New Data Indicate Larger Decline in Morphological Diversity in Split-Footed Lacewing Larvae than Previously Estimated
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Imaging and Documentation
2.3. Image Processing and Presentation
2.4. Measurements
2.5. Outlines
2.6. Shape Analysis
3. Results
3.1. Description of New Fossil Larvae
- (1)
- (2)
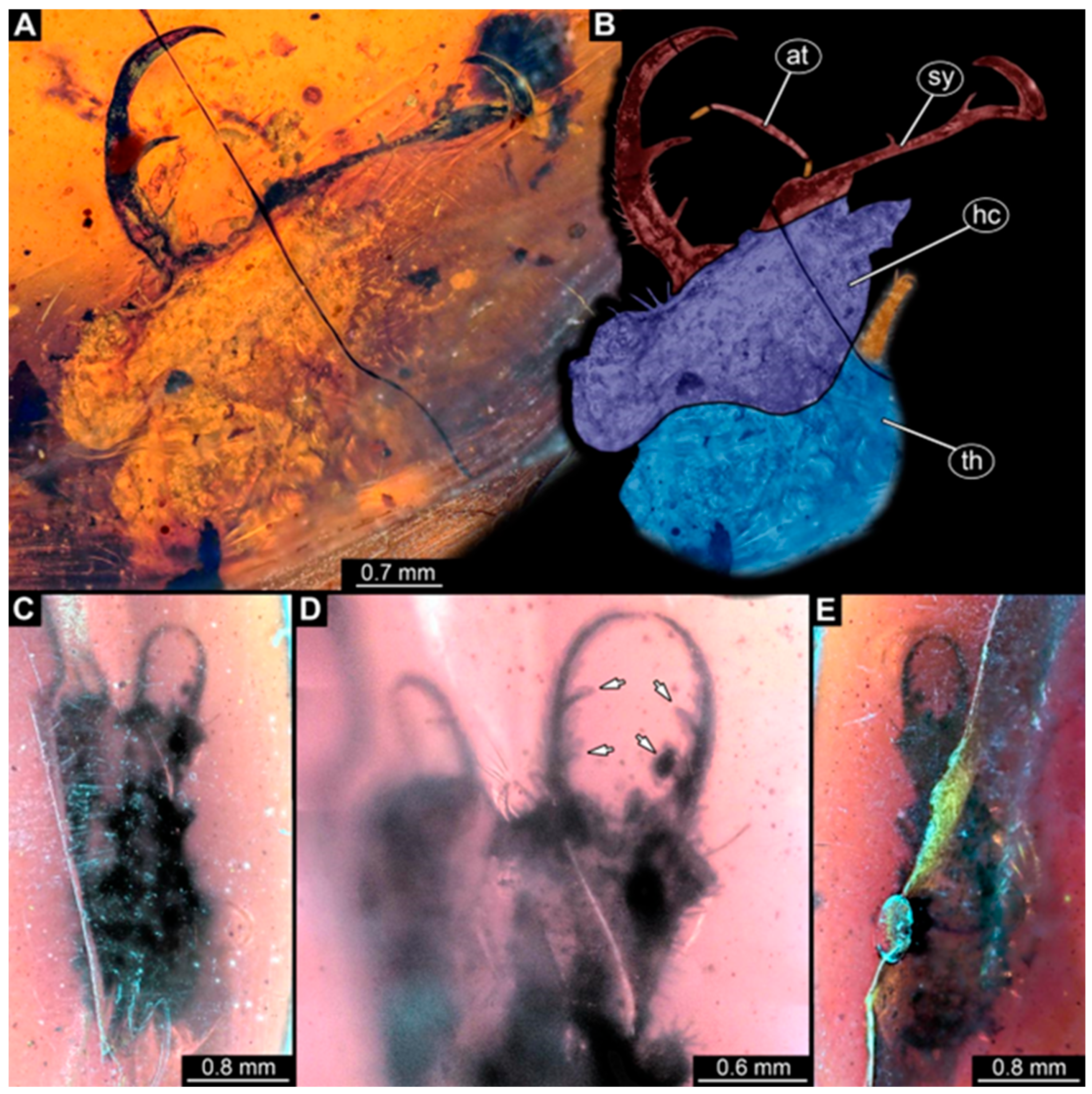
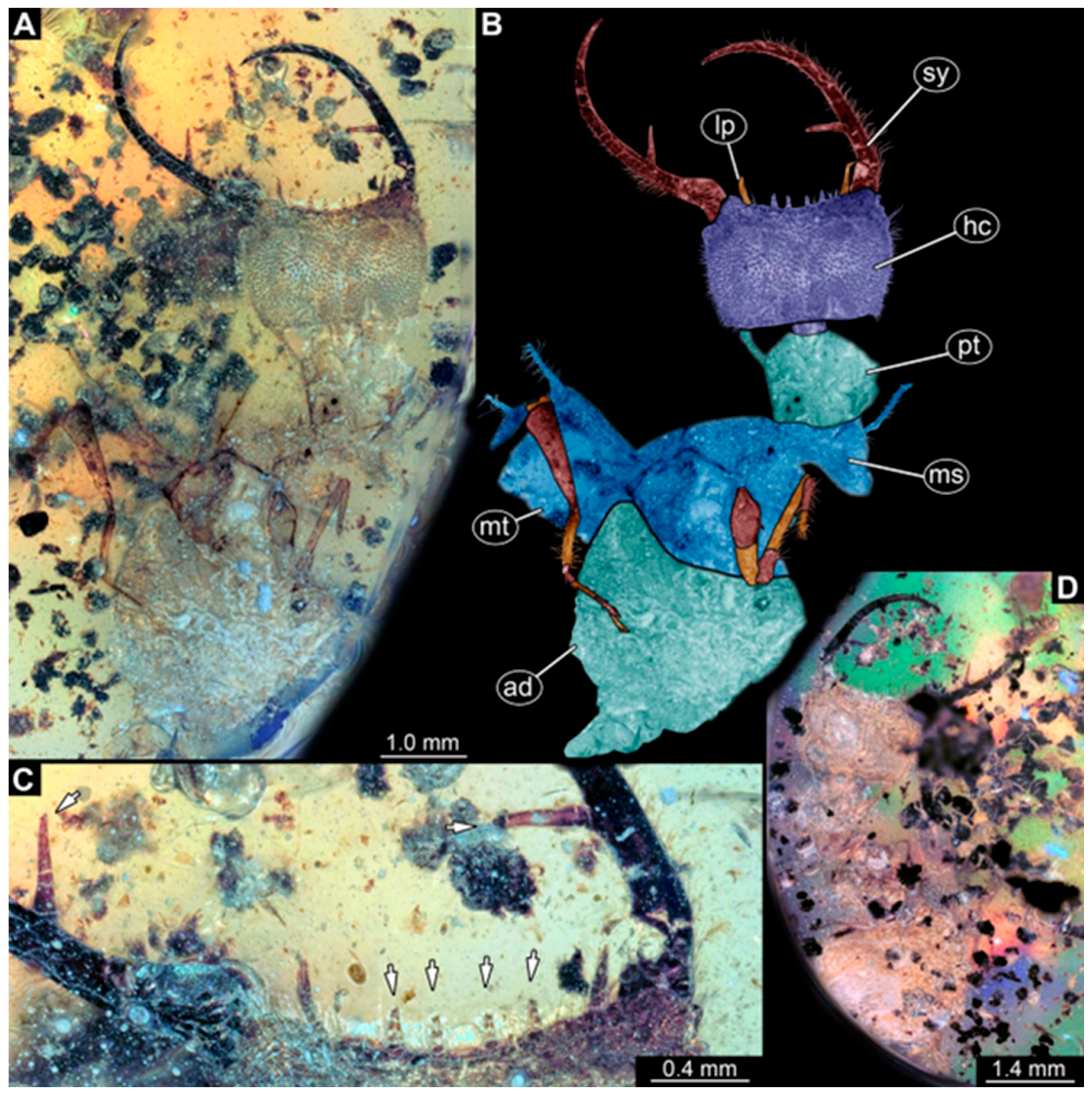
- Specimen 246 (PED 1294) is largely concealed by flaws in the amber in the ventral or dorsal view (Figure 1A,B). Each stylet bears one longer forward-inward-curved tooth and a shorter distally pointing tooth. The trunk seems to be almost completely missing. The specimen has a length of about 3.9 mm.
