Monitoring the Capacity of Microsporidia MB Transgenerational Spread in Anopheles arabiensis Populations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Collection and Rearing
2.2. Microsporidia MB-Infected Anopheles arabiensis Mosquito Populations
2.3. Deoxyribonucleic Acid (DNA) Extraction and Molecular Species Identification
2.4. Microsporidia MB Screening and Intensity Determination
2.5. Data Analysis
3. Results
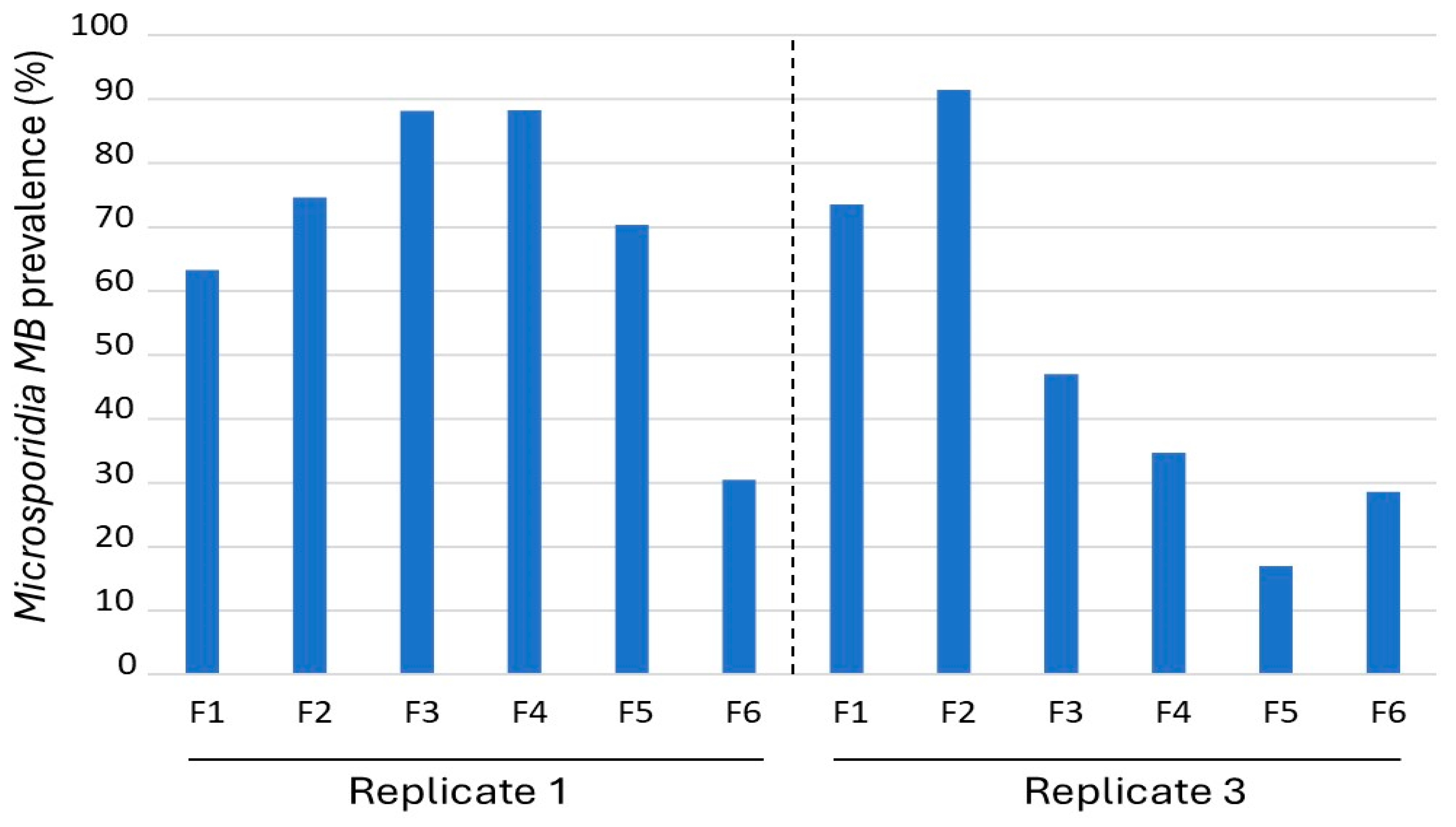
3.1. Overview of Microsporidia MB Mosquito Generations
3.2. Microsporidia MB Prevalence Across Generations and Impact of Temperature and Humidity
3.3. Microsporidia MB Intensity Across Generations and Sex
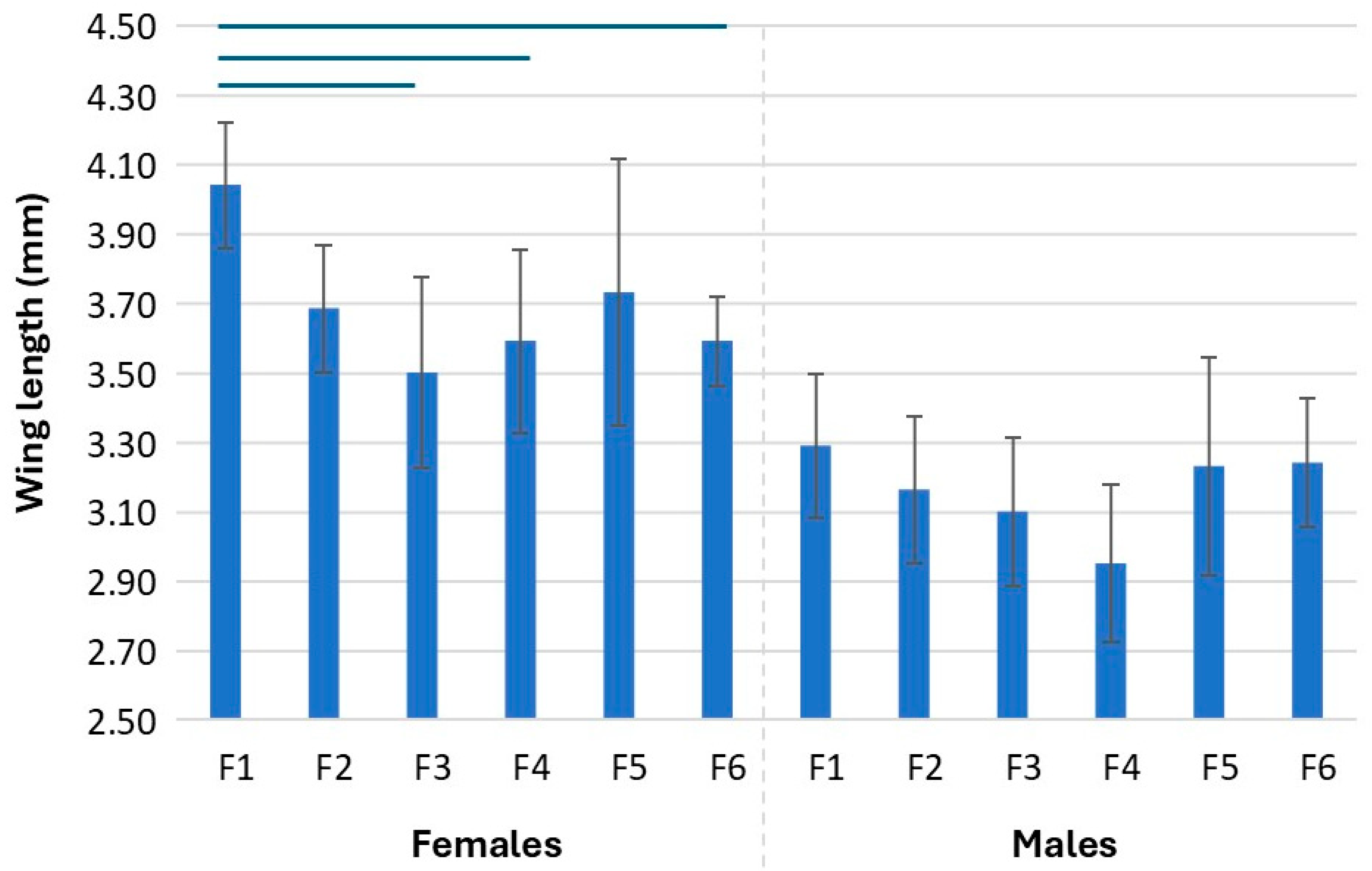
3.4. Wing Size of Mosquitoes Across Generations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bukhari, T.; Pevsner, R.; Herren, J.K. Microsporidia: A promising vector control tool for residual malaria transmission. Front. Trop. Dis. 2022, 3, 957109. [Google Scholar] [CrossRef]
- Herren, J.K.; Mbaisi, L.; Mararo, E.; Makhulu, E.E.; Mobegi, V.A.; Butungi, H.; Mancini, M.V.; Oundo, J.W.; Teal, E.T.; Pinaud, S.; et al. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat. Commun. 2020, 11, 2187. [Google Scholar] [CrossRef]
- Maina, T.; Shisia, A.; Gichuhi, J.; Bargul, J.L.; Herren, J.K.; Bukhari, T. Maximizing horizontal transmission through mating: Increased mating frequency and mating competitiveness associated with Microsporidia MB-infected Anopheles arabiensis males. Malar. J. 2025, 24, 114. [Google Scholar] [CrossRef]
- Nattoh, G.; Onyango, B.; Makhulu, E.E.; Omoke, D.; Ang’ang’o, L.M.; Kamau, L.; Gesuge, M.M.; Ochomo, E.; Herren, J.K. Microsporidia MB in the primary malaria vector Anopheles gambiae sensu stricto is avirulent and undergoes maternal and horizontal transmission. Parasites Vectors 2023, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Nattoh, G.; Maina, T.; Makhulu, E.E.; Mbaisi, L.; Mararo, E.; Otieno, F.G.; Bukhari, T.; Onchuru, T.O.; Teal, E.; Paredes, J.; et al. Horizontal Transmission of the Symbiont Microsporidia MB in Anopheles arabiensis. Front. Microbiol. 2021, 12, 647183. [Google Scholar] [CrossRef]
