1. Introduction
Understanding the ecology and biology of insect pests is fundamental for developing effective management strategies [
1,
2,
3]. Abiotic factors such as temperature, humidity, and photoperiod exert direct effects on insect performance, viability, development, and survival [
4]. Among the main pest species, the green-belly stink bug
Diceraeus melacanthus (Dallas, 1851) (Heteroptera: Pentatomidae) is an hemimetabolous (five nymphal instars) and polyphagous insect capable of feeding on several crops, starting from early nymphal instars, particularly soybean [
Glycine max (L.) Merr.], maize (
Zea mays L.), and wheat (
Triticum aestivum L.) [
1,
5,
6,
7]. These crops are cultivated throughout the year in the states of Mato Grosso do Sul, Paraná, and São Paulo, with wheat production being especially relevant in Paraná state. As a result,
D. melacanthus has continuous feeding opportunities across these agricultural regions [
8].
Climatic conditions in these states are diverse. Paraná is located in a transition zone between a humid subtropical climate with hot summers (Cfa) and a temperate humid climate with mild summers (Cfb). Mato Grosso do Sul is predominantly characterized by a tropical wet climate with rainy summers and dry winters (Aw). São Paulo is mainly classified as humid subtropical with dry winters and rainy summers (Cwa). These acronyms are in accordance with the Köppen–Geiger climatic classifications [
9]. These climatic variations support the cultivation of crops with distinct thermal and hydric requirements [
8,
10]. At the same time, they influence insect distribution by restricting the availability of host plants or imposing temperature conditions that may prevent insects from completing their developmental cycle.
Successful insect development requires favorable combinations of nutrient availability, moisture, and temperature [
11]. Photoperiod and temperature are closely linked to insect metabolism, and each species exhibits specific thresholds that determine the duration of its life cycle [
4]. For
D. melacanthus, the average growth cycle is 27 days at 25 °C, becoming shorter at higher temperatures (31 °C) and longer at lower ones (16 °C); this is directly influenced by the upper and lower developmental base temperatures [
12,
13]. The reproductive potential of
D. melacanthus is relatively unknown. Such knowledge is essential to explain population outbreaks in some regions and the absence of this species in others.
The potential number of insect generations can be represented using climatic suitability maps, which identify areas with higher or lower generational frequency [
14,
15,
16,
17,
18]. These maps are constructed from historical temperature records and are spatially explicit, considering latitude, longitude, and altitude. The construction and availability of these climatic suitability maps enhance current and future integrated pest management (IPM) programs. Understanding pest ecology and thermal biology is key for the proper performance of IPM strategies, given that insect adaptability and survival influence potential plant damage and production loss [
15,
16]. Maps that represent insect phenology may also be used to guide pest management decision-making [
19].
The objective of this study was to determine the lower (Tb) and upper (Tup) developmental base temperatures, the thermal constant (K), and degree-days for D. melacanthus, then to verify if geographic characteristics influence the specimen’s development, and finally, to estimate the potential annual generations in the states of Mato Grosso do Sul, Paraná, and São Paulo, represented with phenology maps.
2. Materials and Methods
2.1. Study Environment
The data used in this study were obtained from previously published research, which investigated the biology of
Diceraeus melacanthus through two experimental trials (2017 and 2019) [
13]. In that work, the green-belly stink bug was reared under five constant temperatures (16, 21, 26, 31, and 36 °C ± 1 °C) with a 14:10 h (light/dark) photoperiod in climate-controlled chambers and was fed with peanut seeds (
Arachis hypogaea), soybean grains, and bean pods (
Phaseolus vulgaris).
2.2. Evaluation Concept
Ecdysis, instar change, and total development time for
D. melacanthus were tabulated (
Table 1).
Based on the total developmental period of D. melacanthus, the following biological parameters were calculated: lower developmental threshold (Tb), upper developmental threshold (Tup), and thermal constant (K). The biofix was set at egg hatching. Subsequently, values of degree-days (DD), accumulated degree-days (ADD), and the potential annual generations (PAG) were estimated. These data were later used to construct phenology maps showing the potential generations of D. melacanthus in the states of Mato Grosso do Sul, Paraná, and São Paulo.
Meteorological data for the state of Mato Grosso do Sul were obtained from the online database of the National Institute of Meteorology (INMET), comprising historical records (2008–2023) from 23 municipalities: Água Clara, Amambai, Aquidauana, Bataguassu, Campo Grande, Cassilândia, Corumbá, Costa Rica, Coxim, Dourados, Itaquirai, Ivinhema, Jardim, Juti, Maracaju, Miranda, Ponta Porã, Porto Murtinho, Rio Brilhante, São Gabriel do Oeste, Sete Quedas, Sonora, and Três Lagoas.
Meteorological data for the state of Paraná were provided by the Meteorology Department of the Instituto de Desenvolvimento Rural do Paraná IAPAR-EMATER (IDR-Paraná). The dataset covered a 39-year period (1976–2015) across 28 municipalities: Antonina, Bandeirantes, Bela Vista do Paraíso, Cambará, Cândido de Abreu, Cascavel, Cerro Azul, Clevelândia, Fernandes Pinheiro, Francisco Beltrão, Guarapuava, Guaraqueçaba, Ibiporã, Joaquim Távora, Lapa, Londrina, Morretes, Nova Cantú, Palmas, Palotina, Paranavaí, Pato Branco, Pinhais, Planalto, Ponta Grossa, São Miguel do Iguaçu, Telêmaco Borba, and Umuarama. The 2015 cut-off was due to missing records and municipalities in later years.
Meteorological data for the state of São Paulo were also obtained from the INMET database, comprising records from 2003 to 2023 for 31 municipalities: Ariranha, Avaré, Barra Bonita, Barra do Turvo, Barretos, Barueri, Bauru, Campos do Jordão, Casa Branca, Franca, Ibitinga, Iguape, Itapeva, Itapira, Ituverava, Jales, José Bonifácio, Lins, São Paulo, Ourinhos, Piracicaba, Pradópolis, Presidente Prudente, Rancharia, São Carlos, São Luís do Paraitinga, São Miguel Arcanjo, Sorocaba, Taubaté, Valparaíso, and Votuporanga.
