Three New Species of Aphelinus (Hymenoptera: Aphelinidae) from China, with a Note on the japonicus Group †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Parasitoids
2.2. Photograph and Slides of Parasitoids
2.3. DNA Sequencing
2.4. Phylogenetic Reconstruction
2.5. Terminology, Morphological Measurement and Abbreviations
3. Results
3.1. Species Accounts
3.1.1. Aphelinus jinshanensis Wang & Huang, sp.n. (Figure 1)
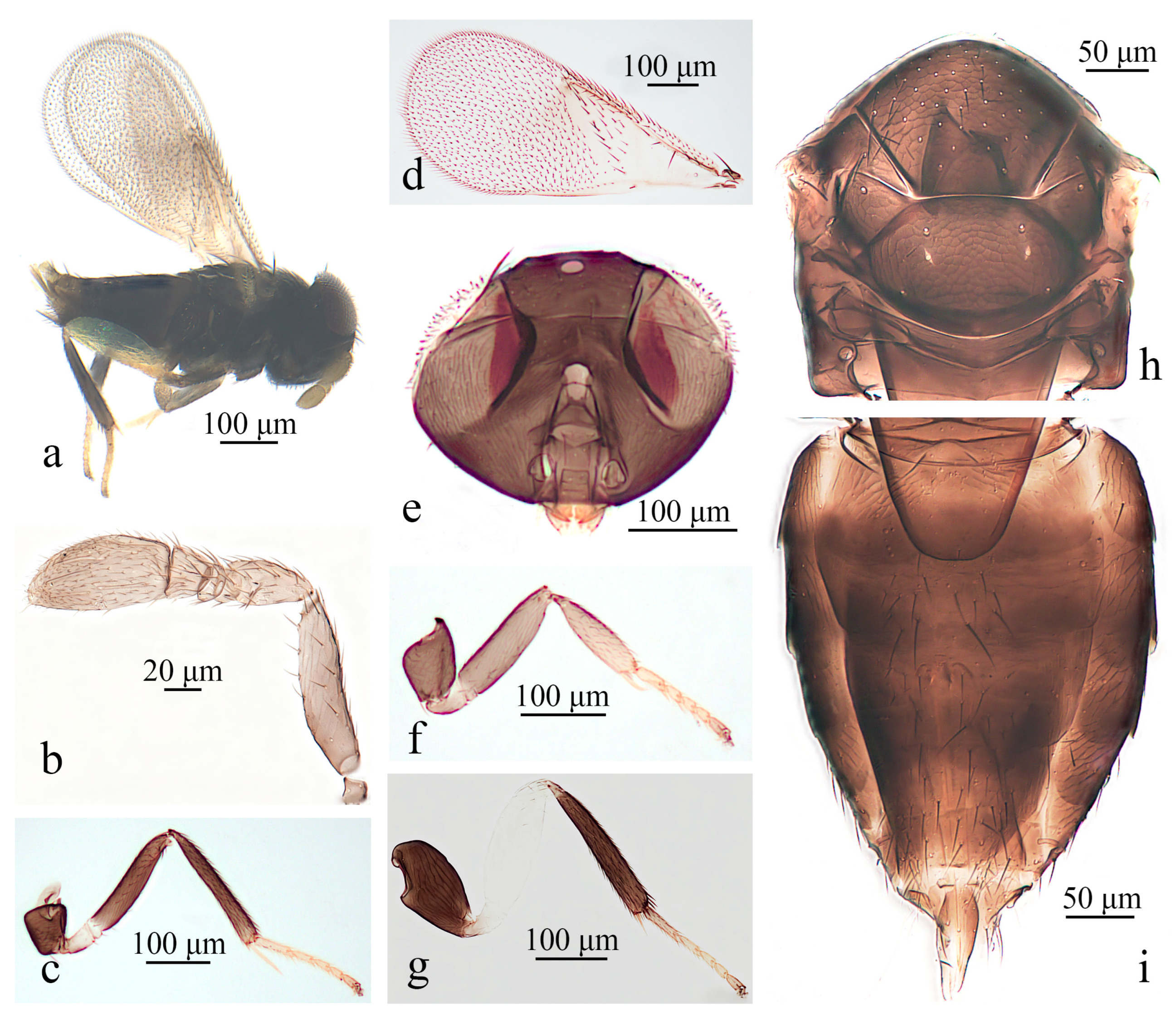
3.1.2. Aphelinus albimaculatus Wang & Huang, sp.n. (Figure 2)

3.2. The Species of the japonicus Group
- Pronotum, mesonotum and most of axillae yellow; scutellum, metanotum and propodeum dark brown to black. digitus of the genitalia with two claspers of the male........................................................................................A. japonicus
- Pronotum, mesonotum, axillae, scutellum, metanotum and propodeum black. digitus of the genitalia with three claspers of the male....................................................................................A.varius, sp.n.