- Akorli, J.; Akorli, E.A.; Tetteh, S.N.; Amlalo, G.K.; Opoku, M.; Pwalia, R.; Adimazoya, M.; Atibilla, D.; Pi-Bansa, S.; Chabi, J.; et al. Microsporidia MB is found predominantly associated with Anopheles gambiae s.s. and Anopheles coluzzii in Ghana. Sci. Rep. 2021, 11, 18658. [Google Scholar] [CrossRef] [PubMed]
- Moustapha, L.M.; Sadou, I.M.; Arzika, I.I.; Maman, L.I.; Gomgnimbou, M.K.; Konkobo, M.; Diabate, A.; Bilgo, E. First identification of Microsporidia MB in Anopheles coluzzii from Zinder City, Niger. Parasites Vectors 2024, 17, 39. [Google Scholar] [CrossRef]
- Moustapha, L.M.; Mukhtar, M.M.; Sanda, A.N.; Adamu, S.; Aliyu, Y.Y.; Einoi, H.K.; Maigari, M.U.; Okeke, P.C.; Nwele, D.E.; Obembe, A.; et al. Spatial Distribution of Microsporidia MB Along Clinal Gradient and the Impact of Its Infection on Pyrethroid Resistance in Anopheles gambiae s.l. Mosquitoes from Nigeria and Niger Republic. Parasitologia 2025, 5, 31. [Google Scholar] [CrossRef]
- Tchigossou, G.; Lontsi-Demano, M.; Tossou, E.; Sovegnon, P.M.; Akoton, R.; Adanzounon, D.; Dossou, C.; Koto, M.; Ogbon, A.; Gouété, M.; et al. Seasonal variation of Microsporidia MB infection in Anopheles gambiae and Anopheles coluzzii in two different geographical localities in Benin. Malar. J. 2025, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- El Jarkass, H.T.; Reinke, A.W. The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cell. Microbiol. 2020, 22, e13247. [Google Scholar] [CrossRef]
- Texier, C.; Vidau, C.; Viguès, B.; El Alaoui, H.; Delbac, F. Microsporidia: A model for minimal parasite-host interactions. Curr. Opin. Microbiol. 2010, 13, 443–449. [Google Scholar] [CrossRef]
- Vávra, J.; Lukeš, J. Microsporidia and ‘The Art of Living Together’. Adv. Parasitol. 2013, 82, 253–319. [Google Scholar]
- Puerta-Guardo, H.; Contreras-Perera, Y.; Perez-Carrillo, S.; Che-Mendoza, A.; Ayora-Talavera, G.; Vazquez-Prokopec, G.; Martin-Park, A.; Zhang, D.; Manrique-Saide, P.; UCBE-LCB Team. Wolbachia in native populations of Aedes albopictus (Diptera: Culicidae) from Yucatan Peninsula, Mexico. J. Insect Sci. 2020, 20, 16. [Google Scholar] [CrossRef]