- (3)
- (4)
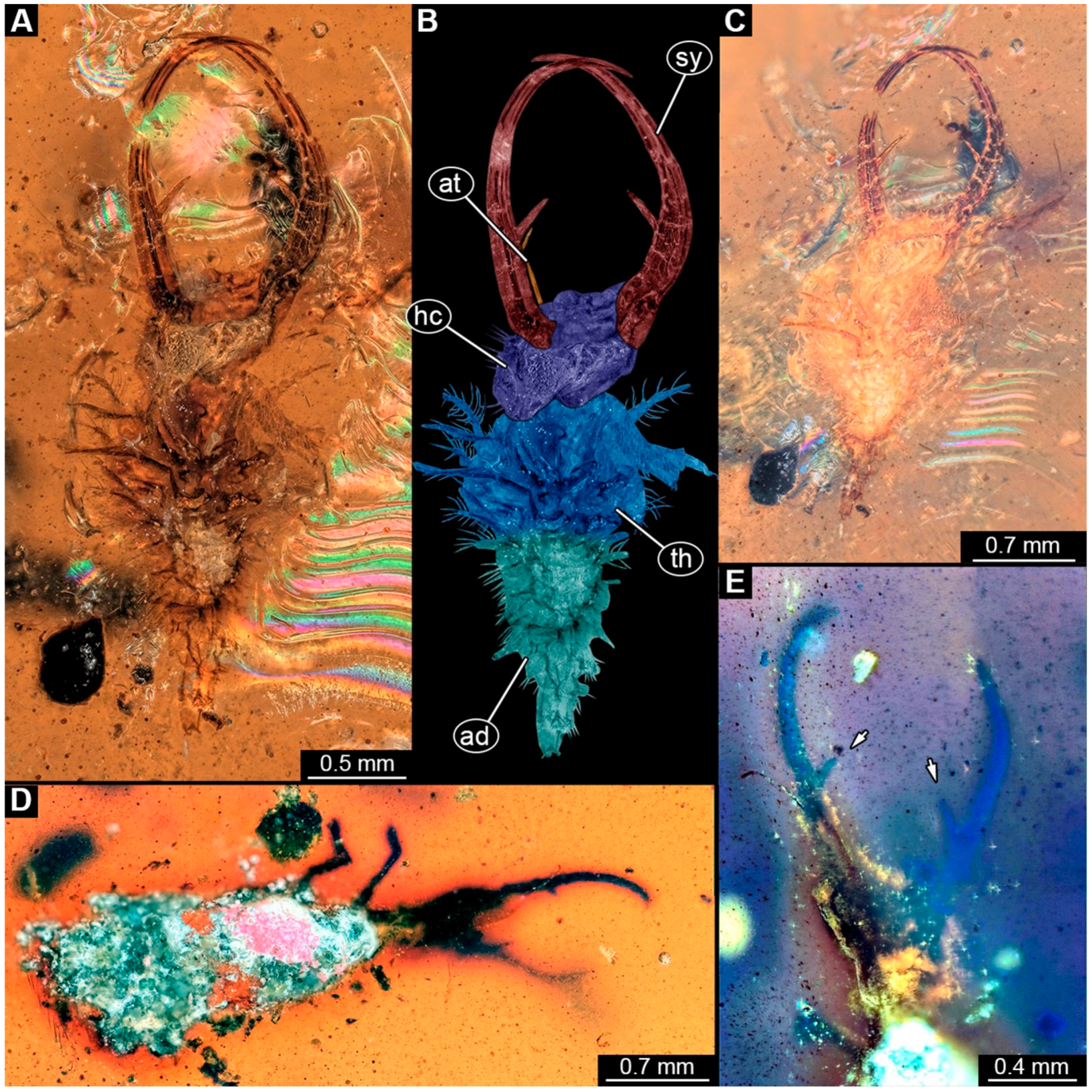
- Specimen 249 (PED 1777) is well accessible in the ventral (Figure 3A,B) and dorsal view (Figure 3C,D). Each stylet bears one longer forward-inward-curved tooth and a shorter distally pointing tooth. Four protrusions are visible on the anterior rim of the head. A syninclusion with a different insect (probably Orthoptera) is present (Figure 3D). The specimen has a length of about 3.5 mm.
- (5)
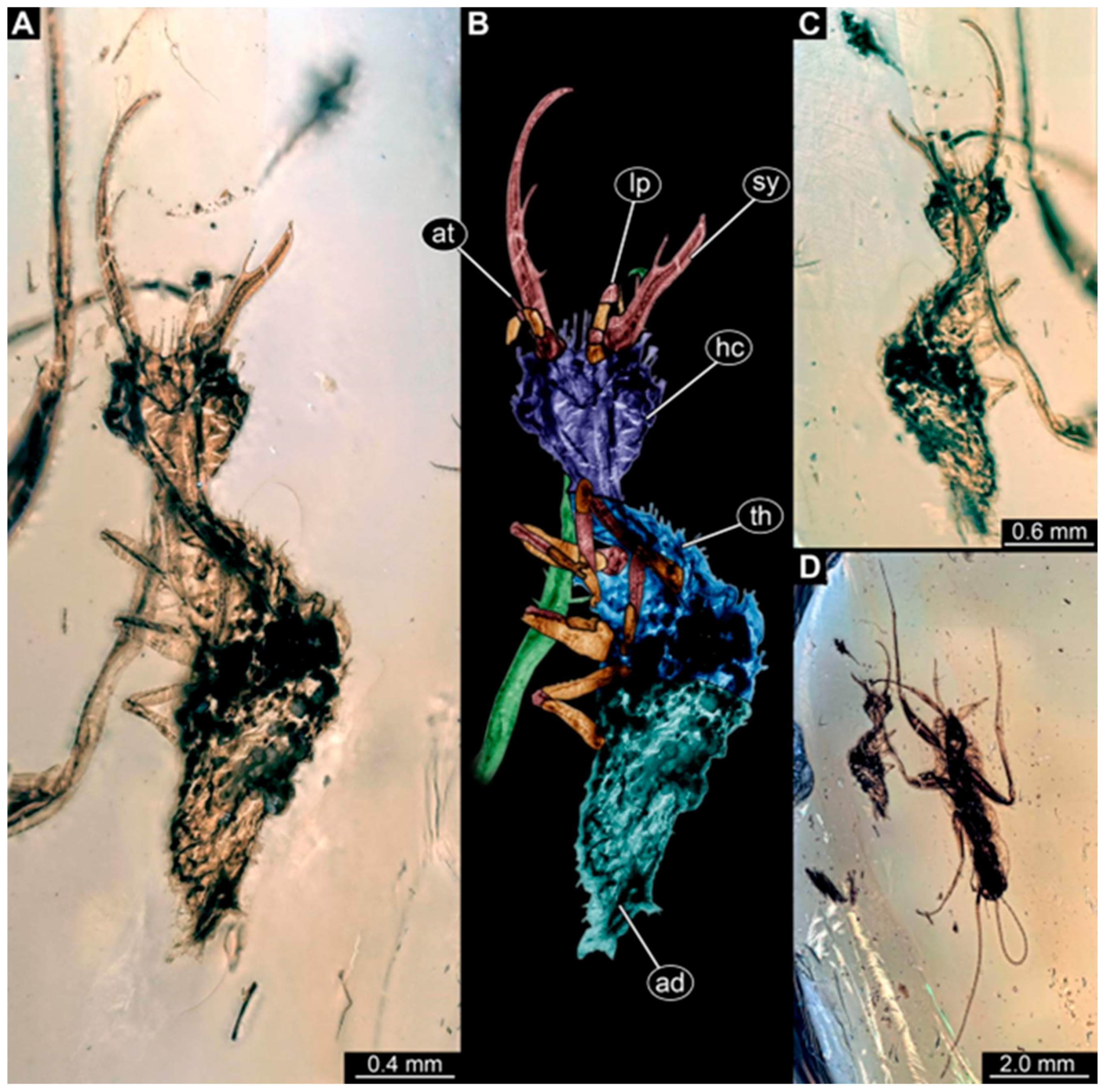
- Specimen 250 (PED 2211) is well accessible in the ventral or dorsal view (Figure 2A–C). Each stylet bears one slightly forward-inward-curved tooth. Protrusions (possible scoli) are visible at some trunk segments. The specimen has a length of about 4.5 mm.
