancrustacea. Integr. Comp. Biol. 2015, 55, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Chou, J.; Garvey, S.L.; Wang, V.R.; Yanes, K.O. Evolution and regulation of limb regeneration in arthropods. In Evo-Devo: Non-Model Species in Cell and Developmental Biology; Results and Problems in Cell Differentiation, Volume 68; Tworzydlo, W., Bilinski, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 419–454. [Google Scholar] [CrossRef]
- Klowden, M.J.; Palli, S.R. Physiological Systems in Insects, 4th ed.; Academic Press: London, UK, 2023. [Google Scholar]
- Parle, E.; Dirks, J.H.; Taylor, D. Damage, repair and regeneration in insect cuticle: The story so far, and possibilities for the future. Arthropod Struct. Dev. 2017, 46, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Parle, E.; Dirks, J.H.; Taylor, D. Bridging the gap: Wound healing in insects restores mechanical strength by targeted cuticle deposition. J. R. Soc. Interface 2016, 13, 20150984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heisenberg, C.P. Dorsal closure in Drosophila: Cells cannot get out of the tight spot. Bioessays 2009, 31, 1284–1287. [Google Scholar] [CrossRef]
- Repiso, A.; Bergantiños, C.; Corominas, M.; Serras, F. Tissue repair and regeneration in Drosophila imaginal discs. Dev. Growth Differ. 2011, 53, 177–185. [Google Scholar] [CrossRef]
- Worley, M.I.; Hariharan, I.K. Imaginal disc regeneration: Something old, something new. Cold Spring Harb. Perspect. Biol. 2022, 14, a040733. [Google Scholar] [CrossRef] [PubMed]
- Belacortu, Y.; Paricio, N. Drosophila as a model of wound healing and tissue regeneration in vertebrates. Dev. Dyn. 2011, 240, 2379–2404. [Google Scholar] [CrossRef]
- Jacinto, A.; Martinez-Arias, A.; Martin, P. Mechanisms of epithelial fusion and repair. Nat. Cell Biol. 2001, 3, E117–E123. [Google Scholar] [CrossRef]
- Hariharan, I.K.; Serras, F. Imaginal disc regeneration takes flight. Curr. Opin. Cell Biol. 2017, 48, 10–16. [Google Scholar] [CrossRef]
- Fox, D.T.; Cohen, E.; Smith-Bolton, R. Model systems for regeneration: Drosophila. Development 2020, 147, dev173781. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, R.; Harris, T.J.C. Contractile and expansive actin networks in Drosophila: Developmental cell biology controlled by network polarization and higher-order interactions. Curr. Top. Dev. Biol. 2023, 154, 99–129. [Google Scholar] [CrossRef]
- Antunes, M.; Pereira, T.; Cordeiro, J.V.; Almeida, L.; Jacinto, A. Coordinated waves of actomyosin flow and apical cell constriction immediately after wounding. J. Cell Biol. 2013, 202, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, B.; Campos, I.; Geiger, J.; Santos, A.C.; Jacinto, A. Epithelial resealing. Int. J. Dev. Biol. 2009, 53, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Redd, M.J.; Cooper, L.; Wood, W.; Stramer, B.; Martin, P. Wound healing and inflammation: Embryos reveal the way to perfect repair. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.V.; Lee, D.M.; Harris, T.J.; Fernandez-Gonzalez, R. Polarized E-cadherin endocytosis directs actomyosin remodeling during embryonic wound repair. J. Cell Biol. 2015, 210, 801–816. [Google Scholar] [CrossRef]
- Nakamura, M.; Parkhurst, S.M. Calcium influx rapidly establishes distinct spatial recruitments of Annexins to cell wounds. Genetics 2024, 227, iyae101. [Google Scholar] [CrossRef] [PubMed]
- Turley, J.; Robertson, F.; Chenchiah, I.V.; Liverpool, T.B.; Weavers, H.; Martin, P. Deep learning reveals a damage signalling hierarchy that coordinates different cell behaviours driving wound re-epithelialisation. Development 2024, 151, dev202943. [Google Scholar] [CrossRef]
- Restrepo, S.; Basler, K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP3R-mediated intercellular calcium waves. Nat. Commun. 2016, 7, 12450. [Google Scholar] [CrossRef] [PubMed]