3.2.1. Aphelinus japonicus Ashmead [20,21,29] (Figure 3 and Figure 4)

- Note. Aphelinus japonicus Ashmead, a little known species collected from Japan, was redescribed by Hayat (1991) [20]. Hayat (1991) pointed out that A. japonicus differs from all the known species of the genus by the partly dark color of the thorax, the numerous setae on mid-lobe not arranged in symmetry, the short ovipositor, and the hypopygium not reaching the apex of the gaster, and regarded A. japonicus as a species of a separate group, the japonicus group, not placed in the three subgenera recognized by Hayat (1990) [19]. The male of A. japonicus was described by Hayat (1994) [21] as having a 3-segmented flagellum and clearly different from all other described species of Aphelinus which have a 4-segmented flagellum in both males and females. In China, A. japonicus was first recorded by Chen & Li (2016) [30] from the female, and this is the second report of the female.

3.2.2. Aphelinus varius Wang & Huang, sp.n. (Figure 5 and Figure 6)


3.3. Phylogenetic Analysis
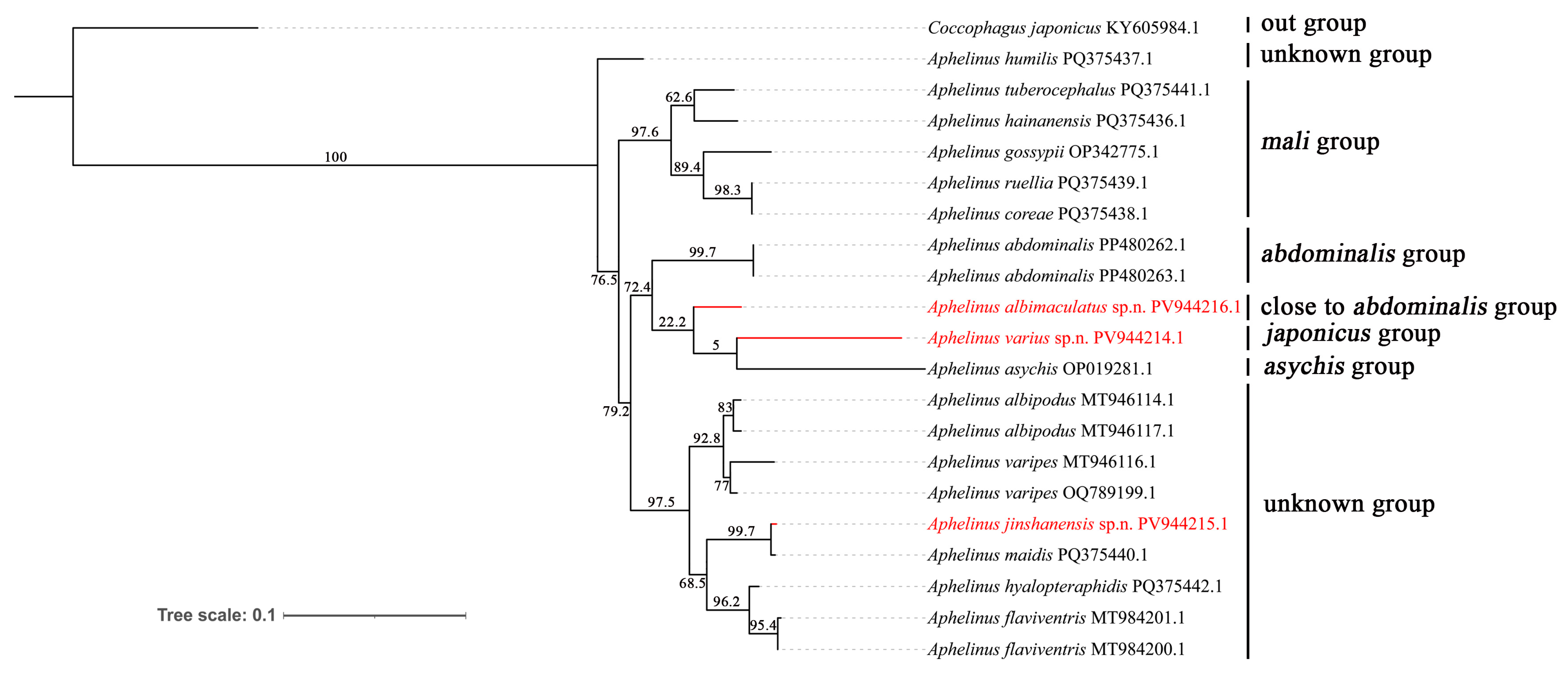
4. Discussions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrer-Suay, M.; Barreda, M.; Rakhshani, E.; Rodrigo, E.; Selfa, J.; Polaszek, A. A review of aphid parasitoids, with an identification key to the genera of economic importance. Insects 2025, 16, 648. [Google Scholar] [CrossRef]
- Huang, J. Systematic studies on Aphelinidae of China (Hymenoptera: Chalcidoidea); Chongqing Publishing House: Chongqing, China, 1994; pp. 1–348. [Google Scholar]
- Long, C.D.; Wang, Y.P.; Tang, P.Z. Study on biological characteristics and utilization of Aphelinus mali. Acta Entomol. Sin. 1960, 1, 1–39. [Google Scholar] [CrossRef]
- Bangels, E.; Alhmedi, A.; Akkermans, W.; Bylemans, D.; Belien, T. Towards a knowledge-based decision support system forintegrated control of woolly apple aphid, Eriosoma lanigerum, with maximal biological suppression by the parasitoid Aphelinus mali. Insects 2021, 12, 479. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Roskam, M.M.; Timmer, R. Commercial mass production and pricing of organisms for biological control of pests in Europe. Biol. Control 1997, 10, 143–149. [Google Scholar] [CrossRef]
- Strong, K.L. Electrophoretic analysis of two strains of Aphelinus varipes (Hymenoptera: Aphelinidae). J. Aust. Entomol. Soc. 1993, 32, 21–22. [Google Scholar] [CrossRef]
- Yashima, K.; Murai, T. Development and reproduction of a potential biological control agent, Aphelinus varipes (Hymenoptera: Aphelinidae), at different temperatures. Appl. Entomol. Zool. 2013, 48, 21–26. [Google Scholar] [CrossRef]
- Hayat, M. Aphelinidae of India (Hymenoptera: Chalcidoidea): A Taxonomic Revision; Associated Publishers: Gainesville, FL, USA, 1998; pp. 1–416. [Google Scholar]
- Noyes, J.S.; Universal Chalcidoidea Database. World Wide Web Electronic Publication. 2019. Available online: https://ucd.chalcid.org (accessed on 30 October 2025).