- Vinayagam, S.; Nirmolia, T.; Chetry, S.; Kumar, N.P.; Saini, P.; Bhattacharyya, D.; Bhowmick, I.P.; Sattu, K.; Patgiri, S.J. Molecular Evidence of Wolbachia Species in Wild-Caught Aedes albopictus and Aedes aegypti Mosquitoes in Four States of Northeast India. J. Trop. Med. 2023, 1, 6678627. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Rath, A.; Pradhan, N.; Panda, B.B.; Mohapatra, P.K.; Hazra, R.K. Prevalence and transmission potential of Wolbachia in Aedes albopictus population circulating in endemic coastal districts of Odisha, India. J. Vector Borne Dis. 2021, 58, 297–305. [Google Scholar] [CrossRef]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; De Bruyne, J.T.; Kien, D.H.; Hoang, N.L.; Chau, N.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef] [PubMed]
- Bayoh, M.N.; Lindsay, S.W. Effect of temperature on the development of the aquatic stages of Anopheles gambiae sensu stricto (Diptera: Culicidae). Bull. Entomol. Res. 2003, 93, 375–381. [Google Scholar] [CrossRef]
- Mouton, L.; Henri, H.; Bouletreau, M.; Vavre, F. Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 2006, 132, 49–56. [Google Scholar] [CrossRef]
- Moghadam, N.N.; Thorshauge, P.M.; Kristensen, T.N.; de Jonge, N.; Bahrndorff, S.; Kjeldal, H.; Nielsen, J.L. Strong responses of Drosophila melanogaster microbiota to developmental temperature. Fly 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Doremus, M.R.; Kelly, S.E.; Hunter, M.S. Exposure to opposing temperature extremes causes comparable effects on Cardinium density but contrasting effects on Cardinium induced cytoplasmic incompatibility. PLoS Pathog. 2019, 15, e1008022. [Google Scholar] [CrossRef]
- Boanyah, G.Y.; Koekemoer, L.L.; Herren, J.K.; Bukhari, T. Effect of Microsporidia MB infection on the development and fitness of Anopheles arabiensis under different diet regimes. Parasites Vectors 2024, 17, 294. [Google Scholar] [CrossRef]
- Carvajal-Lago, L.; Ruiz-López, M.J.; Figuerola, J.; Martínez-de la Puente, J. Implications of diet on mosquito life history traits and pathogen transmission. Environ. Res. 2021, 195, 110893. [Google Scholar] [CrossRef]
- Jiggins, F.M. The spread of Wolbachia through mosquito populations. PLOS Biol. 2017, 15, e2002780. [Google Scholar] [CrossRef]
- Wiwatanaratanabutr, I.; Kittayapong, P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr. Pathol. 2009, 102, 220–224. [Google Scholar] [CrossRef]