The latitude, longitude, and altitude of each municipality corresponded to the location of its respective meteorological station.
2.3. Data Analysis
Given the limited available data on
D. melacanthus development in different temperatures, the lower base temperature (Tb) was calculated using a linear regression where the abscissa represented the rearing temperatures and the ordinates represented the development rate (DR).
The Tb is found where the linear regression crosses the abscissa, in other words, where the DR is null.
The thermal constant (K) was found through the linear regression’s angular coefficient (b) multiplicative inverse.
The upper base temperature was obtained through the sum of the Tb with the square root of the thermal constant.
The optimal temperature range for development was determined with the following formulas, resulting in the upper and lower limits (UL and LL):
The required development days for the upper limit are equal to its value, but the units are in days. To calculate the required development days for the inferior limit, the following formula was used:
The degree-days were calculated following the method as shown by De Melo, Tenente, and de Olivera [
20]:
where
DD = Degree-days
ADD = Accumulated degree-days
Tmax = Maximum temperature recorded during the day
Tmin = Minimal temperature recorded during the day
Tb = Lower base organism development temperature
Tup = Upper base organism development temperature
n = Number of accumulated days
i = Index variable of number of days used in degree-day calculation
The degree-day (DD) calculation was accomplished using the averaged biological parameters (
Table 2). The DD for each state’s municipality was calculated, and their sum represented the municipality’s accumulated degree-days (ADD). This value was then divided by the total years of historical meteorological data, resulting in the average municipality accumulated degree-days (AMADD). Finally, these means were divided by the thermal constant (K), resulting in the municipality’s potential annual generations (PAG); these results are represented in tables for each state.
The PAG results were then extrapolated to cover each state utilizing a linear multiple regression, considering the variations in latitude (decimal degrees), longitude (decimal degrees), and altitude (meters):
where a = equation constant; b = latitude factor variable; c = longitude factor variable; and d = altitude factor variable.
Three linear multiple regressions were created, one for each state, and each of these regressions was calculated utilizing the latitude, longitude, and altitude of the previously cited municipalities.
2.4. Software Used
The biological temperature standards, degree-day macros, and multiple linear regression were all calculated utilizing the Microsoft Office Excel (Microsoft 365) software. The macros were used to automate degree-day calculation by using recorded daily temperatures, present in several datasheets from varying dates, and automating data cross-referencing. The linear multiple regressions were calculated utilizing the Office Excel function of INDEX combined with LINEST. The
D. melacanthus phenology maps were later produced utilizing the free-access QGIS (v3.28.12) software [
21]. Potential annual generations (PAG) and average municipal accumulated degree-days (AMADD), derived from 25 randomly selected municipalities, for the Mato Grosso do Sul, Paraná, and São Paulo states, were analyzed for normality, homoscedasticity, and ANOVA mean comparisons, following Scott–Knott (α < 0.01), using the free-access Sisvar software (version 5.8) [
22].
2.5. QGIS Methodology and Operation
First, the Coordinate Reference System (CRS) was standardized to the World Geodetic System (WGS 1984), authority identifier EPSG:4326. Elevation data for the three states were obtained from NASA’s global topographic program, the Shuttle Radar Topography Mission (SRTM) [
23]. Elevations were represented as raster layers in Float32 (GeoTIFF) format for each state using the SRTM-Downloader plugin, developed by Dr. Horst Düster. Each elevation raster layer was subsequently clipped to the boundary of the respective state using the “clip raster by mask layer” tool. The mask layers were territorial grids of federal units (released in 2022) for the states of Mato Grosso do Sul, Paraná, and São Paulo, provided by the Brazilian Institute of Geography and Statistics (IBGE).
Latitude and longitude data for each state were obtained by importing a Comma-Separated Values (CSV) file into QGIS through the Data Source Manager. The file was initially introduced as delimited text with geometry defined by latitude and longitude values in the respective columns. Latitude and longitude values were stored in the attribute table in decimal degrees. This file was then exported as a GeoPackage to facilitate further manipulation. Next, rasterization of these data was performed for each GeoPackage file to generate individual raster layers of latitude and longitude for each state. This was accomplished using the SAGA GIS Provider plugin, developed by Víctor Olaya, applying its polynomial regression function configured to produce a simple planar surface (GeoTIFF) with 1000 × 1000 pixels.
Finally, multiple linear regressions generated from biological and meteorological data for each state were applied using the QGIS raster calculator, in which latitude, longitude, and altitude were represented by their respective raster layers (Float32). This process resulted in a Float32 file containing the extrapolated regression outputs across the entire state territory. These files were subsequently symbolized to display fluctuations in the potential number of D. melacanthus annual generations in each state. Visualization was performed using single-band false-color rendering with discrete interpolation, an inverted spectral color gradient, and five classes defined. The maps for each state were ultimately plotted both individually and jointly, with the scale of potential annual generations of D. melacanthus standardized across all outputs.