- (6)
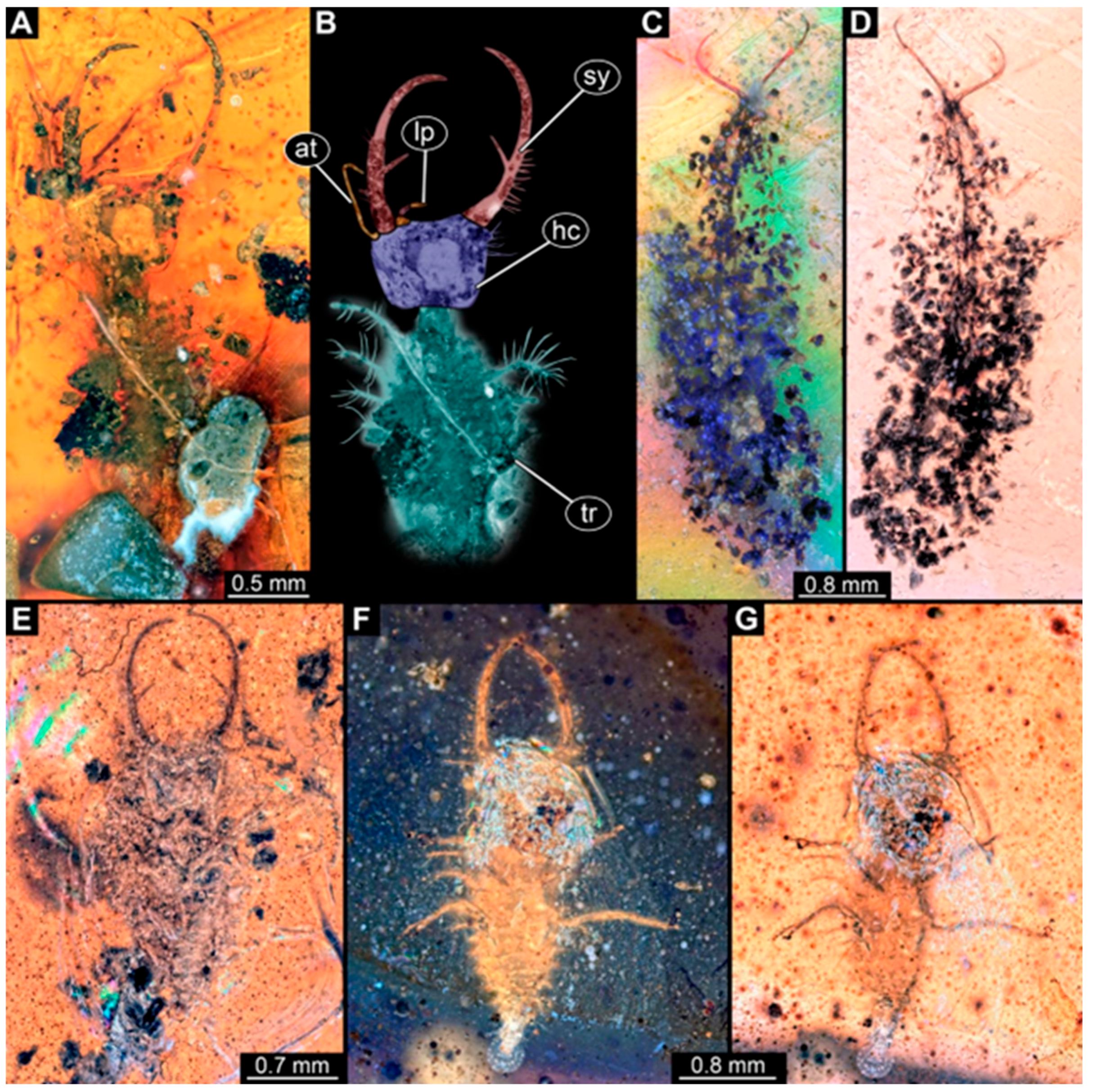
- Specimen 251 (PED 2242) is largely concealed in the ventral or dorsal view by cloudiness and dirt particles (Figure 4A,B). Each stylet bears one rather straight tooth. A rather slender cervix seems to be present. The posterior trunk seems to be missing. The specimen has a length of about 3.6 mm.
- (7)
- Specimen 253 (PED 2626) is largely concealed by cloudiness and dirt particles in the ventral or dorsal view; only the rough outline is apparent (Figure 4E). Each stylet seems to bear a single tooth. The specimen has a length of about 3.0 mm.
- (8)
- Specimen 254 (PED 2627) is largely concealed by cloudiness and dirt particles in the ventral and dorsal view; only the rough outline is apparent (Figure 4F,G). Each stylet seems to bear a single tooth. The specimen has a length of about 3.9 mm.
- (9)
- Specimen 255 (BuB 4) is accessible in the dorsal (Figure 5A,B) and ventral view (Figure 5C) but partly concealed by dirt and cloudiness in the dorsal and ventral view. Each stylet seems to bear a single forward-inward-curved-tooth. Claws are visible at the end of locomotory appendages. The posterior trunk seems to be missing. The specimen has a length of about 2.2 mm.
- (10)
- (11)
- Specimen 257 (BuB 29) is accessible in the ventral and dorsal view (Figure 4C,D) but largely concealed by dirt particles and cloudiness in the ventral and dorsal view. Each stylet seems to bear one forward-inward curved tooth. The specimen has a length of about 5.1 mm.
- (12)
- Specimen 258 (BuB 12) is well accessible in the dorsal and ventral view (Figure 6A,B,D). Each stylet seems to bear one rather straight tooth (Figure 6C). Four protrusions are visible on the anterior rim of the head (Figure 6C). A rather slender neck seems to be apparent. Protrusions of the trunk (possible scoli) seem to be present. The specimen has a length of about 7.8 mm.
- (13)
- Specimen 260 (PED 2770) is accessible in the ventral or dorsal view (Figure 7A,B) but partly concealed by dirt particles and cloudiness. Each stylet seems to bear one slightly forward-inward curved tooth. Claws are visible at the end of locomotory appendages. The specimen has a length of about 3.4 mm.