- Otieno, F.G.; Barreaux, P.; Belvinos, A.S.; Makhulu, E.E.; Onchuru, T.O.; Wairimu, A.W.; Omboye, S.M.; King’ori, C.N.; Sokame, B.M.; Nyamache, A.; et al. The dissemination potential of Microsporidia MB in Anopheles arabiensis mosquitoes is modulated by temperature. Sci. Rep. 2025, 15, 28839. [Google Scholar] [CrossRef]
- Degefa, T.; Yewhalaw, D.; Zhou, G.; Lee, M.C.; Atieli, H.; Githeko, A.K.; Yan, G. Indoor and outdoor malaria vector surveillance in western Kenya: Implications for better understanding of residual transmission. Malar. J. 2017, 16, 443. [Google Scholar] [CrossRef]
- Nepomichene, T.N.; Andrianaivolambo, L.; Boyer, S.; Bourgouin, C. Efficient method for establishing F1 progeny from wild populations of Anopheles mosquitoes. Malar. J. 2017, 16, 21. [Google Scholar] [CrossRef]
- Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J. 2020, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; della Torre, A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Onchuru, T.O.; Makhulu, E.E.; Ronnie, P.C.; Mandere, S.; Otieno, F.G.; Gichuhi, J.; Herren, J.K. The Plasmodium transmission-blocking symbiont, Microsporidia MB, is vertically transmitted through Anopheles arabiensis germline stem cells. PLoS Pathog. 2024, 20, e1012340. [Google Scholar] [CrossRef]
- Makhulu, E.E.; Onchuru, T.O.; Gichuhi, J.; Otieno, F.G.; Wairimu, A.W.; Muthoni, J.N.; Koekemoer, L.; Herren, J.K. Localization and tissue tropism of the symbiont Microsporidia MB in the germ line and somatic tissues of Anopheles arabiensis. MBio 2024, 15, e0219223. [Google Scholar] [CrossRef]
- Tokash-Peters, A.G.; Jabon, J.D.; Fung, M.E.; Peters, J.A.; Lopez, S.G.; Woodhams, D.C. Trans-Generational Symbiont Transmission Reduced at High Temperatures in a West Nile Virus Vector Mosquito Culex quinquefasciatus. Front. Trop. Dis. 2022, 3, 762132. [Google Scholar] [CrossRef]
- Corbin, C.; Heyworth, E.R.; Ferrari, J.; Hurst, G.D. Heritable symbionts in a world of varying temperature. Heredity 2017, 118, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ameneshewa, B.; Service, M.W. The relationship between female body size and survival rate of the malaria vector Anopheles arabiensis in Ethiopia. Med. Vet. Entomol. 1996, 10, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Cator, L.J.; Zanti, Z. Size, sounds and sex: Interactions between body size and harmonic convergence signals determine mating success in Aedes aegypti. Parasites Vectors 2016, 9, 622. [Google Scholar] [CrossRef]