3. Results
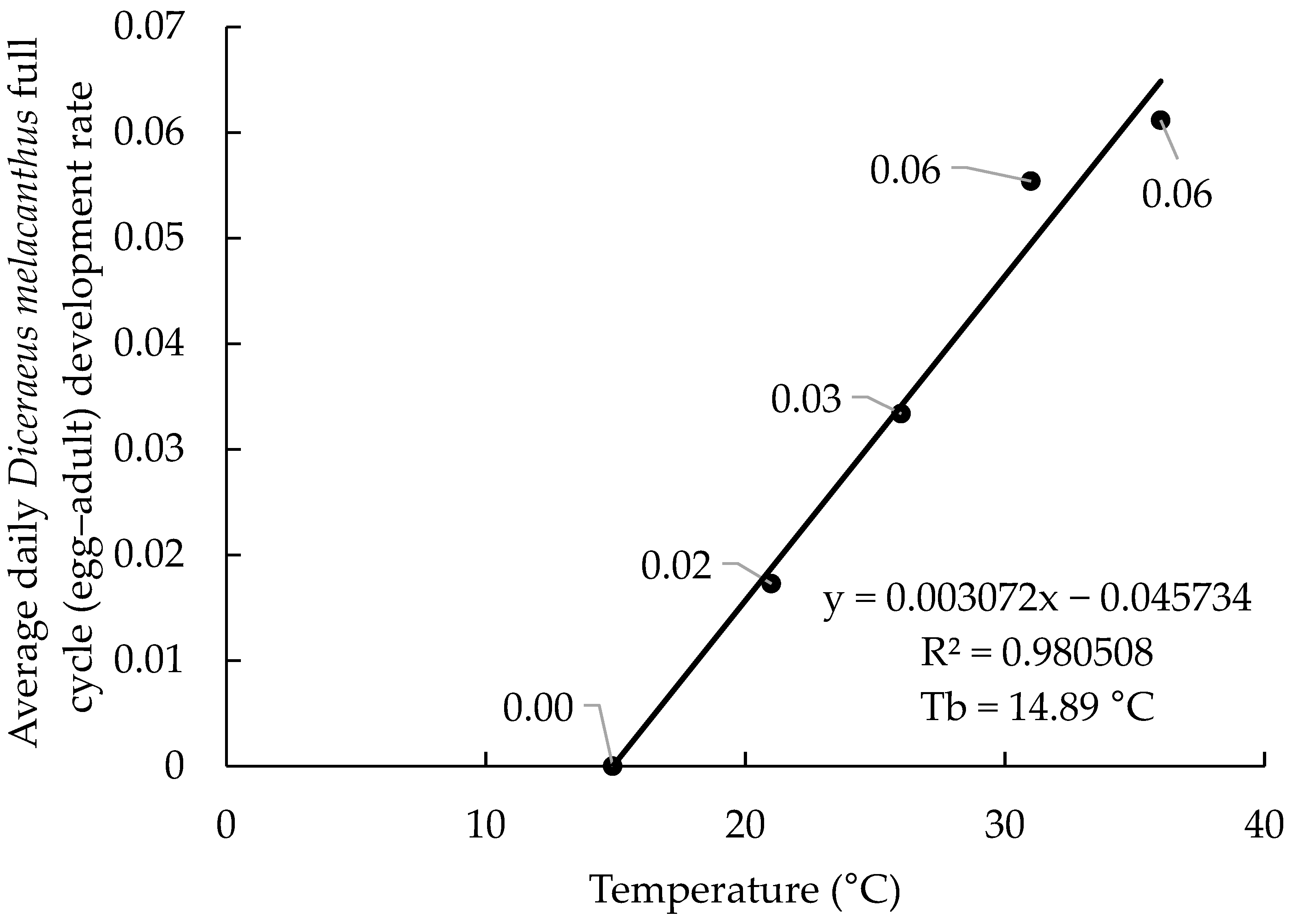
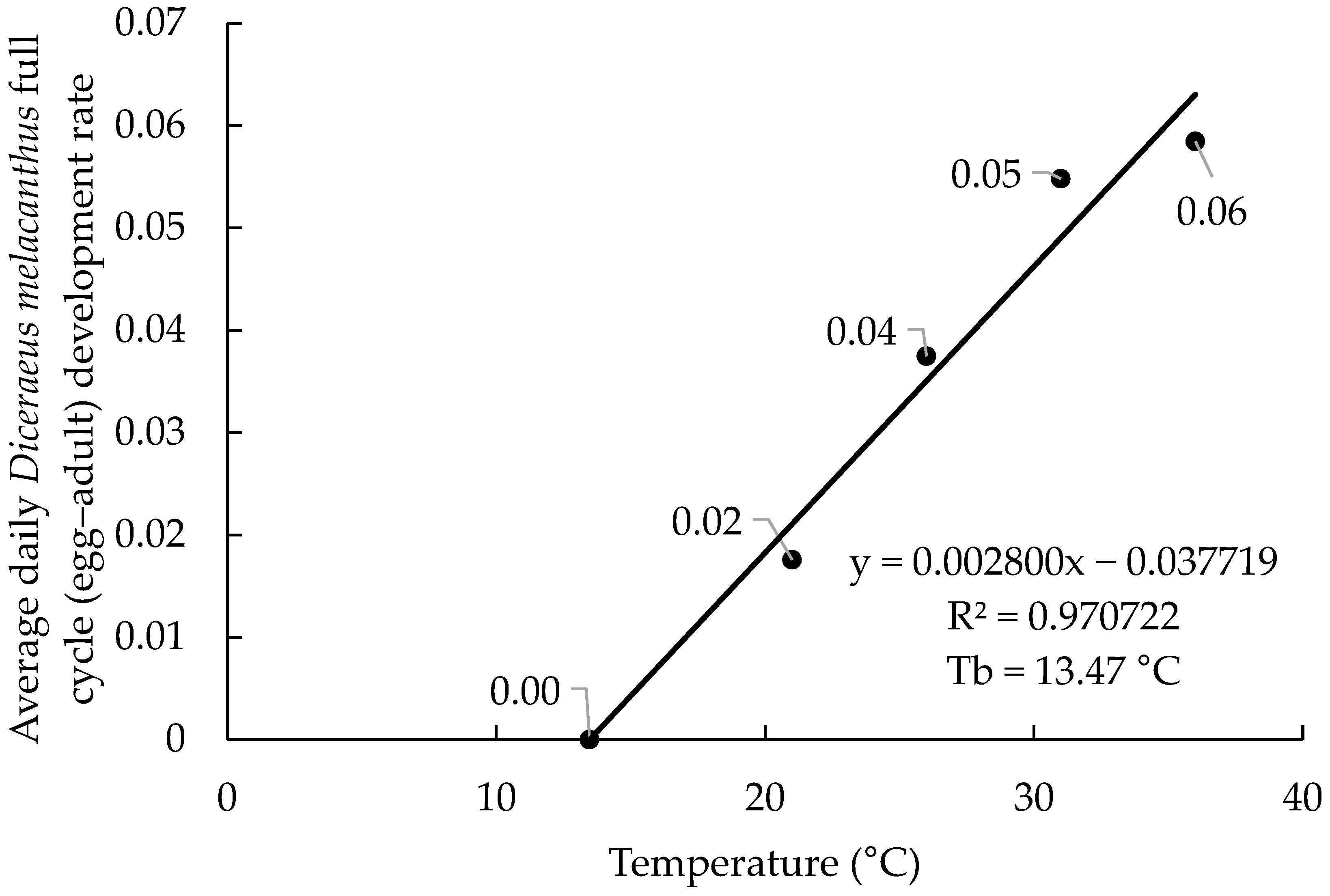
The linear regressions based on the 2017 and 2019 datasets showed highly consistent results, with coefficients of determination (R
2) of 0.98 and 0.97, respectively (
Figure 1 and
Figure 2). The estimated equations were (−0.045734 + 0.003072x) for 2017 and (−0.037719 + 0.002800x) for 2019. The lower base organism development temperature (Tb) was calculated at 14.89 °C (2017) and 13.47 °C (2019), indicating similar biological requirements. In both years, the highest developmental rates were observed at 31 and 36 °C, ranging from 0.055 to 0.061 day
−1. The marginal differences in developmental rate between the 31 and 36 °C temperatures (0.006 day
−1 in 2017 and 0.003 day
−1 in 2019) may suggest that these are neighboring the upper base organism development temperature (Tup).