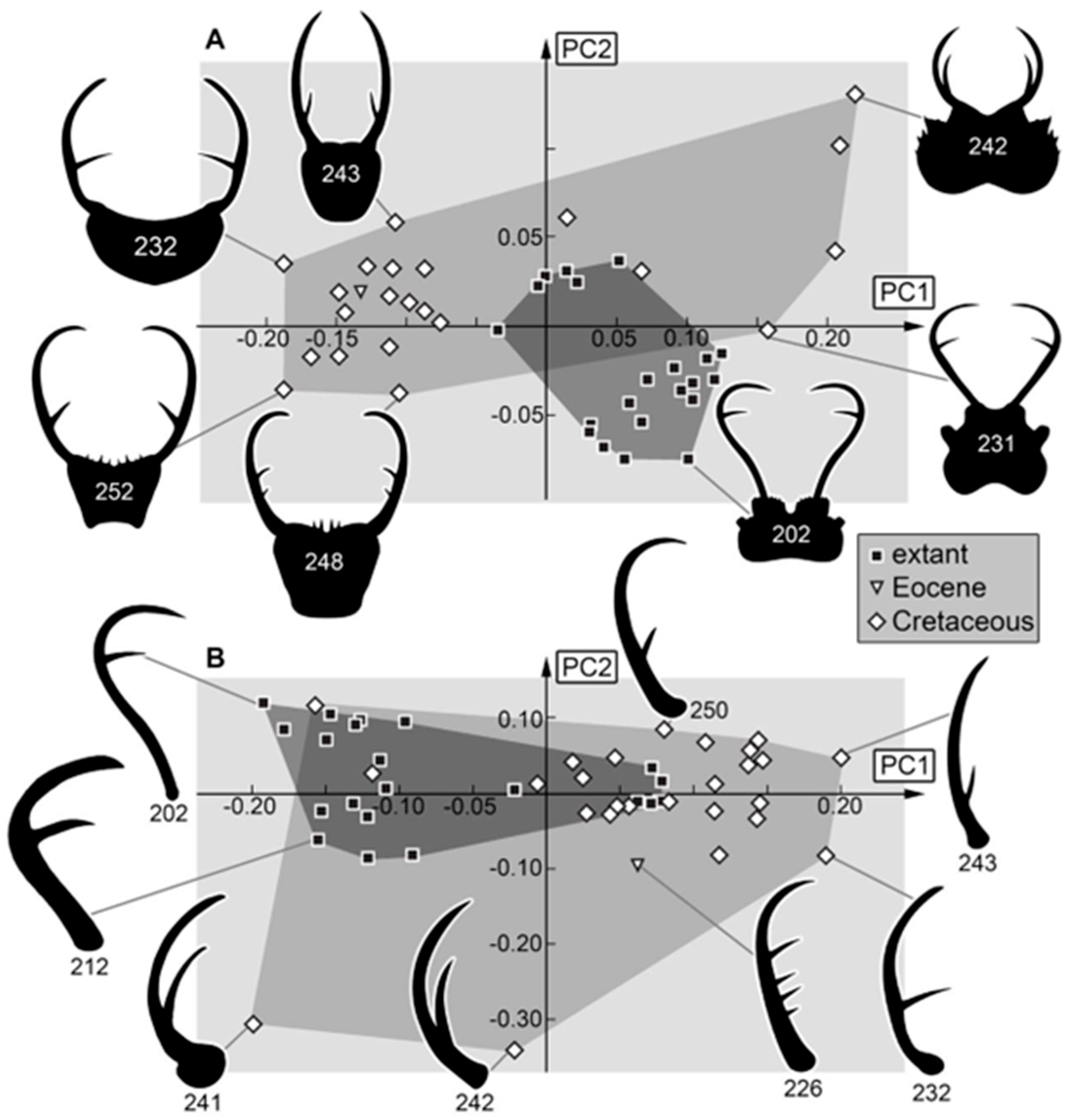
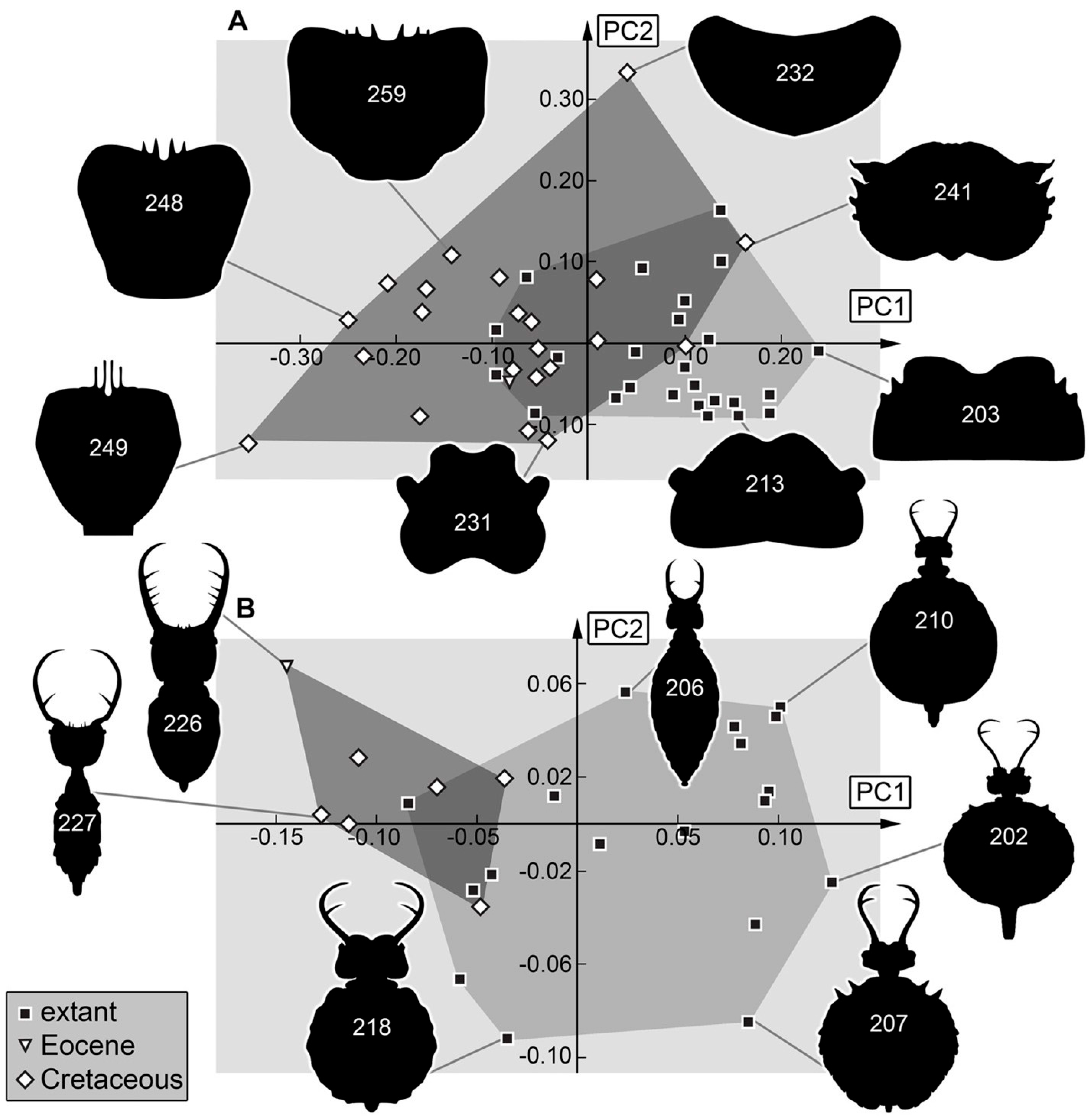
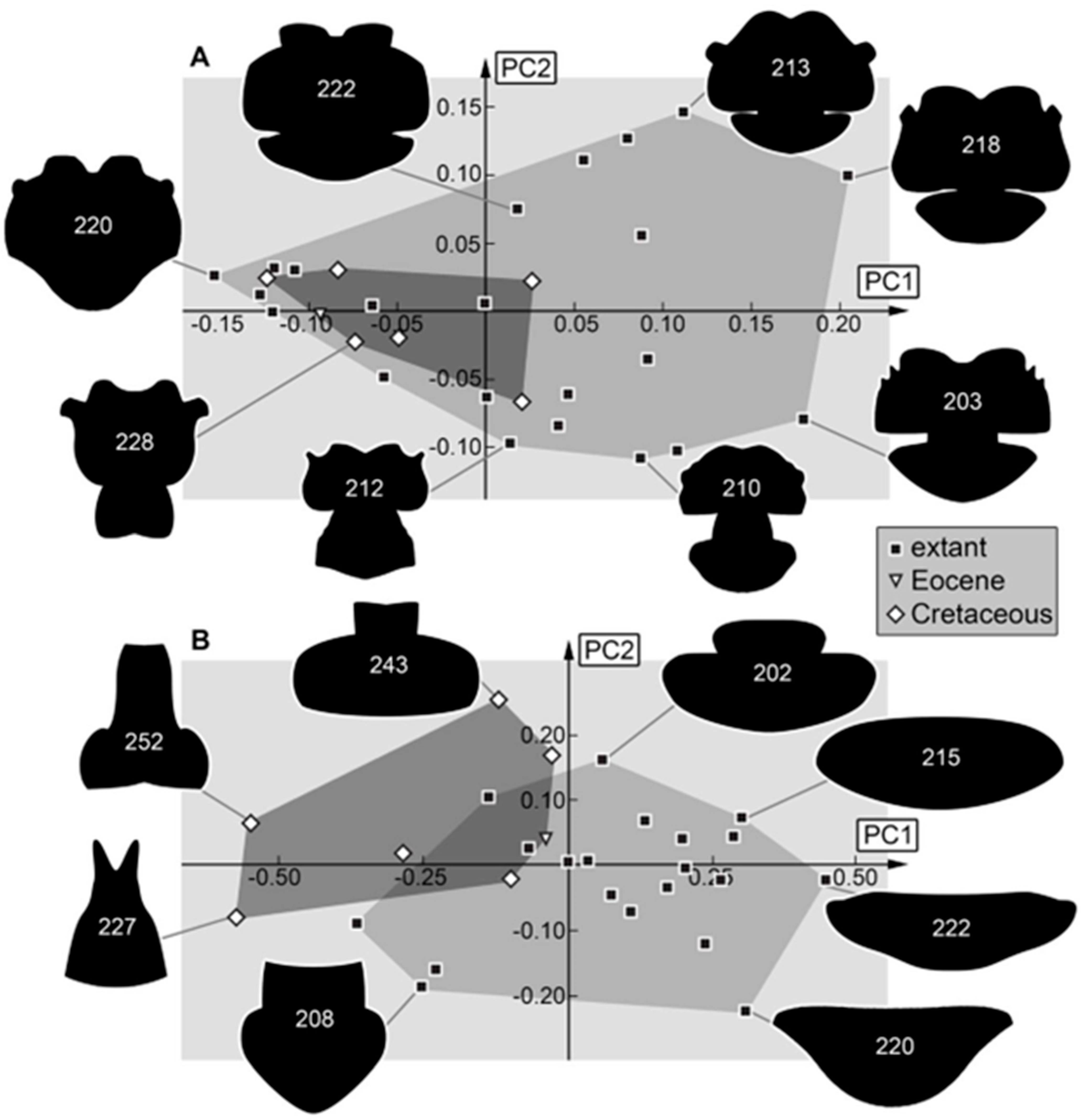
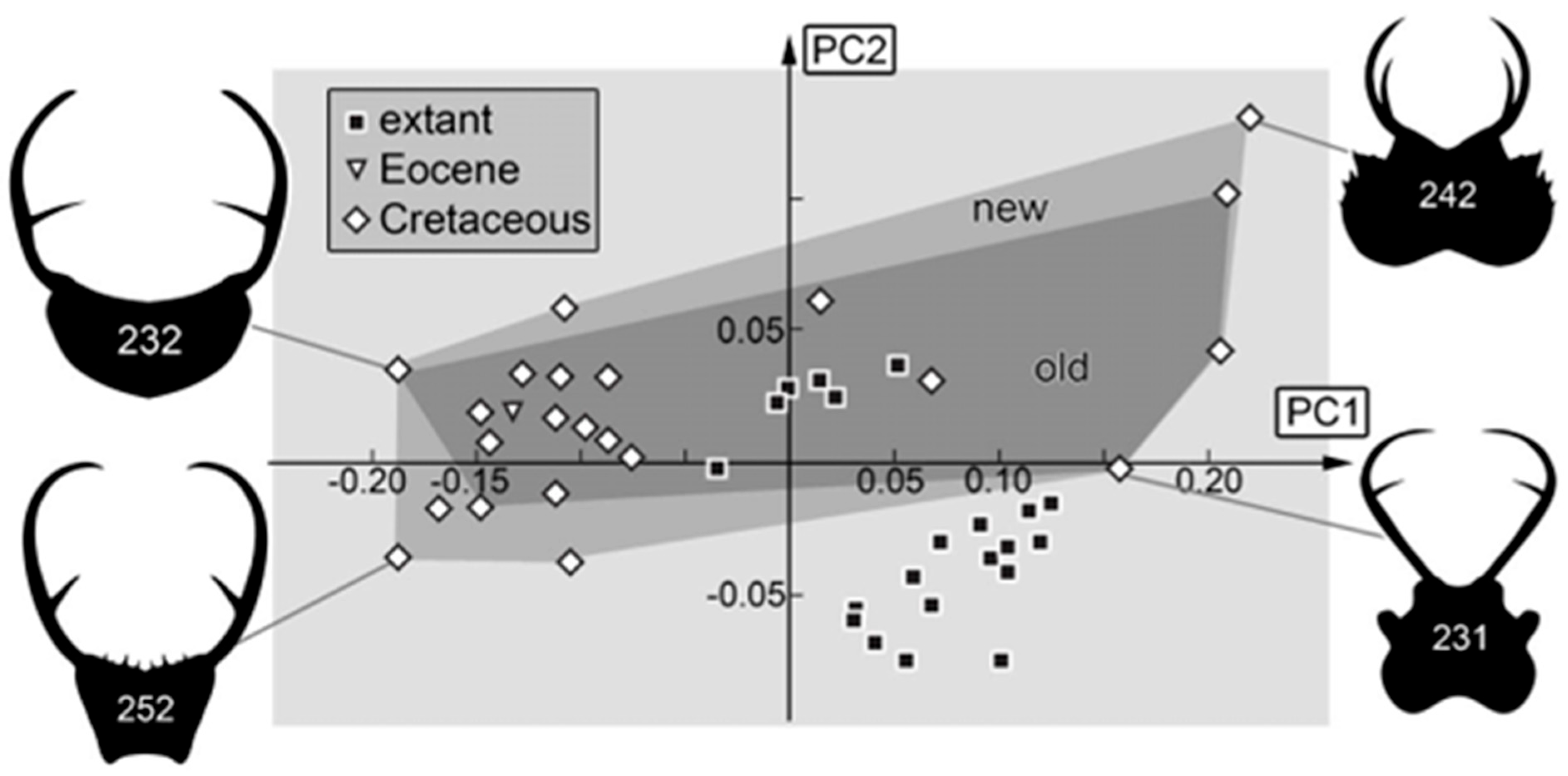
3.2. Shape Analysis
4. Discussion
4.1. Identity of the Specimens
4.2. Differences in Shape Aspects
4.3. Differences to Earlier Studies
4.4. Changes in Morphological Diversity and Abundance
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, R.R. Modern insect extinctions, the neglected majority. Conserv. Biol. 2005, 19, 1030–1036. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Ssymank, A.; Sorg, M.; de Kroon, H.; Jongejans, E. Insect biomass decline scaled to species diversity: General patterns derived from a hoverfly community. Proc. Natl. Acad. Sci. USA 2021, 118, e2002554117. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; Pelton, E.M.; Black, S.H. Declines in insect abundance and diversity: We know enough to act now. Conserv. Sci. Pract. 2019, 1, e80. [Google Scholar] [CrossRef]
- Goulson, D. The insect apocalypse, and why it matters. Curr. Biol. 2019, 29, R967–R971. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.E.; Grooten, M.; Peterson, T. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; World Wildlife Fund: Gland, Switzerland, 2020. [Google Scholar]
- Van der Sluijs, J.P. Insect decline, an emerging global environmental risk. Curr. Opin. Environ. Sustain. 2020, 46, 39–42. [Google Scholar] [CrossRef]
- Fenoglio, M.S.; Calviño, A.; González, E.; Salvo, A.; Videla, M. Urbanisation drivers and underlying mechanisms of terrestrial insect diversity loss in cities. Ecol. Entomol. 2021, 46, 757–771. [Google Scholar] [CrossRef]
- Schachat, S.R.; Labandeira, C.C. Are insects heading toward their first mass extinction? Distinguishing turnover from crises in their fossil record. Ann. Entomol. Soc. Amer. 2021, 114, 99–118. [Google Scholar] [CrossRef]
- Wagner, D.L.; Grames, E.M.; Forister, M.L.; Berenbaum, M.R.; Stopak, D. Insect decline in the Anthropocene: Death by a thousand cuts. Proc. Natl. Acad. Sci. USA 2021, 118, e2023989118. [Google Scholar] [CrossRef] [PubMed]
- Weisser, W.; Blüthgen, N.; Staab, M.; Achury, R.; Müller, J. Experiments are needed to quantify the main causes of insect decline. Biol. Lett. 2023, 19, 20220500. [Google Scholar] [CrossRef]
- Schowalter, T.D.; Noriega, J.A.; Tscharntke, T. Insect effects on ecosystem services—Introduction. Basic Appl. Ecol. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Dangles, O.; Casas, J. Ecosystem services provided by insects for achieving sustainable development goals. Ecosyst. Serv. 2019, 35, 109–115. [Google Scholar] [CrossRef]
