Anthropogenic Impacts on Bark and Ambrosia Beetle Assemblages in Tropical Montane Forest in Northern Borneo
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Statistical Analysis
3. Results
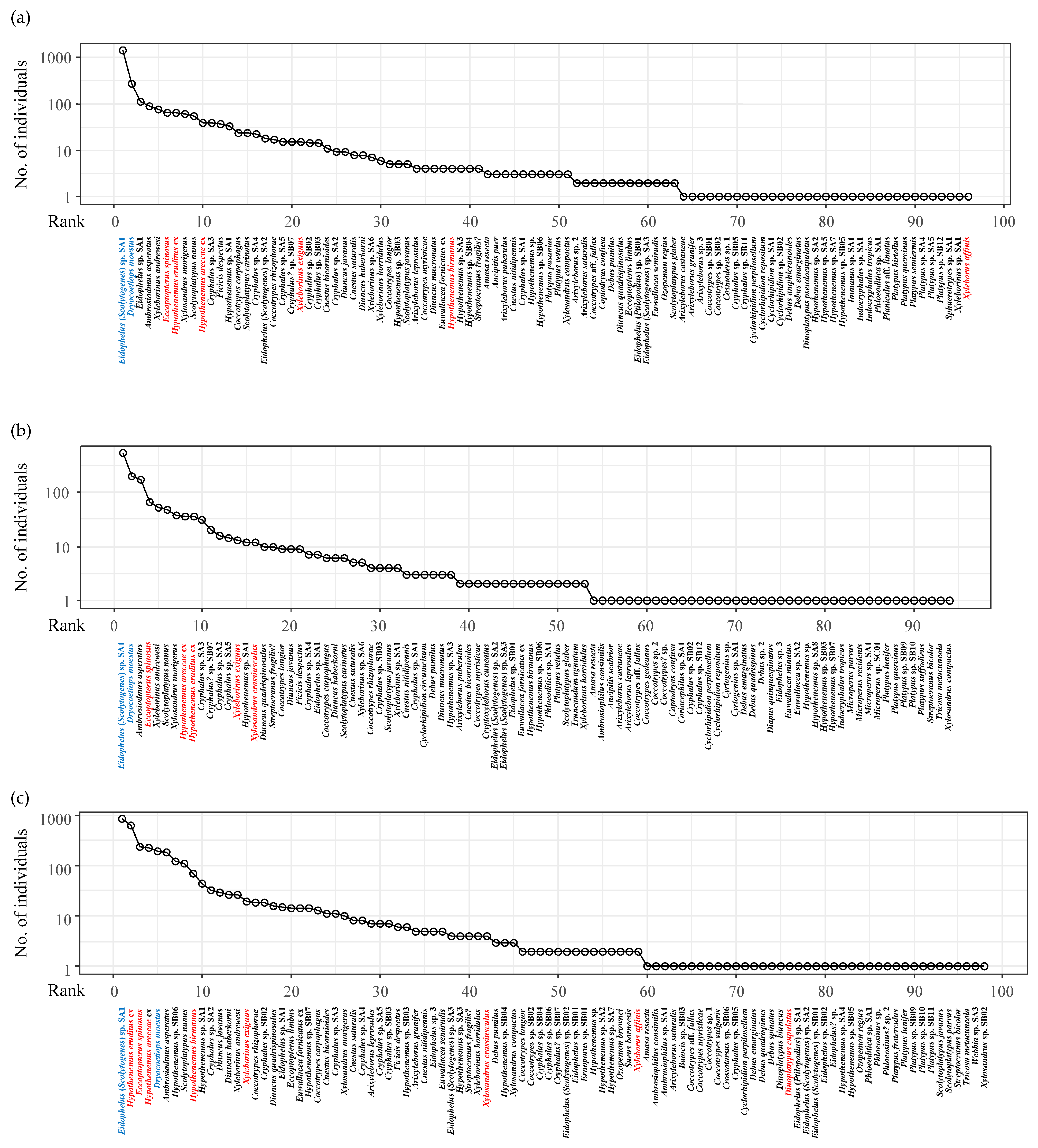
3.1. Bark and Ambrosia Beetles Species Composition
3.2. Trap Captures, Diversity Indices, and Species Estimation
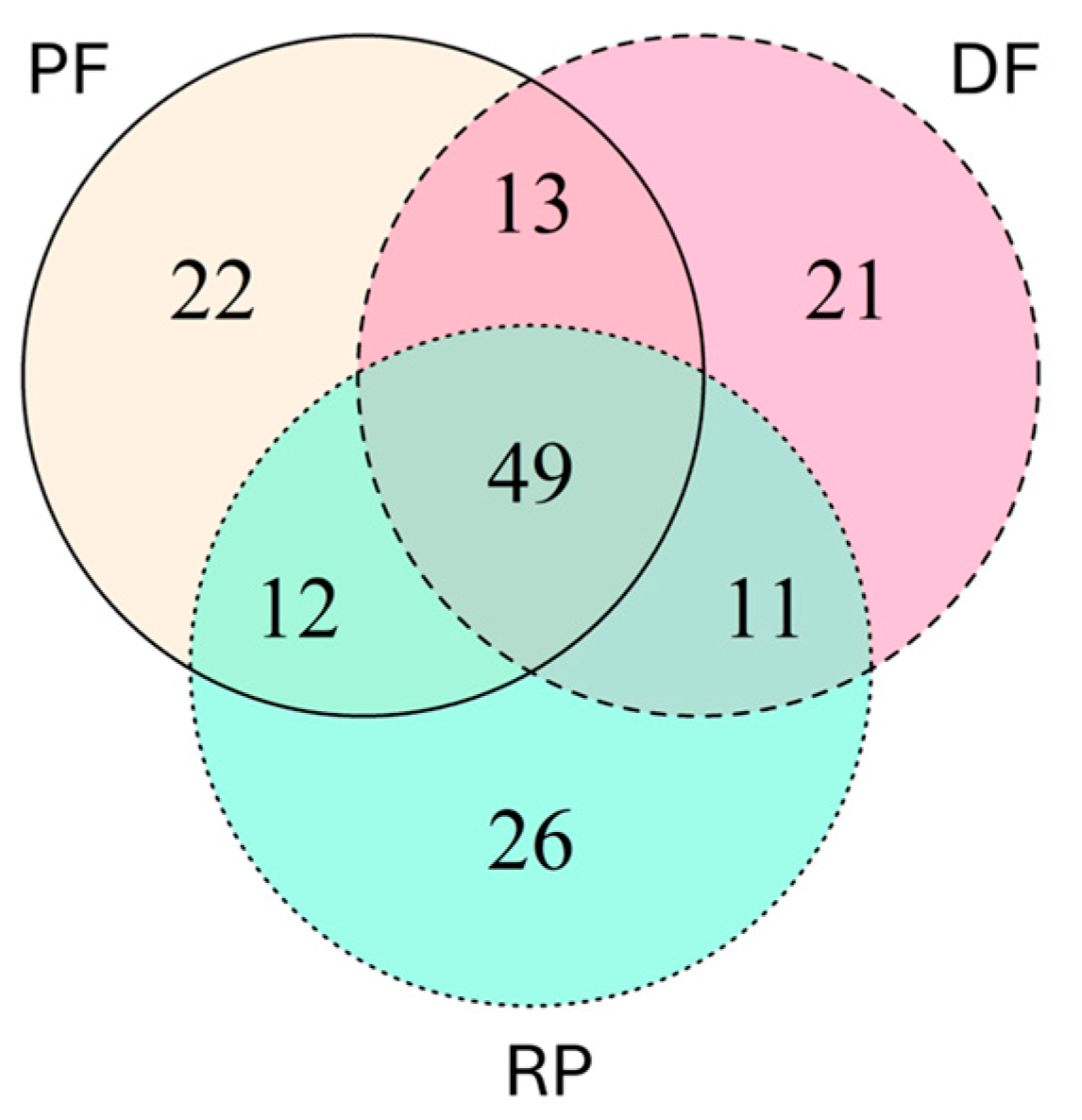
3.3. Dissimilarity of Bark and Ambrosia Beetles in Three Types of Forest
4. Discussion
4.1. High Diversity and Stochasticity of Bark and Ambrosia Beetles in Tropical Rainforest
4.2. Diversity Indices Among Three Forest Types
4.3. Influence of Rubber Trees on Bark and Ambrosia Beetle Assemblage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillay, R.; Venter, M.; Aragon-Osejo, J.; González-del-Pliego, P.; Hansen, A.J.; Watson, J.E.; Venter, O. Tropical forests are home to over half of the world’s vertebrate species. Front. Ecol. Environ. 2021, 20, 10–15. [Google Scholar] [CrossRef]
- Barlow, J.; França, F.; Gardner, T.A.; Hicks, C.C.; Lennox, G.D.; Berenguer, E.; Castello, L.; Economo, E.P.; Ferreira, J.; Guénard, B.; et al. The future of hyperdiverse tropical ecosystems. Nature 2018, 559, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Grégoire, J.-C.; Lindgren, B.S. Natural history and ecology of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: London, UK, 2015; pp. 1–40. [Google Scholar]
- Bouchard, P.; Smith, A.B.T.; Douglas, H.; Gimmel, M.L.; Brunke, A.J.; Kanda, K. Biodiversity of Coleoptera. In Insect Biodiversity: Science and Society; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 337–417. [Google Scholar]
- Jordal, B.H.; Cognato, A.I. Molecular phylogeny of bark and ambrosia beetles reveals multiple origins of fungus farming during periods of global warming. BMC Evol. Biol. 2012, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Cognato, A.I.; Smith, S.M.; Jordal, B.H. Patterns of host tree use within a lineage of saproxylic snout-less weevils (Coleoptera: Curculionidae: Scolytinae: Scolytini). Mol. Phylogenet. Evol. 2021, 159, 107107. [Google Scholar] [CrossRef]
- Peris, D.; Delclòs, X.; Jordal, B. Origin and evolution of fungus farming in wood-boring Coleoptera—A palaeontological perspective. Biol. Rev. 2021, 96, 2476–2488. [Google Scholar] [CrossRef] [PubMed]