The biological parameters estimated from the 2017 and 2019 datasets were highly consistent, with only minor variations between years (
Table 2). The lower base temperatures (Tb) were 14.89 °C in 2017 and 13.47 °C in 2019, while the Tup was nearly identical, at 32.9 °C and 32.4 °C, respectively. The thermal constant (K) showed the largest difference, with 325.5 degree-days in 2017 and 357.1 degree-days in 2019, indicating a longer developmental duration in 2019. The upper limit of the optimal temperature range (UL) was stable across years (27 °C, 27 days), whereas the lower limit (LL) showed greater variation, with 19.1 °C and 77 days in 2017 and 17.8 °C and 82 days in 2019. The UL and LL serve to represent the best development range for the specimen; they do not represent development thresholds. The thresholds are represented by the Tb and Tup values. Together, these values corroborate the regression analyses, reinforcing that 31 and 36 °C are near the Tup.
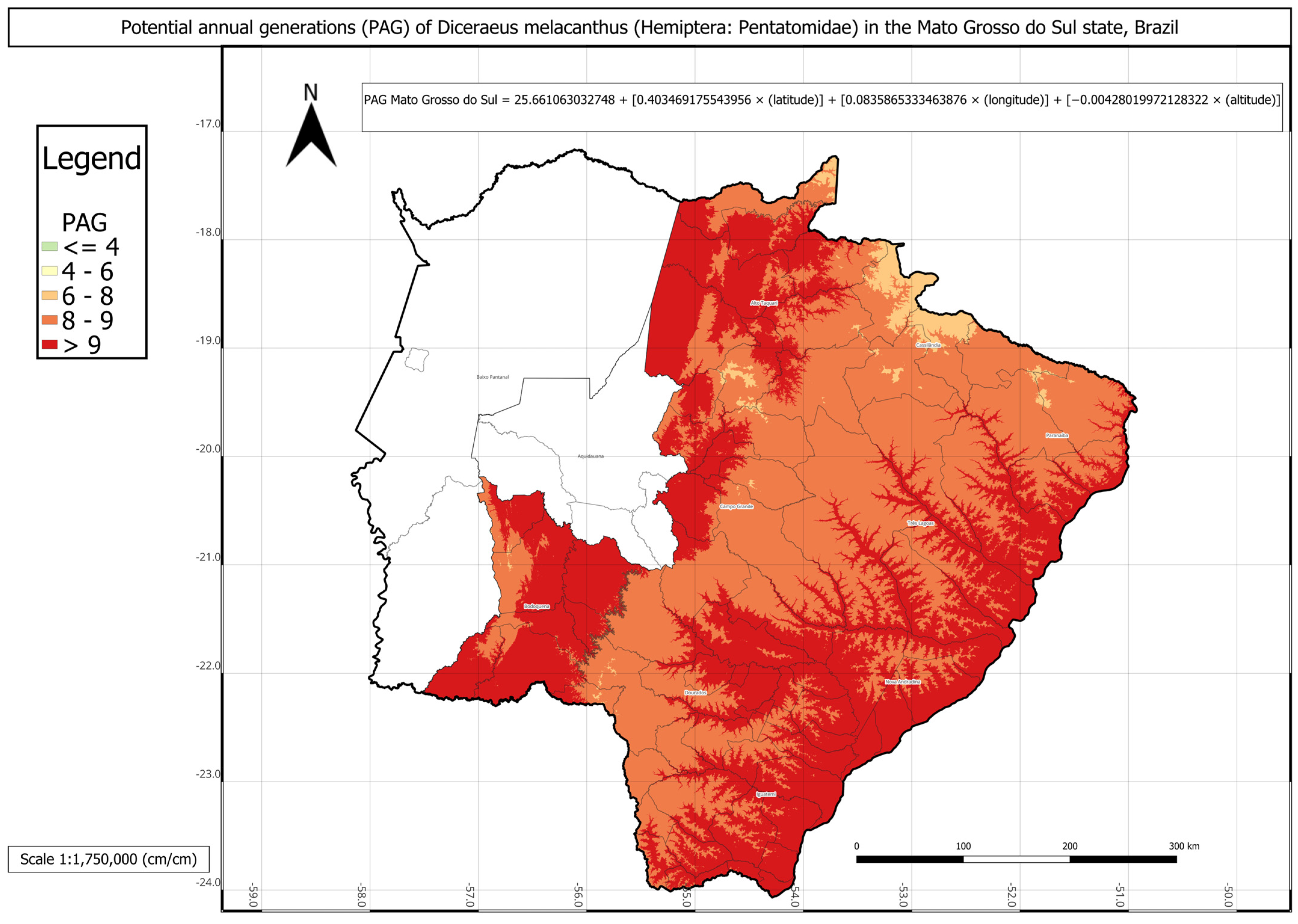
In the state of Mato Grosso do Sul, the potential annual generations (PAG) of
D. melacanthus was the highest among the three states, averaging approximately 11 generations per year, with an average municipal accumulated degree-days (AMADD) value of about 3751 (
Table 3). The municipality of Corumbá presented the highest PAG, with a potential of thirteen generations annually, whereas the lowest values, nine generations per year, were recorded in Amambai, Ponta Porã, and Sete Quedas. It is worth noting that the municipalities of Aquidauana, Anastácio, Corumbá, Dois Irmãos do Buriti, Ladário, Miranda, and Porto Murtinho have little agronomic relevance in the state.