- Morimoto, J. Addressing global challenges with unconventional insect ecosystem services: Why should humanity care about insect larvae? People Nat. 2020, 2, 582–595. [Google Scholar] [CrossRef]
- Mirth, K.M.; Riddiford, L.M. Size assessment and growth control: How adult size is determined in insects. BioEssays 2007, 29, 344–355. [Google Scholar] [CrossRef]
- Zacharuk, R.Y.; Shields, V.D. Sensilla of immature insects. Ann. Rev. 1991, 36, 331–354. [Google Scholar] [CrossRef]
- Sarwar, M. Typical flies: Natural history, lifestyle and diversity of Diptera. In Life Cycle and Development of Diptera; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK, 2005; p. 755. [Google Scholar]
- Lawrence, J.F.; Ślipiski, A.; Seago, A.E.; Thayer, M.K.; Newton, A.F.; Marvaldi, A.E. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 2011, 61, 1–217. [Google Scholar] [CrossRef]
- Yuan, M.L.; Zhang, Q.L.; Zhang, L.; Guo, Z.L.; Liu, Y.J.; Shen, Y.Y.; Shao, R. High-level phylogeny of the Coleoptera inferred with mitochondrial genome sequences. Mol. Phyl. Evol. 2016, 104, 99–111. [Google Scholar] [CrossRef]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef] [PubMed]
- Yeates, D.K.; Wiegmann, B.M.; Courtney, G.W.; Meier, R.; Lambkin, C.; Pape, T. Phylogeny and systematics of Diptera: Two decades of progress and prospects. Zootaxa 2007, 1668, 565–590. [Google Scholar] [CrossRef]
- Lambkin, C.L.; Sinclair, B.J.; Pape, T.; Courtney, G.W.; Skevington, J.H.; Meier, R.; Yeates, D.K.; Blagoderov, V.; Wiegmann, B.M. The phylogenetic relationships among infraorders and superfamilies of Diptera based on morphological evidence. Syst. Entomol. 2013, 38, 164–179. [Google Scholar] [CrossRef]
- Courtney, G.W.; Pape, T.; Skevington, J.H.; Sinclair, B.J. Biodiversity of Diptera. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 229–278. [Google Scholar]
- Timmermans, M.J.; Lees, D.C.; Simonsen, T.J. Towards a mitogenomic phylogeny of Lepidoptera. Mol. Phyl. Evol. 2014, 79, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, P.Z. Diversity and significance of Lepidoptera: A phylogenetic perspective. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 463–495. [Google Scholar]
- Mitter, C.; Davis, D.R.; Cummings, M.P. Phylogeny and evolution of Lepidoptera. Ann. Rev. Entomol. 2017, 62, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.T. Biodiversity of Hymenoptera. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017; pp. 419–461. [Google Scholar]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary history of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Shih, C.; Gao, T.; Ren, D. Chapter 22: Hymenoptera—Sawflies and Wasps. In Rhythms of Insect Evolution: Evidence from the Jurassic and Cretaceous in Northern China; Ren, D., Shih, C.K., Gao, T., Yao, Y., Wang, Y., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; pp. 429–496. [Google Scholar] [CrossRef]