- Grousset, F.; Grégoire, J.-C.; Jactel, H.; Battisti, A.; Benko Beloglavec, A.; Hrašovec, B.; Hulcr, J.; Inward, D.; Orlinski, A.; Petter, F. The risk of bark and ambrosia beetles associated with imported non-coniferous wood and potential horizontal phytosanitary measures. Forests 2020, 11, 342. [Google Scholar] [CrossRef]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.F.; Riggins, J.J.; Cognato, A.I. Bark Beetles. In Forest Entomology and Pathology; Allison, D., Paine, T.D., Slippers, B., Wingfield, M.J., Eds.; Springer: Cham, Switzerland, 2023; pp. 299–337. [Google Scholar]
- Kühnholz, S.; Borden, J.H.; Uzunovic, A. Secondary ambrosia beetles in apparently healthy trees: Adaptations, potential causes, and suggested research. Integr. Pest Manag. Rev. 2001, 6, 209–219. [Google Scholar] [CrossRef]
- Kamata, N.; Esaki, K.; Kato, K.; Igeta, Y.; Wada, K. Potential impact of global warming on deciduous oak dieback caused by ambrosia fungus Raffaelea sp. carried by ambrosia beetle Platypus quercivorus (Coleoptera: Platypodidae) in Japan. Bull. Entomol. Res. 2002, 92, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Choi, Y.J.; Seo, S.T.; Shin, H.D. Raffaelea quercus-mongolicae sp. nov. associated with Platypus koryoensis on oak in Korea. Mycotaxon 2009, 110, 189–197. [Google Scholar] [CrossRef]
- Fraedrich, S.W. California laurel is susceptible to laurel wilt caused by Raffaelea lauricola. Plant Dis. 2008, 92, 1469. [Google Scholar] [CrossRef]
- De Castro, A.I.; Ehsani, R.; Ploetz, R.C.; Crane, J.H.; Buchanon, S. Detection of laurel wilt disease in avocado using low altitude aerial imaging. PLoS ONE 2015, 10, e0124642. [Google Scholar] [CrossRef] [PubMed]
- Eskalen, A.; Gonzalez, A.; Wang, D.H.; Twizeyimana, M.; Mayorquin, J.S.; Lynch, S.G. First report of a Fusarium sp. and its vector tea shot hole borer (Euwallacea fornicatus) causing fusarium dieback on avocado in California. Plant Dis. 2012, 96, 1070. [Google Scholar] [CrossRef]
- Umeda, C.; Eskalen, A.; Paine, T.D. Polyphagous shot hole borer and Fusarium dieback in California. In Insects and Diseases of Mediterranean Forest Systems; Paine, T., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 757–767. [Google Scholar]