- Narciso, C.E.; Contento, N.M.; Storey, T.J.; Hoelzle, D.J.; Zartman, J.J. Release of applied mechanical loading stimulates intercellular calcium waves in Drosophila wing discs. Biophys. J. 2017, 113, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.C.; O’Connor, J.T.; Pumford, A.D.; Page-McCaw, A.; Hutson, M.S. A mathematical model of calcium signals around laser-induced epithelial wounds. Mol. Biol. Cell 2023, 34, ar49. [Google Scholar] [CrossRef] [PubMed]
- Ponte, S.; Carvalho, L.; Gagliardi, M.; Campos, I.; Oliveira, P.J.; Jacinto, A. Drp1-mediated mitochondrial fission regulates calcium and F-actin dynamics during wound healing. Biol. Open 2020, 9, bio048629. [Google Scholar] [CrossRef]
- Zulueta-Coarasa, T.; Fernandez-Gonzalez, R. Tension (re)builds: Biophysical mechanisms of embryonic wound repair. Mech. Dev. 2017, 144, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Narciso, C.; Wu, Q.; Brodskiy, P.; Garston, G.; Baker, R.; Fletcher, A.; Zartman, J. Patterning of wound-induced intercellular Ca2+ flashes in a developing epithelium. Phys. Biol. 2015, 12, 056005. [Google Scholar] [CrossRef] [PubMed]
- Razzell, W.; Evans, I.R.; Martin, P.; Wood, W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 2013, 23, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Parkhurst, S.M. Parallels between tissue repair and embryo morphogenesis. Development 2004, 131, 3021–3034. [Google Scholar] [CrossRef] [PubMed]
- Kiehart, D.P.; Galbraith, C.G.; Edwards, K.A.; Rickoll, W.L.; Montague, R.A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 2000, 149, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.; Solon, J. Drosophila dorsal closure: An orchestra of forces to zip shut the embryo. Mech. Dev. 2017, 144, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Paci, G.; Mao, Y. Forced into shape: Mechanical forces in Drosophila development and homeostasis. Semin. Cell Dev. Biol. 2021, 120, 160–170. [Google Scholar] [CrossRef]
- Tsai, C.R.; Wang, Y.; Galko, M.J. Crawling wounded: Molecular genetic insights into wound healing from Drosophila larvae. Int. J. Dev. Biol. 2018, 62, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Pandita, S.; Singh, S.; Bajpai, S.K.; Mishra, G.; Saxena, G.; Verma, P.C. Molecular aspects of regeneration in insects. Dev. Biol. 2024, 507, 64–72. [Google Scholar] [CrossRef]
- Harden, N. Signaling pathways directing the movement and fusion of epithelial sheets: Lessons from dorsal closure in Drosophila. Differentiation 2002, 70, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Arenas Gómez, C.M.; Sabin, K.Z.; Echeverri, K. Wound healing across the animal kingdom: Crosstalk between the immune system and the extracellular matrix. Dev. Dyn. 2020, 249, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, Z.; Liu, D.; Gregory, S. Sterile inflammation in Drosophila. Mediators Inflamm. 2015, 2015, 369286. [Google Scholar] [CrossRef]
- Yu, J.C.; Fernandez-Gonzalez, R. Quantitative modelling of epithelial morphogenesis: Integrating cell mechanics and molecular dynamics. Semin. Cell Dev. Biol. 2017, 67, 153–160. [Google Scholar] [CrossRef]
- Iwanaga, S.; Lee, B.L. Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 2005, 38, 128–150. [Google Scholar] [CrossRef]
- Williams, M.J. Drosophila hemopoiesis and cellular immunity. J. Immunol. 2007, 178, 4711–4716. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Revenis, C. Role and importance of phenoloxidase in insect hemostasis. J. Innate Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef]
- Marieshwari, B.N.; Bhuvaragavan, S.; Sruthi, K.; Mullainadhan, P.; Janarthanan, S. Insect phenoloxidase and its diverse roles: Melanogenesis and beyond. J. Comp. Physiol. B. 2023, 193, 1–23. [Google Scholar] [CrossRef]
- Hodgetts, R.B.; O’Keefe, S.L. Dopa decarboxylase: A model gene-enzyme system for studying development, behavior, and systematics. Annu. Rev. Entomol. 2006, 51, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Wago, H.; Kitano, H. Morphological and functional characterization of the larval hemocytes of the cabbage white butterfly, Pieris rapae crucivora. Appl. Entomol. Zool. 1985, 20, 1–7. [Google Scholar] [CrossRef][Green Version]