- Benefer, C.; Andrew, P.; Blackshaw, R.; Ellis, J.; Knight, M. The spatial distribution of phytophagous insect larvae in grassland soils. Appl. Soil Ecol. 2010, 45, 269–274. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The ecology of stream insects. Ann. Rev. Entomol. 1970, 15, 25–42. [Google Scholar] [CrossRef]
- Hershey, A.E.; Lamberti, G.A.; Chaloner, D.T.; Northington, R.M. Aquatic insect ecology. In Ecology and Classification of North American Freshwater Invertebrates; Academic Press: Amsterdam, The Netherlands, 2010; pp. 659–694. [Google Scholar]
- Aspöck, U.; Aspöck, H. Kamelhälse, Schlammfliegen, Ameisenlöwen. Wer sind sie? (Insecta: Neuropterida: Raphidioptera, Megaloptera, Neuroptera). Stapfia 1999, 60, 1–34. [Google Scholar]
- Aspöck, U.; Aspöck, H. Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia 2007, 20, 451–516. [Google Scholar]
- Aspöck, U.; Haring, E.; Aspöck, H. The phylogeny of the Neuropterida: Long lasting and current controversies and challenges (Insecta: Endopterygota). Arthropod Syst. Phyl. 2012, 70, 119–129. [Google Scholar] [CrossRef]
- Aspöck, U.; Plant, J.D.; Nemeschkal, H.L. Cladistic analysis of Neuroptera and their systematic position within Neuropterida (Insecta: Holometabola: Neuropterida: Neuroptera). Syst. Entomol. 2001, 26, 73–86. [Google Scholar] [CrossRef]
- Winterton, S.L.; Hardy, N.B.; Wiegmann, B.M. On wings of lace: Phylogeny and Bayesian divergence time estimates of Neuropterida (Insecta) based on morphological and molecular data. Syst. Entomol. 2010, 35, 349–378. [Google Scholar] [CrossRef]
- Winterton, S.L.; Lemmon, A.R.; Gillung, J.P.; Garzon, I.J.; Badano, D.; Bakkes, D.K.; Breitkreuz, L.C.V.; Engel, M.; Moriarty, E.M.; Liu, X.; et al. Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Syst. Entomol. 2018, 43, 330–354. [Google Scholar] [CrossRef]
- Engel, M.S.; Winterton, S.L.; Breitkreuz, L.C. Phylogeny and evolution of Neuropterida: Where have wings of lace taken us? Ann. Rev. Entomol. 2018, 63, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Vasilikopoulos, A.; Misof, B.; Meusemann, K.; Lieberz, D.; Flouri, T.; Beutel, R.G.; Niehuis, O.; Wappler, T.; Rust, J.; Peters, R.S.; et al. An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evol. Biol. 2020, 20, 64. [Google Scholar] [CrossRef]
- Oswald, J.D. Neuropterida Species of the World. Lacewing Digital Library, Research Publication, 1. 2018. Available online: https://lacewing.tamu.edu/neuropterida/neur_bibliography/edoc12/oswald2024ref13078MainVer8-26549.pdf (accessed on 13 March 2024).