- Carrillo, D.; Cruz, L.; Kendra, P.; Narvaez, T.; Montgomery, W.; Monterroso, A.; De Grave, C.; Cooperband, M. Distribution, pest status, and fungal associates of Euwallacea nr. fornicatus in Florida avocado groves. Insects 2016, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Beaver, R.A. The invasive neotropical ambrosia beetle Euplatypus parallelus (Fabricius, 1801) in the oriental region and its pest status (Coleoptera: Curculionidae: Platypodinae). Entomol. Mon. Mag. 2013, 149, 143–154. [Google Scholar]
- Schowalter, T.D. Ecology and management of bark beetles (Coleoptera: Curculionidae: Scolytinae) in southern pine forests. J. Integr. Pest Manag. 2012, 3, A1–A7. [Google Scholar] [CrossRef]
- Onokpise, O.U. Natural rubber, Hevea brasiliensis (Willd. Ex A. Juss.) Müll. Arg., germplasm collection in the Amazon basin, Brazil: A retrospective. Econ. Bot. 2004, 58, 544–555. [Google Scholar] [CrossRef]
- Noin, J. The Development of Rubber Planting in Sabah 1892–1941. SEJARAH J. Dep. Hist. 2017, 19, 19. (In Malay) [Google Scholar]
- Chung, A.A.Y.; Paul, V.; Ibrahim, N.A.; Japir, R.; Pang, K.K.N.; Mohammad, A. Introduction to Forest Plantation Pest Insects in Sabah; Sabah Forest Department: Sabah, Malaysia; Jabatan Perhutanan Sabah: Sabah, Malaysia, 2021. (In Malay) [Google Scholar]
- De Jesus Eufrade Junior, H.; Ohto, J.M.; da Silva, L.L.; Palma, H.A.L.; Ballarin, A.W. Potential of rubberwood (Hevea brasiliensis) for structural use after the period of latex extraction: A case study in Brazil. J. Wood Sci. 2015, 61, 384–390. [Google Scholar] [CrossRef]
- Min, S.; Waibel, H.; Cadisch, G.; Langenberger, G.; Bai, J.; Huang, J. The economics of smallholder rubber farming in a mountainous region of southwest China: Elevation, ethnicity, and risk. Mt. Res. Dev. 2017, 37, 281–293. [Google Scholar] [CrossRef]
- Vijayan, D.; Girindran, R.; Sam, A.S.; Sathyan, A.R.; Kaechele, H. The large-Scale expansion of rubber plantations in southern India: Major impacts and the changing nature of drivers. Environ. Monit. Assess. 2024, 196, 356. [Google Scholar] [CrossRef] [PubMed]
- Sittichaya, W.; Beaver, R. Rubberwood-destroying beetles in the eastern and gulf areas of Thailand (Coleoptera: Bostrichidae, Curculionidae: Scolytinae and Platypodinae). Songklanakarin J. Sci. Technol. 2009, 31, 381–387. [Google Scholar]
- Sarikaya, O. Notes on bark and wood-boring beetles (Coleoptera: Bostrichidae, Curculionidae; Platypodinae and Scolytinae) of the sweetgum (Liquidambar orientalis Mill.) forest nature protection area, with a new record for Turkish fauna. J. Food Agric. Environ. 2013, 11, 2178–2185. [Google Scholar]