- Liao, D.X.; Li, X.L.; Pang, X.F.; Chen, T.L. Economic Insect Fauna of China 34: Hymenoptera Chalcidoidea (1); Science Press: Beijing, China, 1987; pp. 1–241. [Google Scholar]
- Pan, M.X. A new species of Aphelinus Dalman from Zhejiang Province, China (Hymenoptera: Aphelinidae). Acta Zoot. Sin. 1992, 17, 75–77. [Google Scholar]
- Li, C.D.; Langor, D.W. A new species of Aphelinus (Hymenoptera: Aphelinidae) from northeastern China. Can. Entomol. 1998, 130, 799–801. [Google Scholar] [CrossRef]
- Li, C.D.; Zhao, S.L. A new species of Aphelinus Dalman (Hymenoptera: Aphelinidae) from Northeastern China. Entomotaxonomia 1998, 20, 150–152. [Google Scholar]
- Li, C.D.; Zhang, S. A new species and a new record of Aphelinus Dalman (Hymenoptera: Aphelinidae) from China. Entomotaxonomia 2005, 27, 69–73. [Google Scholar]
- Geng, S.Y.; Li, C.D. A new record species of Aphelinus Dalman (Hymenoptera: Aphelinidae) from China. J. Northeast. For. Univ. 2011, 39, 117–118. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.D. Three new species and new distributional data for five rare species of Aphelinus (Hymenoptera: Aphelinidae) from China. Zootaxa 2016, 4092, 258–272. [Google Scholar] [CrossRef]
- Shih, Y.T.; Ko, C.C. Fauna Taiwan: Insecta Hymenoptera Aphelinidae; National Taiwan University: Taipei, China, 2020; pp. 1–679. [Google Scholar]
- Dong, Z.G.; Luo, Y.; Ge, J.Q.; Huang, J.; Polaszek, A.; Wang, Z.H. The species of the mali-group of Aphelinus (Hymenoptera: Aphelinidae), with descriptions of three new species, DNA sequence data and one newly-recorded species from China. Insects 2024, 15, 945. [Google Scholar] [CrossRef]
- Hayat, M. Taxonomic studies on Aphelinus (Hymenoptera: Aphelinidae). 2. A new subgenus from India with comments on Mesidia and Mesidiopsis. Orient. Insects 1990, 24, 253–257. [Google Scholar] [CrossRef]
- Hayat, M. Taxonomic studies on Aphelinus (Hymenoptera: Aphelinidae) III. Notes on A. japonicus Ashmead and A. howardii Ashmead. Entomon 1991, 16, 179–181. [Google Scholar]
- Hayat, M. Taxonomic studies on Aphelinus (Homoptera: Aphelinidae): 7. A new species from Nepal and records of three known species. Entomon 1994, 19, 85–89. [Google Scholar]
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Polaszek, A.; Ayshford, T.; Yahya, B.E.; Fusu, L. Wallaceaphytis: An unusual new genus of parasitoid wasp (Hymenoptera: Aphelinidae) from Borneo. J. Nat. Hist. 2013, 48, 1111–1123. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 35, 294–299. [Google Scholar]
- Xiang, C.Y.; Gao, F.L.; Jakovlic, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree–based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ashmead, W.H. Descriptions of new Hymenoptera from Japan. II. J. N. Y. Entomol. Soc. 1904, 12, 146–165. Available online: https://www.jstor.org/stable/25003087 (accessed on 20 September 2025).