- MacLeod, E.G. The Neuroptera of the Baltic Amber. I. Ascalaphidae, Nymphidae, and Psychopsidae. Psyche 1970, 77, 147–180. [Google Scholar] [CrossRef]
- Larsson, S.G. Baltic Amber: A Palaeobiological Study; Scandinavian Science Press: Klampenborg, Denmark, 1978; p. 192. [Google Scholar]
- Grimaldi, D.A. A diverse fauna of Neuropteroidea in amber from the Cretaceous of New Jersey. In Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey; Grimaldi, D.A., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2000; pp. 259–303. [Google Scholar]
- Grimaldi, D.A.; Engel, M.S.; Nascimbene, P.C. Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Amer. Mus. Nov. 2002, 3361, 1–71. [Google Scholar] [CrossRef]
- Weitschat, W.; Wichard, W. Atlas of Plants and Animals in Baltic Amber; Dr. Friedrich Pfeil: München, Germany, 2002; p. 256. [Google Scholar]
- Perrichot, V. Environnements Paraliques à Ambre et à Végétaux du Crétacé Nord-Aquitain (Charentes, Sud-Ouest de la France). Ph.D. Thesis, Université Rennes, Rennes, France, 2003. Available online: https://tel.archives-ouvertes.fr/tel-00011639/file/Memoire_GS_Web.pdf (accessed on 26 January 2025).
- Scheven, J. Bernstein-Einschlüsse: Eine Untergegangene Welt Bezeugt die Schöpfung. Erinnerungen an die Welt vor der Sintflut; Kuratorium Lebendige Vorwelt e.V.: Hofheim, Germany, 2004; p. 166. [Google Scholar]
- Engel, M.S.; Grimaldi, D.A. The neuropterid fauna of Dominican and Mexican amber (Neuropterida: Megaloptera, Neuroptera). Amer. Mus. Nov. 2007, 3587, 1–58. [Google Scholar] [CrossRef]
- Engel, M.S.; Grimaldi, D.A. Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nova Suppl. Entomol. 2008, 20, 1–86. [Google Scholar]
- Wichard, W.; Gröhn, C.; Seredszus, F. Aquatic Insects in Baltic Amber; Kessel: Remagen, Germany, 2009; p. 336. [Google Scholar]
- Ohl, M. Aboard a spider—A complex developmental strategy fossilized in amber. Naturwissenschaften 2011, 98, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Fuente, R.; Delclòs, X.; Peñalver, E.; Speranza, M.; Wierzchos, J.; Ascaso, C.; Engel, M.S. Early evolution and ecology of camouflage in insects. Proc. Natl. Acad. Sci. USA 2012, 109, 21414–21419. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Delclos, X.; Penalver, E.; Engel, M.S. A defensive behavior and plant-insect interaction in Early Cretaceous amber–the case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Struct. Dev. 2016, 45, 133–139. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Peñalver, E.; Azar, D.; Engel, M.S. A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Sci. Rep. 2018, 8, 16663. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Engel, M.S.; Azar, D.; Peñalver, E. The hatching mechanism of 130-million-year-old insects: An association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology 2019, 62, 547–559. [Google Scholar] [CrossRef]
- Pérez-de la Fuente, R.; Engel, M.S.; Delclòs, X.; Peñalver, E. Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretac. Res. 2020, 106, 104200. [Google Scholar] [CrossRef]
- Gröhn, C. Einschlüsse im Baltischen Bernstein; Wachholtz Verlag—Murmann Publishers: Kiel, Germany, 2015; p. 424. [Google Scholar]
- Xia, F.; Yang, G.; Zhang, Q.; Shi, G.; Wang, B. Amber: Life Through Time and Space; Science Press: Beijing, China, 2015; p. 196. [Google Scholar]
- Liu, H.; Luo, C.; Jarzembowski, E.A.; Xiao, C. Acanthochrysa langae gen. et sp. nov., a new lacewing larva (Neuroptera: Chrysopoidea) from mid-Cretaceous Kachin amber. Cretac. Res. 2022, 133, 105146. [Google Scholar] [CrossRef]
- Liu, X.; Shi, G.; Xia, F.; Lu, X.; Wang, B.; Engel, M.S. Liverwort mimesis in a Cretaceous lacewing larva. Curr. Biol. 2018, 28, 1475–1481. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Winterton, S.L.; Breitkreuz, L.C.; Engel, M.S. Early morphological specialization for insect-spider associations in Mesozoic lacewings. Curr. Biol. 2016, 26, 1590–1594. [Google Scholar] [CrossRef]
- Wang, B.; Xia, F.; Engel, M.S.; Perrichot, V.; Shi, G.; Zhang, H.; Chen, J.; Jarzembowski, E.A.; Wappler, T.; Rust, J. Debris-carrying camouflage among diverse lineages of Cretaceous insects. Sci. Adv. 2016, 2, e1501918. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, G.; Xu, C.; Spicer, R.A.; Perrichot, V.; Schmidt, A.R.; Feldberg, K.; Heinrichs, J.; Chény, C.; Pang, H.; et al. The mid-Miocene Zhangpu biota reveals an outstandingly rich rainforest biome in East Asia. Sci. Adv. 2021, 7, eabg0625. [Google Scholar] [CrossRef] [PubMed]
- Wichard, W. Family Nevrorthidae (Insecta, Neuroptera) in mid-Cretaceous Burmese amber. Palaeodiversity 2017, 10, 1–6. [Google Scholar] [CrossRef][Green Version]
- Makarkin, V.N. Re-description of Grammapsychops lebedevi Martynova, 1954 (Neuroptera: Psychopsidae) with notes on the Late Cretaceous psychopsoids. Zootaxa 2018, 4524, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Badano, D.; Engel, M.S.; Basso, A.; Wang, B.; Cerretti, P. Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nat. Comm. 2018, 9, 3257. [Google Scholar] [CrossRef] [PubMed]
- Haug, C.; Zippel, A.; Hassenbach, C.; Haug, G.T.; Haug, J.T. A split-footed lacewing larva from about 100-million-year-old amber indicates a now extinct hunting strategy for neuropterans. Bull. Geosci. 2022, 97, 453–464. [Google Scholar] [CrossRef]
- Haug, G.T.; Haug, C.; van der Wal, S.; Müller, P.; Haug, J.T. Split-footed lacewings declined over time: Indications from the morphological diversity of their antlion-like larvae. PalZ 2022, 96, 29–50. [Google Scholar] [CrossRef]
- Haug, C.; Braig, F.; Haug, J.T. Quantitative analysis of lacewing larvae over more than 100 million years reveals a complex pattern of loss of morphological diversity. Sci. Rep. 2023, 13, 6127. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, H.; Jarzembowski, E.A. High morphological disparity of neuropteran larvae during the Cretaceous revealed by a new large species. Geol. Mag. 2022, 159, 954–962. [Google Scholar] [CrossRef]
- Hassenbach, C.; Buchner, L.; Haug, G.T.; Haug, C.; Haug, J.T. An expanded view on the morphological diversity of long-nosed antlion larvae further supports a decline of silky lacewings in the past 100 million years. Insects 2023, 14, 170. [Google Scholar] [CrossRef]