- Kangkamanee, T.; Sittichaya, W.; Ngampongsai, A.; Permkam, S.; Beaver, R.A. Wood-boring beetles (Coleoptera: Bostrichidae, Curculionidae; Platypodinae and Scolytinae) infesting rubberwood sawn timber in southern Thailand. J. For. Res. 2011, 16, 302–308. [Google Scholar] [CrossRef]
- Liza, A.; Pamunag, M.N.; Lozada, A.O.; Bagaforo, R.O. First report of the pinhole borer (Euplatypus sp.) to cause stem bleeding of rubber trees in the Philippines. J. Rubber Res. 2022, 25, 151–155. [Google Scholar]
- Gunggot, E.; Beaver, R.A.; Lucas, J.J.; George, S.G.; Rasiah, A.; Wong, W.V.C.; Lardizabal, M.L.T.; Kamata, N. List of Scolytinae and Platypodinae (Coleoptera: Curculionidae) Captured by Ethanol-Baited Traps in a Tropical Rainforest in Southern Sabah, Malaysia. Misc. Inf. Univ. Tokyo For. 2025, 71. [Google Scholar]
- Steininger, M.S.; Hulcr, J.; Sigut, M.; Lucky, A. Simple and Efficient Trap for Bark and Ambrosia Beetles (Coleoptera: Curculionidae) to Facilitate Invasive Species Monitoring and Citizen Involvement. J. Econ. Entomol. 2015, 108, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Sanguansub, S.; Buranapanichpan, S.; Beaver, R.; Kamata, N. List of wood-boring beetles (Coleoptera: Bostrichidae, Curculionidae; Platypodinae, and Scolytinae) captured by ethanol-baited traps in a lower montane forest in northern Thailand. Misc. Inform. Univ. Tokyo For. 2020, 62, 15–59. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Chao, A.; Chiu, C.-H. Species richness: Estimation and comparison. In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–26. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. R Package Version 3.0.1. 2024. Available online: https://cran.r-project.org/web/packages/iNEXT/iNEXT.pdf (accessed on 3 August 2024).
- Chao, A. Species Estimation and Applications. In Encyclopedia of Statistical Sciences; Balakrishnan, N., Read, C.B., Vidakovic, B., Eds.; Wiley: New York, NY, USA, 2005; pp. 7907–7916. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-6. 2024. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 11 August 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 11 August 2024).
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots. R Package Version 1.7.3. 2022. Available online: https://cran.r-project.org/web/packages/VennDiagram/VennDiagram.pdf (accessed on 15 August 2024).