- Taira, W.; Toki, M.; Kakinohana, K.; Sakauchi, K.; Otaki, J.M. Developmental and hemocytological effects of ingesting Fukushima’s radiocesium on the cabbage white butterfly Pieris rapae. Sci. Rep. 2019, 9, 2625. [Google Scholar] [CrossRef]
- Nijhout, H.F. Cautery-induced colour patterns in Precis coenia (Lepidoptera: Nymphalidae). J. Embryol. Exp. Morphol. 1985, 86, 191–203. [Google Scholar] [CrossRef]
- Nijhout, H.F. The Development and Evolution of Butterfly Wing Patterns; Smithsonian Institution Press: Washington, DC, USA, 1991. [Google Scholar]
- Brakefield, P.M.; French, V. Eyespot development on butterfly wings: The epidermal response to damage. Dev. Biol. 1995, 168, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Artificially induced changes of butterfly wing colour patterns: Dynamic signal interactions in eyespot development. Sci. Rep. 2011, 1, 111. [Google Scholar] [CrossRef]
- Otaki, J.M. Long-range effects of wing physical damage and distortion on eyespot color patterns in the hindwing of the blue pansy butterfly Junonia orithya. Insects 2018, 9, 195. [Google Scholar] [CrossRef]
- Ohno, Y.; Otaki, J.M. Spontaneous long-range calcium waves in developing butterfly wings. BMC Dev. Biol. 2015, 15, 17. [Google Scholar] [CrossRef]
- Özsu, N.; Monteiro, A. Wound healing, calcium signaling, and other novel pathways are associated with the formation of butterfly eyespots. BMC Genom. 2017, 18, 788. [Google Scholar] [CrossRef]
- Ohno, Y.; Otaki, J.M. Eyespot colour pattern determination by serial induction in fish: Mechanistic convergence with butterfly eyespots. Sci. Rep. 2012, 2, 290. [Google Scholar] [CrossRef]
- Nijhout, H.F. Elements of butterfly wing patterns. J. Exp. Zool. 2001, 291, 213–225. [Google Scholar] [CrossRef]
- Nijhout, H.F. Symmetry systems and compartments in Lepidopteran wings: The evolution of a patterning mechanism. Development 1994, 1994 (Supplement), 225–233. [Google Scholar] [CrossRef]
- Kusaba, K.; Otaki, J.M. Positional dependence of scale size and shape in butterfly wings: Wing-wide phenotypic coordination of color-pattern elements and background. J. Insect Physiol. 2009, 55, 174–182. [Google Scholar] [CrossRef]
- Otaki, J.M. Color-pattern analysis of eyespots in butterfly wings: A critical examination of morphogen gradient models. Zool. Sci. 2011, 28, 403–413. [Google Scholar] [CrossRef]
- Otaki, J.M. Generation of butterfly wing eyespot patterns: A model for morphological determination of eyespot and parafocal element. Zool. Sci. 2011, 28, 817–827. [Google Scholar] [CrossRef]
- Otaki, J.M. Color pattern analysis of nymphalid butterfly wings: Revision of the nymphalid groundplan. Zool. Sci. 2012, 29, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Morphological and spatial diversity of the discal spot on the hindwings of nymphalid butterflies: Revision of the nymphalid groundplan. Insects 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. The fractal geometry of the nymphalid groundplan: Self-similar configuration of color pattern symmetry systems in butterfly wings. Insects 2021, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Structural analysis of eyespots: Dynamics of morphogenic signals that govern elemental positions in butterfly wings. BMC Syst. Biol. 2012, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Self-similarity, distortion waves, and the essence of morphogenesis: A generalized view of color pattern formation in butterfly wings. In Diversity and Evolution of Butterfly Wing Patterns: An Integrative Approach; Sekimura, T., Nijhout, H.F., Eds.; Springer: Singapore, 2018; pp. 119–152. [Google Scholar] [CrossRef]
- Iwata, M.; Otaki, J.M. Insights into eyespot color-pattern formation mechanisms from color gradients, boundary scales, and rudimentary eyespots in butterfly wings. J. Insect Physiol. 2019, 114, 68–82. [Google Scholar] [CrossRef]