- Chen, Y.; Li, C.D. Tow new record species of Aphelinus Dalman (Hymenoptera: Aphelinidae) from China. J. Northeast. For. Univ. 2016, 44, 100–103. [Google Scholar] [CrossRef]
- Hopper, K.R.; Woolley, J.B.; Hoelmer, K.; Wu, K.M.; Qiao, G.X.; Lee, S.H. An identification key to species in the mali complex of Aphelinus (Hymenoptera, Chalcidoidea) with descriptions of three new species. J. Hym. Res. 2012, 26, 73–96. [Google Scholar] [CrossRef]
| Primer | Sequence | Cycling Conditions | |||
|---|---|---|---|---|---|
| LCO1490 | 5′-GGTCAACAAAATCATAAAGATATTGG-3′ | Denaturation | Annealing | Extension | Cycles |
| HCO2198 | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | 94 °C (30 s) | 47 °C (30 s) | 72 °C (1 min) | 35 |
| Family/Species | GenBank Accession No. | Source |
|---|---|---|
| Aphelinidae | ||
| Aphelinus abdominalis | PP480262.1 | GenBank |
| A. abdominalis | PP480263.1 | GenBank |
| A. asychis | OP019281.1 | GenBank |
| A. gossypii | OP342775.1 | GenBank |
| A. varipes | OQ789199.1 | GenBank |
| A. varipes | MT946116.1 | GenBank |
| A. coreae | PQ375438.1 | [18] |
| A. hainanensis | PQ375436.1 | [18] |
| A. humilis | PQ375437.1 | [18] |
| A. hyalopteraphidis | PQ375442.1 | [18] |
| A. maidis | PQ375440.1 | [18] |
| A. ruellia | PQ375439.1 | [18] |
| A. tuberocephalus | PQ375441.1 | [18] |
| A. albipodus | MT946114.1 | GenBank |
| A. albipodus | MT946117.1 | GenBank |
| A. flaviventris | MT984200.1 | GenBank |
| A. flaviventris | MT984201.1 | GenBank |
| A. varius, sp.n. | PV944214.1 | Present manuscript |
| A. jinshanensis, sp.n. | PV944215.1 | Present manuscript |
| A. albimaculatus, sp.n. | PV944216.1 | Present manuscript |
| Coccophagus japonicus | KY605984.1 | GenBank |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Z.; Ge, J.; Huang, J.; Wang, Z. Three New Species of Aphelinus (Hymenoptera: Aphelinidae) from China, with a Note on the japonicus Group. Insects 2025, 16, 1205. https://doi.org/10.3390/insects16121205
Dong Z, Ge J, Huang J, Wang Z. Three New Species of Aphelinus (Hymenoptera: Aphelinidae) from China, with a Note on the japonicus Group. Insects. 2025; 16(12):1205. https://doi.org/10.3390/insects16121205
Chicago/Turabian StyleDong, Zhigang, Junqing Ge, Jian Huang, and Zhuhong Wang. 2025. "Three New Species of Aphelinus (Hymenoptera: Aphelinidae) from China, with a Note on the japonicus Group" Insects 16, no. 12: 1205. https://doi.org/10.3390/insects16121205
APA StyleDong, Z., Ge, J., Huang, J., & Wang, Z. (2025). Three New Species of Aphelinus (Hymenoptera: Aphelinidae) from China, with a Note on the japonicus Group. Insects, 16(12), 1205. https://doi.org/10.3390/insects16121205