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 354–366. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Simpson, G. Permute: Functions for Generating Restricted Permutations of Data. R Package Version 0.9-7. 2022. Available online: https://cran.r-project.org/web/packages/permute/permute.pdf (accessed on 9 October 2024).
- Wylie, F.R.; Speight, M.R. Insect Pests in Tropical Forestry, 2nd ed.; CABI: Wallingford, UK, 2012. [Google Scholar]
- Chung, A.Y.C. Vertical stratification of beetles (Coleoptera) using flight intercept traps in a lowland rainforest of Sabah, Malaysia. Sepilok Bull. 2004, 1, 29–41. [Google Scholar]
- Reding, M.E.; Schultz, P.B.; Ranger, C.M.; Oliver, J.B. Optimizing ethanol-baited traps for monitoring damaging ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in ornamental nurseries. J. Econ. Entomol. 2011, 104, 2017–2024. [Google Scholar] [CrossRef]
- Idzuka, H.; Goto, H.; Yamasaki, M.; Osawa, N. Wood-boring beetles (Coleoptera: Scolytidae, Platypodidae) captured in ethanol-baited traps in a natural forest in Japan. Appl. Entomol. Zool. 2016, 51, 347–352. [Google Scholar] [CrossRef]
- Ricotta, C.; Podani, J. On some properties of the Bray-Curtis dissimilarity and their ecological meaning. Ecol. Complex. 2017, 31, 201–205. [Google Scholar] [CrossRef]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.-J. Abundance-based similarity indices and their estimation when there are unseen species in samples. Biometrics 2006, 62, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.Y.; James, D.; Ioki, K.; Wong, W.V.C.; Tsuyuki, S.; Phua, M.-H. Aboveground Biomass Changes in Tropical Montane Forest of Northern Borneo Estimated Using Spaceborne and Airborne Digital Elevation Data. Remote Sens. 2020, 12, 3677. [Google Scholar] [CrossRef]
- Yutaka, I.; Esaki, K.; Kato, K.; Kamata, N. Influence of light condition on the stand-level distribution and movement of the ambrosia beetle Platypus quercivorus (Coleoptera: Platypodidae). Appl. Entomol. Zool. 2003, 38, 167–175. [Google Scholar]
- Wermelinger, B.; Flückiger, P.F.; Obrist, M.K.; Duelli, P. Horizontal and vertical distribution of saproxylic beetles (Col., Buprestidae, Cerambycidae, Scolytinae) across sections of forest edges. J. Appl. Entomol. 2007, 131, 104–114. [Google Scholar] [CrossRef]