- Iwata, M.; Otaki, J.M. Spatial patterns of correlated scale size and scale color in relation to color pattern elements in butterfly wings. J. Insect Physiol. 2016, 85, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Otaki, J.M. Focusing on butterfly eyespot focus: Uncoupling of white spots from eyespot bodies in nymphalid butterflies. SpringerPlus 2016, 5, 1287. [Google Scholar] [CrossRef]
- Janssen, J.M.; Monteiro, A.; Brakefield, P.M. Correlations between scale structure and pigmentation in butterfly wings. Evol. Dev. 2001, 3, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Ogasawara, T.; Yamamoto, H. Morphological comparison of pupal wing cuticle patterns in butterflies. Zool. Sci. 2005, 22, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Taira, W.; Otaki, J.M. Butterfly wings are three-dimensional: Pupal cuticle focal spots and their associated structures in Junonia butterflies. PLoS ONE 2016, 11, e0146348. [Google Scholar] [CrossRef]
- Nakazato, Y.; Otaki, J.M. Socket array irregularities and wing membrane distortions at the eyespot foci of butterfly wings suggest mechanical signals for color pattern determination. Insects 2024, 15, 535. [Google Scholar] [CrossRef]
- Nijhout, H.F. Pattern formation on lepidopteran wings: Determination of an eyespot. Dev. Biol. 1980, 80, 267–274. [Google Scholar] [CrossRef]
- French, V.; Brakefield, P.M. Eyespot development on butterfly wings: The focal signal. Dev. Biol. 1995, 168, 112–123. [Google Scholar] [CrossRef]
- Iwasaki, M.; Otaki, J.M. Synergistic damage response of the double-focus eyespot in the hindwing of the peacock pansy butterfly. In Lepidoptera; Perveen, F.K., Ed.; InTech: Rijeka, Croatia, 2017; pp. 65–82. [Google Scholar] [CrossRef]
- Otaki, J.M. Contact-mediated eyespot color-pattern changes in the peacock pansy butterfly: Contributions of mechanical force and extracellular matrix to morphogenic signal propagation. In Lepidoptera; Perveen, F.K., Ed.; InTech: Rijeka, Croatia, 2017; pp. 83–102. [Google Scholar] [CrossRef]
- Otaki, J.M. Butterfly eyespot color pattern formation requires physical contact of the pupal wing epithelium with extracellular materials for morphogenic signal propagation. BMC Dev. Biol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Otaki, J.M. Color-pattern modifications of butterfly wings induced by transfusion and oxyanions. J. Insect Physiol. 1998, 44, 1181–1190. [Google Scholar] [CrossRef]
- Otaki, J.M. Physiologically induced color-pattern changes in butterfly wings: Mechanistic and evolutionary implications. J. Insect Physiol. 2008, 54, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Serfas, M.S.; Carroll, S.B. Pharmacologic approaches to butterfly wing patterning: Sulfated polysaccharides mimic or antagonize cold shock and alter the interpretation of gradients of positional information. Dev. Biol. 2005, 287, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Nakazato, Y. Butterfly wing color pattern modification inducers may act on chitin in the apical extracellular site: Implications in morphogenic signals for color pattern determination. Biology 2022, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Otaki, J.M. Real-time in vivo imaging of the developing pupal wing tissues in the pale grass blue butterfly Zizeeria maha: Establishing the lycaenid system for multiscale bioimaging. J. Imaging 2019, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Tsutsumi, M.; Otaki, J.M. Developmental dynamics of butterfly wings: Real-time in vivo whole-wing imaging of twelve butterfly species. Sci. Rep. 2018, 8, 16848. [Google Scholar] [CrossRef]
- Iwata, M.; Ohno, Y.; Otaki, J.M. Real-time in vivo imaging of butterfly wing development: Revealing the cellular dynamics of the pupal wing tissue. PLoS ONE 2014, 9, e89500. [Google Scholar] [CrossRef]
- Ohno, Y.; Otaki, J.M. Live cell imaging of butterfly pupal and larval wings in vivo. PLoS ONE 2015, 10, e0128332. [Google Scholar] [CrossRef]