The PAG of D. melacanthus for Mato Grosso do Sul was estimated using the following values in Equation (11): a = 25.6611; b = 0.4035; c = 0.0836; d = −0.0043, with a determination coefficient (R2) of 0.93. The intercept (a) was relatively high, approximately 26, with the greatest contribution from latitude (b ≈ 0.4) and the lowest from altitude (c ≈ 0.08). Altitude tends to contribute less than the other variables due to the wide variation within the state, ranging from 100 m in the Pantanal lowlands to 1160 m in Maciço do Urucum. No significant difference was found between the PAG calculated using degree-days and the linear multiple regression estimated PAG.
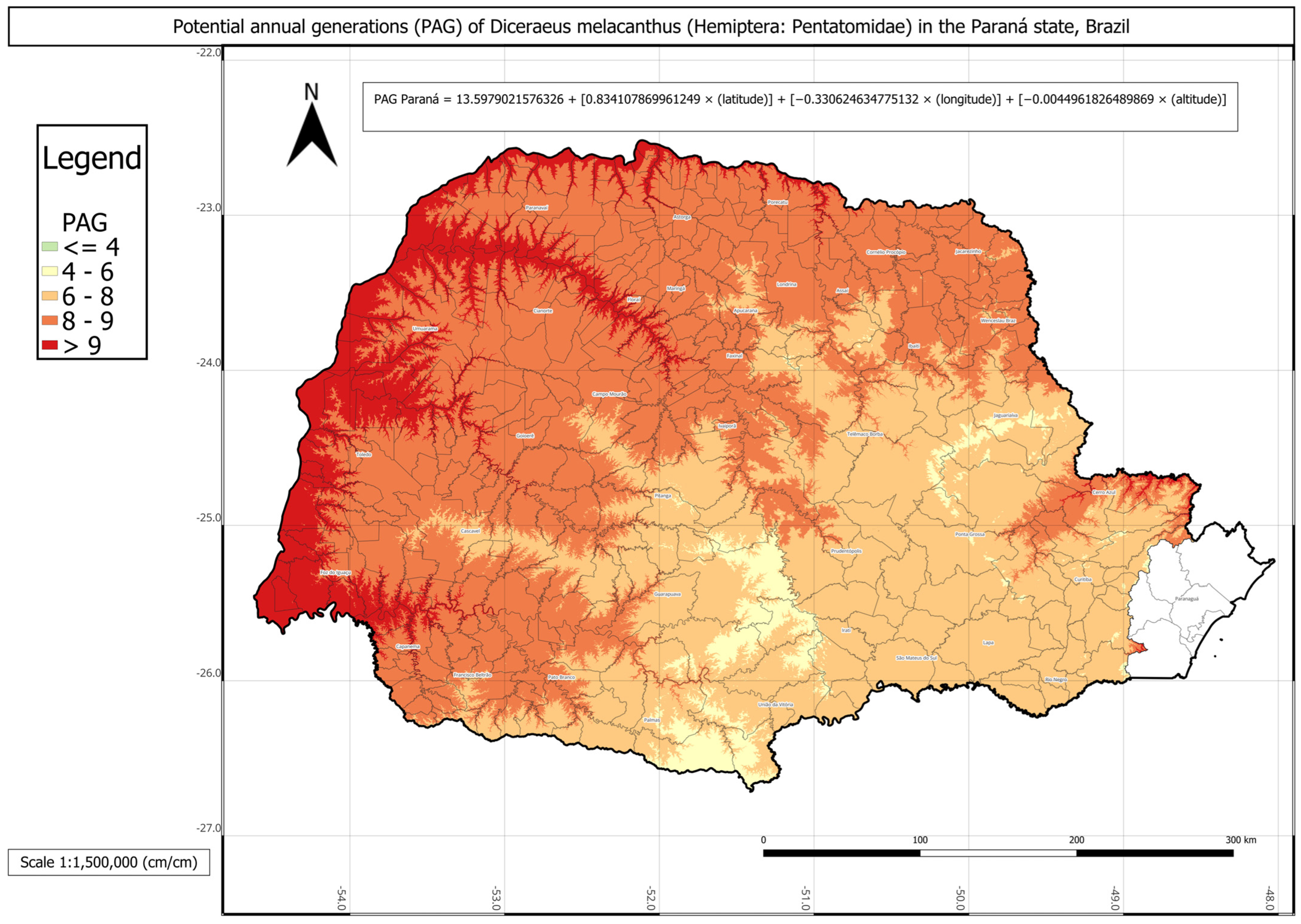
In Paraná state,
D. melacanthus exhibited the lowest mean PAG among the three states, with approximately seven generations per year and an AMADD of about 2482 (
Table 4). The municipality of Paranavaí showed the highest potential, with ten generations annually, whereas the lowest values, four generations per year, were observed in Lapa, Palmas, and Pinhais. It is important to note that the municipalities along the coastal plain of Paraná—Antonina, Guaraqueçaba, Guaratuba, Matinhos, Morretes, Paranaguá, and Pontal do Paraná—are not agriculturally relevant for maize production in the state.
The D. melacanthus PAG in Paraná was obtained using Equation (11) with the following parameters: a = 13.5979; b = 0.8341; c = −0.3306; d = −0.0045 (R2 = 0.93). The intercept (a) was the lowest among the three states, approximately 14. Once again, latitude (b) had the strongest positive contribution (≈0.83), while longitude (c) contributed negatively (≈−0.33). As expected, altitude had the smallest effect, given its wide variation in the state, from sea level in the coastal region to 1877 m at Pico Paraná in Campina Grande do Sul. No significant difference was found between the PAG calculated using degree-days and the linear multiple regression-estimated PAG.