- Johnson, A.J.; Hulcr, J.; Knízek, M.; Atkinson, T.H.; Mandelshtam, M.Y.; Smith, S.M.; Cognato, A.I.; Park, S.; Li, Y.; Jordal, B.H. Revision of the bark beetle genera within the former Cryphalini (Curculionidae: Scolytinae). Insect Syst. Divers. 2020, 4, 1. [Google Scholar] [CrossRef]
- Johnson, A.; LeMay, G.; Hulcr, J. Identification of coffee berry borer from similar bark beetles in Southeast Asia and Oceania. Edis 2022, fr447. Available online: https://edis.ifas.ufl.edu/publication/fr447 (accessed on 19 October 2024). [CrossRef]
- Deyrup, M. Trischidias exigua wood, new to the United States, with notes on the biology of the genus (Coleoptera: Scolytidae). Coleopt. Bull. 1987, 41, 339–343. [Google Scholar]
- Westwood, J.O., VII. Description of a minute coleopterous insect, forming the type of a new subgenus allied to Tomicus, with some observations upon the affinities of the Xylophaga. Trans. R. Entomol. Soc. Lond. 1836, 1, 34–36. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Hulcr, J.; Johnson, A.J.; Lucky, A. Bark Beetle Hypothenemus eruditus Westwood, 1836 (Insecta: Coleoptera: Curculionidae: Scolytinae). Edis 2016, 8, 5. [Google Scholar] [CrossRef]


| Type of Forest | Trap | Elevation (m a.s.l.) | Coordinates |
|---|---|---|---|
| Primary Forest (PF) | PF−1 | 1082 | 4°26′52.6″ N 115°44′11.4″ E |
| PF−2 | 1078 | 4°26′51.7″ N 115°44′11.5″ E | |
| PF−3 | 1098 | 4°26′50.4″ N 115°44′09.5″ E | |
| PF−4 | 1104 | 4°26′50.4″ N 115°44′08.4″ E | |
| Disturbed Forest (DF) | DF−1 | 1024 | 4°27′27.0″ N 115°44′50.0″ E |
| DF−2 | 1016 | 4°27′23.7″ N 115°44′50.0″ E | |
| DF−3 | 1065 | 4°27′41.5″ N 115°44′34.0″ E | |
| DF−4 | 1064 | 4°27′41.2″ N 115°44′34.1″ E | |
| Rubber Plantation Forest (RP) * | RP−1 | 1081 | 4°26′51.7″ N 115°44′15.5″ E |
| RP−2 | 1081 | 4°26′51.4″ N 115°44′16.0″ E | |
| RP−3 | 1047 | 4°26′48.4″ N 115°44′22.5″ E | |
| RP−4 | 1047 | 4°26′47.9″ N 115°44′23.0″ E |
| PF | DF | RP | Overall | |
|---|---|---|---|---|
| Number of individuals | 2697 (2698.9 ± 475.8) a | 1468 (1469.0 ± 253.0) b | 3092 (3093.8 ± 400.9) c | 7257 |
| Species richness | 96 (79.7 ± 9.3) a | 94 (75.2 ± 8.0) b | 98 (80.1 ± 7.7) c | 154 |
| Shannon-Wiener diversity index (H′) | 2.294 (2.27 ± 0.15) a | 2.675 (2.626 ± 0.12) b | 2.637 (2.610 ± 0.08) c | 2.681 |
| Berger-Parker dominance index (D) | 0.515 (0.514 ± 0.040) a | 0.354 (0.353 ± 0.034) b | 0.269 (0.270 ± 0.044) c | 0.377 |
| Species | PF | DF | RP |