- Iwasaki, M.; Ohno, Y.; Otaki, J.M. Butterfly eyespot organiser: In vivo imaging of the prospective focal cells in pupal wing tissues. Sci. Rep. 2017, 7, 40705. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, Y.; Otaki, J.M. Live detection of intracellular chitin in butterfly wing epithelial cells in vivo using fluorescent brightener 28: Implications for the development of scales and color patterns. Insects 2023, 14, 753. [Google Scholar] [CrossRef]
- Carroll, S.B.; Gates, J.; Keys, D.N.; Paddock, S.W.; Panganiban, G.E.; Selegue, J.E.; Williams, J.A. Pattern formation and eyespots determination in butterfly wings. Science 1994, 265, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Brakefield, P.M.; Gates, J.; Keys, D.; Kesbeke, F.; Wijngaarden, P.J.; Monteiro, A.; French, V.; Carroll, S.B. Development, plasticity and evolution of butterfly eyespot patterns. Nature 1996, 384, 236–242. [Google Scholar] [CrossRef]
- Monteiro, A.; Brakefield, P.M.; French, V. The genetics and development of an eyespot pattern in the butterfly Bicyclus anynana: Response to selection for eyespot shape. Genetics 1997, 146, 287–294. [Google Scholar] [CrossRef]
- Murugesan, S.N.; Connahs, H.; Matsuoka, Y.; Das Gupta, M.; Tiong, G.J.L.; Huq, M.; Gowri, V.; Monroe, S.; Deem, K.D.; Werner, T.; et al. Butterfly eyespots evolved via cooption of an ancestral gene-regulatory network that also patterns antennae, legs, and wings. Proc. Natl. Acad. Sci. USA 2022, 119, e2108661119. [Google Scholar] [CrossRef]
- Beldade, P.; Brakefield, P.M.; Long, A.D. Contribution of Distal-less to quantitative variation in butterfly eyespots. Nature 2002, 415, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.C.; Ramos, D.; Prudic, K.L.; Monteiro, A. Temporal gene expression variation associated with eyespot size plasticity in Bicyclus anynana. PLoS ONE 2013, 8, e65830. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Otaki, J.M. A single-wing removal method to assess correspondence between gene expression and phenotype in butterflies. The case of Distal-less. Zool. Sci. 2016, 33, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.R.; Selegue, J.E.; Monteiro, A.; French, V.; Brakefield, P.M.; Carroll, S.B. The generation and diversification of butterfly eyespot color patterns. Curr. Biol. 2001, 11, 1578–1585. [Google Scholar] [CrossRef]
- Monteiro, A.; Chen, B.; Scott, L.C.; Vedder, L.; Prijs, H.J.; Belicha-Villanueva, A.; Brakefield, P.M. The combined effect of two mutations that alter serially homologous color pattern elements on the fore and hindwings of a butterfly. BMC Genet. 2007, 8, 22. [Google Scholar] [CrossRef]
- Reed, R.D.; Serfas, M.S. Butterfly wing pattern evolution is associated with changes in a Notch/Distal-less temporal pattern formation process. Curr. Biol. 2004, 14, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.; Glaser, G.; Stockslager, S.; Glansdorp, N.; Ramos, D. Comparative insights into questions of lepidopteran wing pattern homology. BMC Dev. Biol. 2006, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.L.Q.; Das Banerjee, T.; Prakash, A.; Seah, K.S.; Monteiro, A. Distal-less and spalt are distal organisers of pierid wing patterns. EvoDevo 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.L.Q.; Murugesan, S.N.; Wheat, C.W.; Monteiro, A. The genetic basis of wing spots in Pieris canidia butterflies. BMC Genom. 2023, 24, 169. [Google Scholar] [CrossRef]
- Monteiro, A.; Chen, B.; Ramos, D.M.; Oliver, J.C.; Tong, X.; Guo, M.; Wang, W.-K.; Fazzino, L.; Kamal, F. Distal-less regulates eyespot patterns and melanization in Bicyclus butterflies. J. Exp. Zool. B Mol. Dev. Evol. 2013, 320, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Connahs, H.; Tlili, S.; van Creij, J.; Loo, T.Y.J.; Banerjee, T.D.; Saunders, T.E.; Monteiro, A. Activation of butterfly eyespots by Distal-less is consistent with a reaction-diffusion process. Development 2019, 146, dev169367. [Google Scholar] [CrossRef]
- Dhungel, B.; Ohno, Y.; Matayoshi, R.; Iwasaki, M.; Taira, W.; Adhikari, K.; Gurung, R.; Otaki, J.M. Distal-less induces elemental color patterns in Junonia butterfly wings. Zool. Lett. 2016, 2, 4. [Google Scholar] [CrossRef]