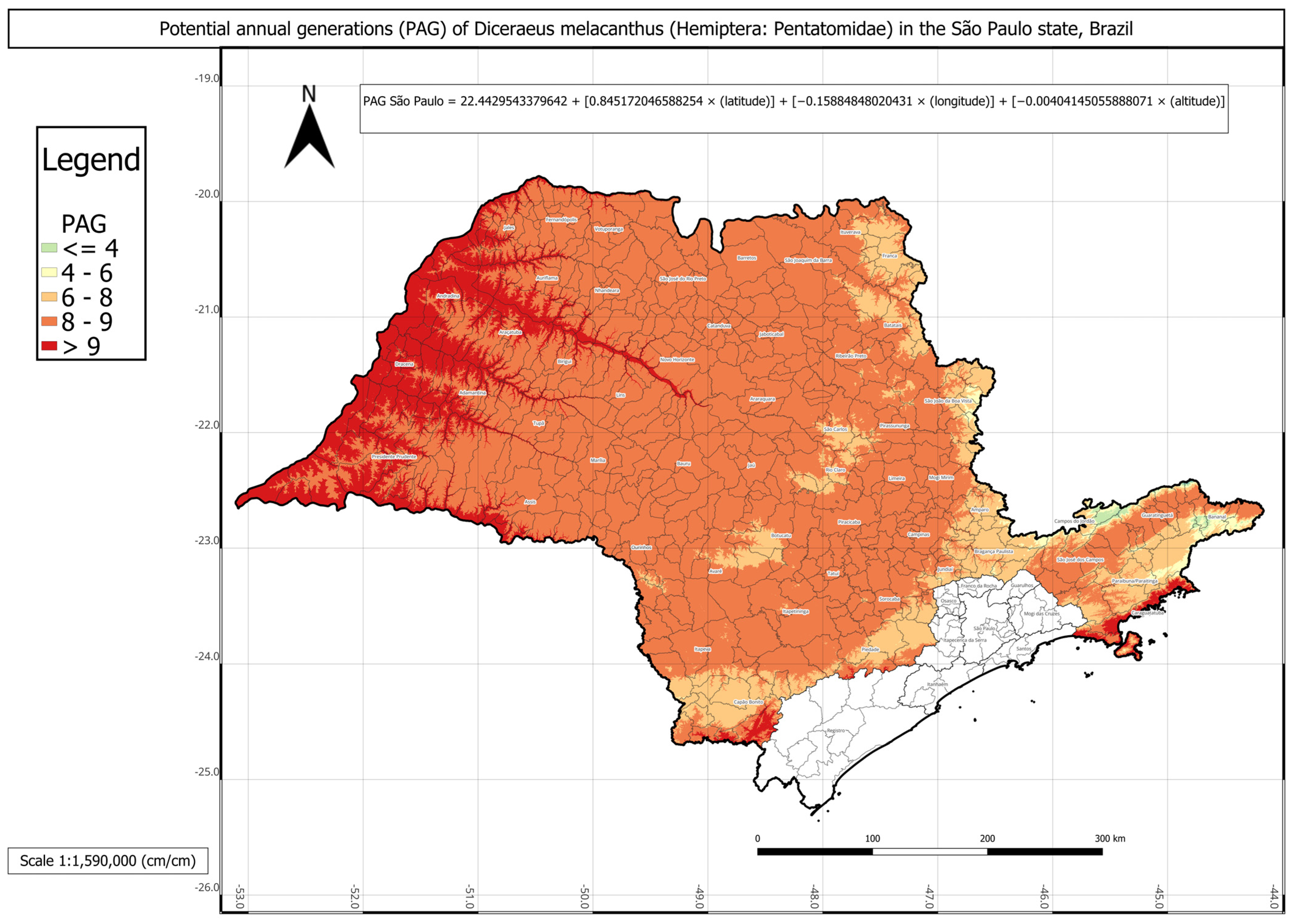
In São Paulo,
D. melacanthus presented an average of approximately nine generations per year, with an AMADD of 2995 (
Table 5). The municipalities of Jales, José Bonifácio, Lins, Presidente Prudente, Valparaíso, and Votuporanga showed the highest PAG values, with up to eleven generations annually, whereas the lowest value, three generations per year, was recorded in Campos do Jordão. It is important to highlight that the mesoregions of the southern seaside and the São Paulo metropolitan region have no significant agricultural relevance in the state.
The PAG of D. melacanthus in São Paulo was estimated using Equation (11) with the following parameters: a = 22.4430; b = 0.8452; c = −0.1589; d = −0.0040 (R2 = 0.90). The intercept (a) represented an intermediate value among the three states, approximately 22. Once again, latitude (b ≈ 0.84) showed the strongest positive effect, whereas longitude (c ≈ −0.15) contributed negatively. Altitude had the smallest effect, as expected, due to its wide range in the state, from sea level along the coast to 2798 m in the Serra da Mantiqueira region. No significant difference was found between the PAG calculated using degree-days and the linear multiple regression-estimated PAG.