- Helinski, M.E.H.; Knols, B.G.J. Mating Competitiveness of Male Anopheles arabiensis Mosquitoes Irradiated with a Partially or Fully Sterilizing Dose in Small and Large Laboratory Cages. J. Med. Entomol. 2008, 45, 698–705. [Google Scholar] [CrossRef]
- Sawadogo, S.P.; Diabaté, A.; Toé, H.K.; Sanon, A.; Lefevre, T.; Baldet, T.; Gilles, J.; Simard, F.; Gibson, G.; Sinkins, S.; et al. Effects of age and size on Anopheles gambiae s.s. male mosquito mating success. J. Med. Entomol. 2013, 50, 285–293. [Google Scholar] [CrossRef]
- Mackay, A.J.; Yan, J.; Kim, C.H.; Barreaux, A.M.; Stone, C.M. Larval diet and temperature alter mosquito immunity and development: Using body size and developmental traits to track carry-over effects on longevity. Parasites Vectors 2023, 16, 434. [Google Scholar] [CrossRef]




| Replicate 1 | Replicate 3 | |||||
|---|---|---|---|---|---|---|
| Generation (F) | Female | Male | Total | Female | Male | Total |
| 1 * | 50 | 10 | 60 | 84 | 98 | 182 |
| 2 | 84 | 106 | 190 | 81 | 72 | 153 |
| 3 | 153 | 178 | 331 | 162 | 119 | 281 |
| 4 | 61 | 55 | 116 | 41 | 31 | 72 |
| 5 | 99 | 101 | 200 | 27 | 26 | 53 |
| 6 | 296 | 278 | 574 | 3 ** | 5 ** | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boanyah, G.Y.; Koekemoer, L.L.; Herren, J.K.; Bukhari, T. Monitoring the Capacity of Microsporidia MB Transgenerational Spread in Anopheles arabiensis Populations. Insects 2025, 16, 1206. https://doi.org/10.3390/insects16121206
Boanyah GY, Koekemoer LL, Herren JK, Bukhari T. Monitoring the Capacity of Microsporidia MB Transgenerational Spread in Anopheles arabiensis Populations. Insects. 2025; 16(12):1206. https://doi.org/10.3390/insects16121206
Chicago/Turabian StyleBoanyah, Godfred Yaw, Lizette L. Koekemoer, Jeremy K. Herren, and Tullu Bukhari. 2025. "Monitoring the Capacity of Microsporidia MB Transgenerational Spread in Anopheles arabiensis Populations" Insects 16, no. 12: 1206. https://doi.org/10.3390/insects16121206
APA StyleBoanyah, G. Y., Koekemoer, L. L., Herren, J. K., & Bukhari, T. (2025). Monitoring the Capacity of Microsporidia MB Transgenerational Spread in Anopheles arabiensis Populations. Insects, 16(12), 1206. https://doi.org/10.3390/insects16121206