- Zhang, L.; Reed, R.D. Genome editing in butterflies reveals that spalt promotes and Distal-less represses eyespot colour patterns. Nat. Commun. 2016, 7, 11769. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Reed, R.D. Wnt signaling underlies evolution and development of the butterfly wing pattern symmetry systems. Dev. Biol. 2014, 395, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hanly, J.J.; Loh, L.S.; Mazo-Vargas, A.; Rivera-Miranda, T.S.; Livraghi, L.; Tendolkar, A.; Day, C.R.; Liutikaite, N.; Earls, E.A.; Corning, O.B.W.H.; et al. Frizzled2 receives WntA signaling during butterfly wing pattern formation. Development 2023, 150, dev201868. [Google Scholar] [CrossRef]
- Banerjee, T.D.; Murugesan, S.N.; Connahs, H.; Monteiro, A. Spatial and temporal regulation of Wnt signaling pathway members in the development of butterfly wing patterns. Sci. Adv. 2023, 9, eadg3877. [Google Scholar] [CrossRef]
- Nakazato, Y.; Otaki, J.M. Antibody-mediated protein knockdown reveals Distal-less functions for eyespots and parafocal elements in butterfly wing color pattern development. Cells 2024, 13, 1476. [Google Scholar] [CrossRef]
- Otaki, J.M.; Ogasawara, T.; Yamamoto, H. Tungstate-induced color-pattern modifications of butterfly wings are independent of stress response and ecdysteroid effect. Zool. Sci. 2005, 22, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Haine, E.R.; Moret, Y.; Siva-Jothy, M.T.; Rolff, J. Antimicrobial defense and persistent infection in insects. Science 2008, 322, 1257–1259. [Google Scholar] [CrossRef]
- Karpen, G.H.; Schubiger, G. Extensive regulatory capabilities of a Drosophila imaginal disk blastema. Nature 1981, 294, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Irvine, K.D. Control of growth during regeneration. Curr. Top. Dev. Biol. 2014, 108, 95–120. [Google Scholar] [CrossRef]
- Otaki, J.M.; Tanaka, A.; Hirose, E. Butterfly pupal wing tissue with an eyespot organizer. Cells Dev. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Broekemeier, K.M.; Krebsbach, R.J.; Pfeiffer, D.R. Inhibition of the mitochondrial Ca2+ uniporter by pure and impure ruthenium red. Mol. Cell. Biochem. 1994, 139, 33–40. [Google Scholar] [CrossRef]
- Tapia, R.; Velasco, I. Ruthenium red as a tool to study calcium channels, neuronal death and the function of neural pathways. Neurochem. Int. 1997, 30, 137–147. [Google Scholar] [CrossRef]
- Kargacin, G.J.; Ali, Z.; Kargacin, M.E. Ruthenium red reduces the Ca2+ sensitivity of Ca2+ uptake into cardiac sarcoplasmic reticulum. Pflug. Arch. 1998, 436, 338–342. [Google Scholar] [CrossRef]
- Cibulsky, S.M.; Sather, W.A. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J. Pharmacol. Exp. Ther. 1999, 289, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.H.; Park, J.W.; Kwon, T.K. Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem. Biophys. Res. Commun. 2003, 303, 1073–1079. [Google Scholar] [CrossRef]
- Pumroy, R.A.; De Jesús-Pérez, J.J.; Protopopova, A.D.; Rocereta, J.A.; Fluck, E.C.; Fricke, T.; Lee, B.H.; Rohacs, T.; Leffler, A.; Moiseenkova-Bell, V. Molecular details of ruthenium red pore block in TRPV channels. EMBO Rep. 2024, 25, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Thien, N.D.; Hai-Nam, N.; Anh, D.T.; Baecker, D. Piezo1 and its inhibitors: Overview and perspectives. Eur. J. Med. Chem. 2024, 273, 116502. [Google Scholar] [CrossRef] [PubMed]
- Thayer, R.C.; Allen, F.I.; Patel, N.H. Structural color in Junonia butterflies evolves by tuning scale lamina thickness. eLife 2020, 9, e52187. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, V.J.; Burg, S.L.; Harizanova, J.; Garcia, E.; Hill, O.; Enciso-Romero, J.; Cooper, R.L.; Flenner, S.; Longo, E.; Greving, I.; et al. The actin cytoskeleton plays multiple roles in structural colour formation in butterfly wing scales. Nat. Commun. 2024, 15, 4073. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Fukuda, N. Regulation of myocardial contraction as revealed by intracellular Ca2+ measurements using aequorin. J. Physiol. Sci. 2024, 74, 12. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B. Calcium and regulation of contraction: A short review. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. S1), S9–S13. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.P.J.; Fouchard, J.; Lisica, A.; Khalilgharibi, N.; Baum, B.; Recho, P.; Kabla, A.J.; Charras, G.T. Actomyosin controls planarity and folding of epithelia in response to compression. Nat. Mater. 2020, 19, 109–117. [Google Scholar] [CrossRef]