The comparative analysis among the three states revealed clear differences in
D. melacanthus PAG (
Table 6 and
Table 7). The data was considered normally distributed in accordance with the Kolmogorov–Smirnov test (α < 0.01) and adequate homoscedasticity (
Table 6). The PAG ANOVA analysis presented significant (F α < 0.01 = 4.91) variations between each state (F = 34.6). The AMADD ANOVA analysis also found significant (F α < 0.01 = 4.91) variations between each state (F = 34.6). Mato Grosso do Sul exhibited the highest values, with a mean of approximately 11 generations and an AMADD of about 3751, both statistically significant (α < 0.01). São Paulo showed intermediate values, averaging 9 generations annually and an AMADD of 2995, while Paraná presented the lowest estimates, with a mean of approximately 7.25 generations and an AMADD of 2473, also significant at α < 0.01. In all models, latitude exerted the strongest positive effect, while altitude contributed the least, reflecting the wide altitudinal ranges within each state. These findings indicate that climatic and topographic gradients strongly influence the potential population growth of
D. melacanthus, with the warmer and lower-altitude regions of Mato Grosso do Sul and western São Paulo offering the most favorable conditions for higher generational turnover, whereas Paraná presents more restrictive environments for population expansion
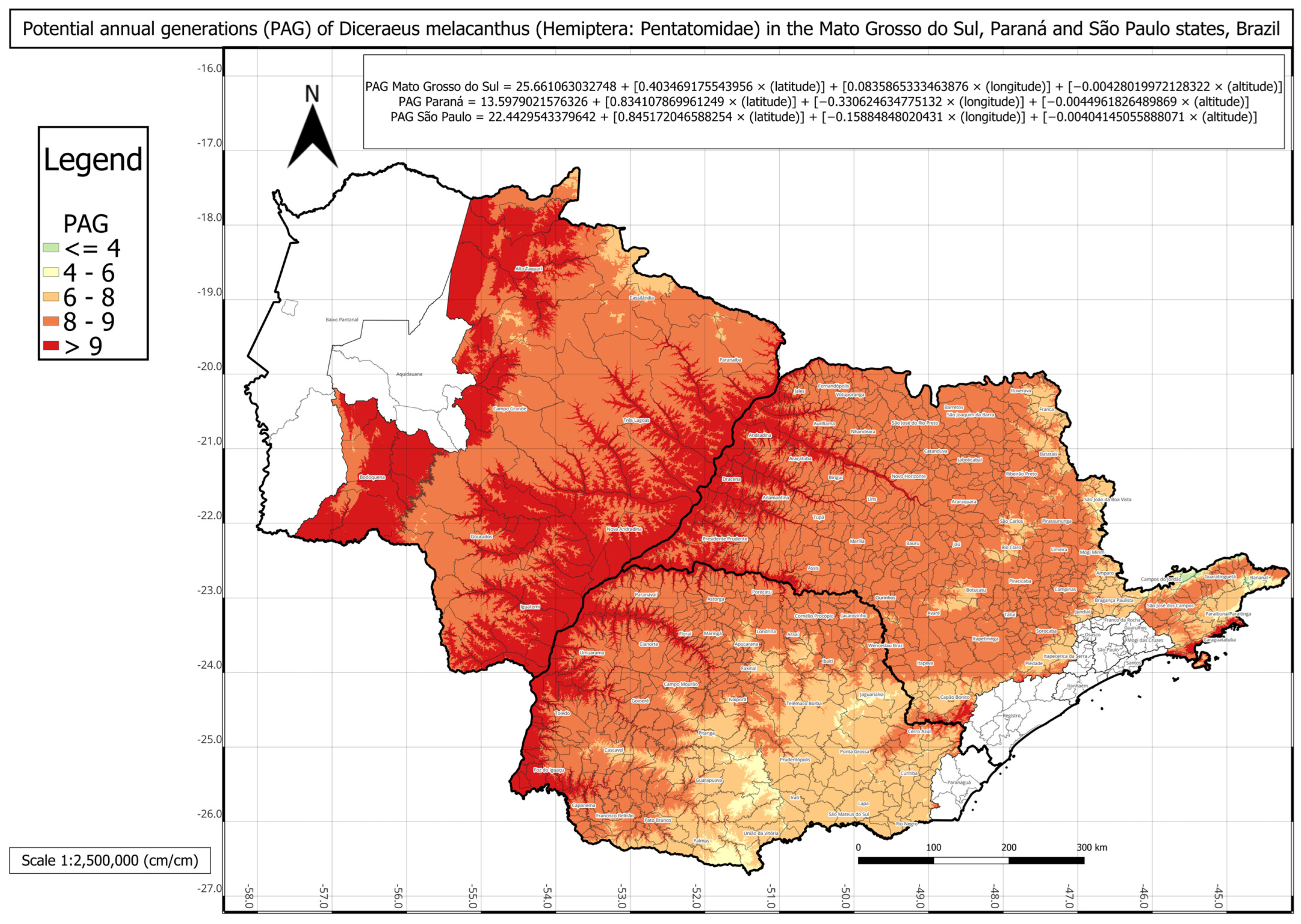
The statistical comparison between PAG values obtained directly from historical climate records and those estimated using the respective multivariate regressions for each state showed no significant differences. The regression-based PAG models were represented in three individual phenology maps for each state and one combined map for all three states (
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
The phenology map analysis revealed distinct spatial patterns of
D. melacanthus PAG across the three states. Mato Grosso do Sul consistently exhibited the highest PAG values, with most municipalities sustaining more than eight generations per year and several exceeding nine, particularly along the borders with Paraná, São Paulo, and Paraguay (
Figure 3). In contrast, Paraná showed the lowest overall PAG values, averaging between four and eight generations in the central, southern, and metropolitan regions, while values above nine were restricted to municipalities bordering Paraguay, Argentina, and northern São Paulo (
Figure 4). São Paulo state presented intermediate conditions, with a PAG above eight in most mesoregions, except in the Vale do Paraíba Paulista, where values fell below four, especially in Bananal and Campos do Jordão (
Figure 5). The highest PAG values in São Paulo were concentrated in the western regions bordering Mato Grosso do Sul and Paraná. These results demonstrate the strong influence of latitude, altitude, and regional climatic gradients on the distribution of
D. melacanthus generational potential, with warmer, lower-altitude regions favoring higher PAG and cooler, elevated areas acting as natural constraints (
Figure 6).
4. Discussion
Degree-days is a useful estimation, calculated through the combination of time and temperature; it ultimately represents the organism development rate during specific phenological stages. The degree-days calculated for the
Diceraeus melacanthus potential annual generations (PAG) was accomplished following the method discussed by De Melo, Tenente, and de Olivera [
20]. This method was chosen because of its high precision, since it considers daily temperature fluctuations and specimen-specific lower and upper temperature thresholds [
20]. It is important to highlight that all these calculations are based on data from insects reared in constant temperatures; although this is not common in nature, it is common in controlled insect rearing conditions, which offer the best conditions for proper development, greater viability, and healthy reproduction [
13]. Food availability, survivability, and biological control were not evaluated during this study.