|---|---|---|---|
| Observed | 96 | 94 | 98 |
| Estimated | 141 ± 21.6 | 149 ± 23.9 | 152 ± 23.8 |
| % Observed | 70.6 | 64.8 | 66.7 |
| PF–DF | PF–RP | DF–RP | |
|---|---|---|---|
| Bray-Curtis Dissimilarity Index | 0.375 (0.394 ± 0.067) a | 0.435 (0.444 ± 0.072) b | 0.439 (0.454 ± 0.059) c |
| Chao Dissimilarity Index | 0.035 (0.0492 ± 0.033) a | 0.033 (0.0557 ± 0.049) b | 0.016 (0.071 ± 0.056) c |
| PF | DF | RP | |
|---|---|---|---|
| Singletons | 18 | 18 | 20 |
| Doubletons | 2 | 2 | 6 |
| Tripletons | 2 | 1 | 0 |
| Total | 22 | 21 | 26 |
| Forest Type | Species | Indicator Value | p-Value |
|---|---|---|---|
| PF | Eidophelus (Scolytogenes) sp. SA1 | 0.319 | 0.0001 |
| Scolytoplatypus carinatus Bright | 0.306 | 0.0001 | |
| Ficicis despectus (Walker) | 0.268 | 0.0001 | |
| RP | Hypothenemus eruditus cx Westwood | 0.582 | 0.0001 |
| Hypothenemus areccae cx (Hornung) | 0.468 | 0.0001 | |
| Hypothenemus sp. SB06 | 0.412 | 0.0001 | |
| Hypothenemus birmanus (Eichhoff) | 0.397 | 0.0001 | |
| Eccoptopterus limbus Sampson | 0.273 | 0.0003 | |
| Eccoptopterus spinosus (Olivier) | 0.265 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunggot, E.; Beaver, R.A.; Lucas, J.J.; George, S.G.; Rasiah, A.; Wong, W.V.C.; Lardizabal, M.L.T.; Kamata, N. Anthropogenic Impacts on Bark and Ambrosia Beetle Assemblages in Tropical Montane Forest in Northern Borneo. Insects 2025, 16, 121. https://doi.org/10.3390/insects16020121
Gunggot E, Beaver RA, Lucas JJ, George SG, Rasiah A, Wong WVC, Lardizabal MLT, Kamata N. Anthropogenic Impacts on Bark and Ambrosia Beetle Assemblages in Tropical Montane Forest in Northern Borneo. Insects. 2025; 16(2):121. https://doi.org/10.3390/insects16020121
Chicago/Turabian StyleGunggot, Evahtira, Roger A. Beaver, Jonathan Jimmey Lucas, Sandra Geogina George, Anastasia Rasiah, Wilson V. C. Wong, Maria Lourdes T. Lardizabal, and Naoto Kamata. 2025. "Anthropogenic Impacts on Bark and Ambrosia Beetle Assemblages in Tropical Montane Forest in Northern Borneo" Insects 16, no. 2: 121. https://doi.org/10.3390/insects16020121
APA StyleGunggot, E., Beaver, R. A., Lucas, J. J., George, S. G., Rasiah, A., Wong, W. V. C., Lardizabal, M. L. T., & Kamata, N. (2025). Anthropogenic Impacts on Bark and Ambrosia Beetle Assemblages in Tropical Montane Forest in Northern Borneo. Insects, 16(2), 121. https://doi.org/10.3390/insects16020121