- Mengel, L.; Linhart, S.; Haug, G.T.; Weiterschan, T.; Müller, P.; Hoffeins, C.; Hoffeins, H.-W.; Baranov, V.; Haug, C.; Haug, J.T. The morphological diversity of dragon lacewing larvae (Nevrorthidae, Neuroptera) changed more over geological time scales than anticipated. Insects 2023, 14, 749. [Google Scholar] [CrossRef]
- Buchner, L.; Linhart, S.; Kalmar, G.; Arce, S.; Haug, G.T.; Haug, J.T.; Haug, C. New fossil lacewing larvae with trumpet-shaped elongate empodia provide insight into the evolution of this attachment structure. Riv. Ital. Paleontol. Stratigr. 2024, 130, 67–80. [Google Scholar] [CrossRef]
- Oswald, J.D.; Machado, R.J.P. Biodiversity of the Neuropterida (Insecta: Neuroptera, Megaloptera, and Raphidioptera). In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2008; Volume 2, pp. 627–672. [Google Scholar]
- Satar, A.; Tusun, S.; Bozdogan, H. Third instars larvae of Gepus gibbosus Hölzel, 1968 (Neuroptera: Myrmeleontindae). Zootaxa 2014, 3793, 281–285. [Google Scholar] [CrossRef]
- Humeau, A.; Rougé, J.; Casas, J. Optimal range of prey size for antlions. Ecol. Entomol. 2015, 40, 776–781. [Google Scholar] [CrossRef]
- MacLeod, E.G. A Comparative Morphological Study of the Head Capsule and Cervix of Larval Neuroptera (Insecta). Ph.D. Thesis, Harvard University, Cambridge, MA, USA, 1964; p. 528. [Google Scholar]
- Zimmermann, D.; Randolf, S.; Aspöck, U. From chewing to sucking via phylogeny—From sucking to chewing via ontogeny: Mouthparts of Neuroptera. In Insect Mouthparts: Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 361–385. [Google Scholar]
- New, T.R. The larva of Nymphes Leach (Neuroptera: Nymphidae). Neuroptera Internat. 1982, 2, 79–84. [Google Scholar]
- Froggatt, W.W. Australian Insects; William Brooks & Company Ltd.: Sydney, Australia, 1907; p. 449. [Google Scholar]
- Riek, E.F. Neuroptera (Lacewings). In Insects of Australia: A Textbook for Students and Research Workers; Melbourne University Press: Melbourne, Australia, 1970; pp. 472–494. [Google Scholar]
- Van der Weele, H.W. Ascalaphiden. Collections Zoologiques du Baron Edm. de Selys Longchamps. Catal. Syst. Descr. 1908, 8, 1–326. [Google Scholar]
- Tillyard, R.J. The Insects of Australia and New Zealand; Angus and Robertson: Sydney, Australia, 1926; p. 560. [Google Scholar]
- Ross, E.S. Insects Close Up: A Pictorial Guide for the Photographer and Collector Featuring 125 Photographs and Drawings; University of California Press: Los Angeles, CA, USA, 1953; p. 80. [Google Scholar]
- New, T.R. Some early stages of Osmylops (Neuroptera: Nymphidae). Syst. Entomol. 1983, 8, 121–126. [Google Scholar] [CrossRef]
- Gepp, J. Erforschungsstand der Neuropteren. Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). Prog. World’s Neuropterol. 1984, 183–239. Available online: https://www.zobodat.at/pdf/MONO-ENT-NEURO_MEN1_0183-0239.pdf (accessed on 25 November 2024).
- New, T.R.; Lambkin, K.J. The larva of Norfolius (Neuroptera: Nymphidae). Syst. Entomol. 1989, 14, 93–98. [Google Scholar] [CrossRef]
- Shi, C.; Winterton, S.L.; Ren, D. Phylogeny of split-footed lacewings (Neuroptera, Nymphidae), with descriptions of new Cretaceous fossil species from China. Cladistics 2015, 31, 455–490. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Badano, D.; Aspöck, U.; Aspöck, H.; Cerretti, P. Phylogeny of Myrmeleontiformia based on larval morphology (Neuropterida: Neuroptera). Syst. Entomol. 2017, 42, 94–117. [Google Scholar] [CrossRef]
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, V.; Picq, S.; Gaucherel, C.; Claude, J. Momocs: Outline analysis using R. J. Stat. Softw. 2014, 56, 1–24. Available online: http://www.jstatsoft.org/v56/i13/ (accessed on 25 November 2024). [CrossRef]
- Ax, P. Das System der Metazoa Ein Lehrbuch der phylogenetischen Systematik; Gustav Fischer: Stuttgart, Germany; Jena, Germany; New York, NY, USA, 1995; p. 226. [Google Scholar]
- Donoghue, P.C. Saving the stem group—A contradiction in terms? Paleobiology 2005, 31, 553–558. [Google Scholar]
- Jefferies, R.P.S. The origin of chordates: A methodological essay. In The Origin of Major Invertebrate Groups. Systematics Association Special Volume 12; House, M.R., Ed.; Academic Press: London, UK, 1979; pp. 443–447. [Google Scholar]
- Liu, H.; Beutel, R.G.; Makarov, K.V.; Jarzembowski, E.A.; Xiao, C.; Luo, C. The first larval record of Migadopinae (Coleoptera: Adephaga: Carabidae) from mid-Cretaceous Kachin amber, northern Myanmar. Cretac. Res. 2023, 142, 105413. [Google Scholar] [CrossRef]
- Liu, H.; Makarov, K.V.; Jarzembowski, E.A.; Xiao, C.; Luo, C. Cretoloricera electra gen. et sp. nov., the oldest record of Loricerini (Coleoptera: Adephaga: Carabidae: Loricerinae) from mid-Cretaceous Kachin amber. Cretac. Res. 2023, 148, 105540. [Google Scholar] [CrossRef]
- Rosová, K.; Prokop, J.; Hammel, J.U.; Beutel, R.G. The earliest evidence of Omophroninae (Coleoptera: Carabidae) from mid-Cretaceous Kachin amber and the description of a larva of a new genus. Arthropod Syst. Phyl. 2023, 81, 689–704. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchner, L.; Linhart, S.; Braig, F.; Haug, G.T.; Weiterschan, T.; Haug, C.; Haug, J.T. New Data Indicate Larger Decline in Morphological Diversity in Split-Footed Lacewing Larvae than Previously Estimated. Insects 2025, 16, 125. https://doi.org/10.3390/insects16020125
Buchner L, Linhart S, Braig F, Haug GT, Weiterschan T, Haug C, Haug JT. New Data Indicate Larger Decline in Morphological Diversity in Split-Footed Lacewing Larvae than Previously Estimated. Insects. 2025; 16(2):125. https://doi.org/10.3390/insects16020125
Chicago/Turabian StyleBuchner, Laura, Simon Linhart, Florian Braig, Gideon T. Haug, Thomas Weiterschan, Carolin Haug, and Joachim T. Haug. 2025. "New Data Indicate Larger Decline in Morphological Diversity in Split-Footed Lacewing Larvae than Previously Estimated" Insects 16, no. 2: 125. https://doi.org/10.3390/insects16020125
APA StyleBuchner, L., Linhart, S., Braig, F., Haug, G. T., Weiterschan, T., Haug, C., & Haug, J. T. (2025). New Data Indicate Larger Decline in Morphological Diversity in Split-Footed Lacewing Larvae than Previously Estimated. Insects, 16(2), 125. https://doi.org/10.3390/insects16020125