The results of this study demonstrate that the potential annual generations of
D. melacanthus is strongly influenced by climatic gradients, particularly temperature and altitude, which is consistent with patterns reported for other Pentatomidae species. Previous research has shown that thermal requirements and degree-day models are effective tools for predicting population dynamics of stink bugs in different agroecosystems [
24,
25,
26]. The higher PAG observed in Mato Grosso do Sul and western São Paulo reflects the predominance of warmer climates and lower altitudes, which accelerate developmental rates and shorten generation times. In contrast, the lower PAG in Paraná, especially in cooler and elevated regions, indicates natural climatic restrictions to population growth. These findings highlight the importance of regional climatic conditions in shaping the distribution and potential risk of
D. melacanthus infestations across major agricultural areas. It is important to highlight that the data and maps produced in this study were generated considering
D. melacanthus biological data, and regional meteorological standards, insect survivability, and behavior were not considered. Also, the data and maps represent the PAG values assuming optimal development and survivability conditions. Thus, further research should be conducted examining true
D. melacanthus migration, survivability, and establishment throughout areas with agricultural importance.
Diceraeus melacanthus was first described in Venezuela [
27,
28]. Initially regarded as a secondary pest of soybean in Brazil, it later became recognized as a key pest in the early developmental stages of maize [
5,
29]. The first record of damage caused by this species to maize seedlings in Brazil occurred in 1993 in Rio Brilhante, Mato Grosso do Sul [
30]. Currently, the insect is widely distributed across Brazil, with higher incidence in states of major agricultural importance, including Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Paraná, and São Paulo. The expansion and persistence of its populations have been favored by factors such as the adoption of no-tillage systems in soybean–maize rotations, which enhance survival conditions, and the use of transgenic maize cultivars, either Bt or glyphosate-tolerant, which reduced insecticide applications and hindered control of volunteer maize plants [
1,
31].
Additional factors contributing to the persistence of
D. melacanthus populations include seed and ear losses during harvest and transport, as well as irregular germination, which promote volunteer plants in fields, roadsides, and adjacent areas. These hosts ensure population continuity during both crop and off-season periods. In this context, maize seed treatment has become fundamental to reducing damage in the early stages of germination. Conversely, pre-emergence insecticide applications have shown low efficacy, and post-emergence interventions are recommended only when population levels exceed 0.8 insects per linear meter [
32].
It is important to note that another species of the same genus,
Diceraeus furcatus (F., 1775) (Hemiptera: Pentatomidae), also has agricultural significance. This species occurs more frequently in southern Brazil and Argentina, possibly due to differences in thermal requirements [
33]. Comparative studies on both species are therefore essential to better understand the distribution and reproductive potential of the genus
Diceraeus in South America.
From a comparative perspective, the brown stink bug
Euschistus heros, a major soybean pest, develops optimally at temperatures between 22 and 28 °C, whereas
D. melacanthus exhibits greater adaptability to warmer regions [
13,
18,
24]. Both species share a lower developmental threshold close to 14 °C, but
D. melacanthus maintains higher developmental rates above 28 °C, unlike
E. heros (
Table 2). The brown marmorated stink bug
Halyomorpha halys (Stål, 1855) (Hemiptera: Pentatomidae), in turn, requires 537.6 degree-days to complete its life cycle, a value significantly higher than that of
D. melacanthus, reflecting interspecific differences in thermal requirements [
34]. The upper base organism development temperature (Tup) represents the temperature threshold at which the specimen still develops healthy and may reproduce; temperatures above this threshold may result in maldevelopment and infertile adults [
13].
The findings of the present study may serve as a basis for risk assessment of maize damage, particularly in high-reproductive-potential regions, as border areas between the three states showed the greatest PAG values (
Figure 6). Additional information on the phenology of
D. melacanthus may support the identification of critical cultivation periods and guide decision-making, similar to initiatives such as the National Phenology Network [
19]. According to Tonnang et al. (2015), insect phenological mapping also requires knowledge of diet and alternative host plants [
35]. For
D. melacanthus, alternative hosts include soybean, wheat, and certain weeds such as
Commelina benghalensis and
Crotalaria lanceolata [
6,
36].
Finally, it is noteworthy that the three states analyzed—Mato Grosso do Sul (central-west), São Paulo (southeast), and Paraná (south)—not only represent major agricultural regions but also encompass nationally relevant biomes: Cerrado, Floresta atlântica, and Pantanal, which account for approximately 23%, 13%, and 2% of Brazil’s territory, respectively. These states also represent four distinct Köppen climate types: Mato Grosso do Sul is predominantly tropical wet with rainy summers and dry winters (Aw); Paraná lies in a transition between humid subtropical with hot summers (Cfa) and temperate humid with mild summers (Cfb); and São Paulo is mainly humid subtropical with dry winters and rainy summers (Cwa) [
9]. The ability of
D. melacanthus to adapt across such diverse climatic and ecological zones underscores the need for further studies to assess its potential expansion not only to other Brazilian regions but also to neighboring South American countries such as Argentina, Bolivia, Colombia, Paraguay, and Venezuela.
This study determined the lower and upper developmental thresholds, thermal constant, and degree-day requirements of Diceraeus melacanthus and used these parameters to estimate the potential annual generations across the Mato Grosso do Sul, Paraná, and São Paulo states in Brazil. The results demonstrated clear regional differences, with higher generational potential in the warmer, low-altitude areas of Mato Grosso do Sul and western São Paulo, and lower values in cooler, elevated regions of Paraná. The adaptability of D. melacanthus to diverse climatic conditions highlights its importance as a key pest in maize and reinforces the need for integrated management strategies. These findings provide a valuable basis for predicting population risk and guiding control decisions, while also emphasizing the necessity of further studies on insect pest distribution, host range, and phenology across agriculturally important territories.