- Nelson, C.M. On buckling morphogenesis. J. Biomech. Eng. 2016, 138, 021005. [Google Scholar] [CrossRef]
- Kim, J.M.; Jo, Y.; Jung, J.W.; Park, K. A mechanogenetic role for the actomyosin complex in branching morphogenesis of epithelial organs. Development 2021, 148, dev190785. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.L. Expansion of ring-shaped supracellular contractile cables induces epithelial sheet folding. Phys. Rev. E 2022, 106, 064403. [Google Scholar] [CrossRef] [PubMed]
- Fierling, J.; John, A.; Delorme, B.; Torzynski, A.; Blanchard, G.B.; Lye, C.M.; Popkova, A.; Malandain, G.; Sanson, B.; Étienne, J.; et al. Embryo-scale epithelial buckling forms a propagating furrow that initiates gastrulation. Nat. Commun. 2022, 13, 3348. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Narumi, R.; Akiyama, R.; Vitiello, E.; Shirai, T.; Tanimura, N.; Kuromiya, K.; Ishikawa, S.; Kajita, M.; Tada, M.; et al. Calcium wave promotes cell extrusion. Curr. Biol. 2020, 30, 670–681.e6. [Google Scholar] [CrossRef] [PubMed]
- Kuromiya, K.; Aoki, K.; Ishibashi, K.; Yotabun, M.; Sekai, M.; Tanimura, N.; Iijima, S.; Ishikawa, S.; Kamasaki, T.; Akieda, Y.; et al. Calcium sparks enhance the tissue fluidity within epithelial layers and promote apical extrusion of transformed cells. Cell Rep. 2022, 40, 111078. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R. The Hierarchical Genome and Differentiation Waves: Novel Unification of Development, Genetics and Evolution; Volume I and Volume II. Series on Mathematical Biology and Medicine, Volume 3; World Scientific: Singapore, 1999. [Google Scholar]
- Gordon, N.K.; Gordon, R. Embryogenesis Explained; World Scientific: Singapore, 2016. [Google Scholar]
- Gordon, R. Are we on the cusp of a new paradigm for biology? The illogic of molecular developmental biology versus Janus-faced control of embryogenesis via differentiation waves. BioSystems 2021, 203, 104367. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.; Stone, R. A short tutorial on the Janus-faced logic of differentiation waves and differentiation trees and their evolution. BioSystems 2021, 205, 104414. [Google Scholar] [CrossRef]
- Otaki, J.M. Reversed type of color-pattern modifications of butterfly wings: A physiological mechanism of wing-wide color-pattern determination. J. Insect Physiol. 2007, 53, 526–537. [Google Scholar] [CrossRef]
- Otaki, J.M. Stress-induced color-pattern modifications and evolution of the painted lady butterflies Vanessa cardui and Vanessa kershawi. Zool. Sci. 2007, 24, 811–819. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, S.; Otaki, J.M. Wound Healing in Butterfly Pupal Wing Tissues: Real-Time In Vivo Imaging of Long-Range Cell Migration, Cluster Formation, and Calcium Oscillations. Insects 2025, 16, 124. https://doi.org/10.3390/insects16020124
Nagai S, Otaki JM. Wound Healing in Butterfly Pupal Wing Tissues: Real-Time In Vivo Imaging of Long-Range Cell Migration, Cluster Formation, and Calcium Oscillations. Insects. 2025; 16(2):124. https://doi.org/10.3390/insects16020124
Chicago/Turabian StyleNagai, Shuka, and Joji M. Otaki. 2025. "Wound Healing in Butterfly Pupal Wing Tissues: Real-Time In Vivo Imaging of Long-Range Cell Migration, Cluster Formation, and Calcium Oscillations" Insects 16, no. 2: 124. https://doi.org/10.3390/insects16020124
APA StyleNagai, S., & Otaki, J. M. (2025). Wound Healing in Butterfly Pupal Wing Tissues: Real-Time In Vivo Imaging of Long-Range Cell Migration, Cluster Formation, and Calcium Oscillations. Insects, 16(2), 124. https://doi.org/10.3390/insects16020124






