Eastern Arc of Glacial Relict Species—Population Genetics of Violet Copper Lycaena helle Butterfly in East-Central Europe
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
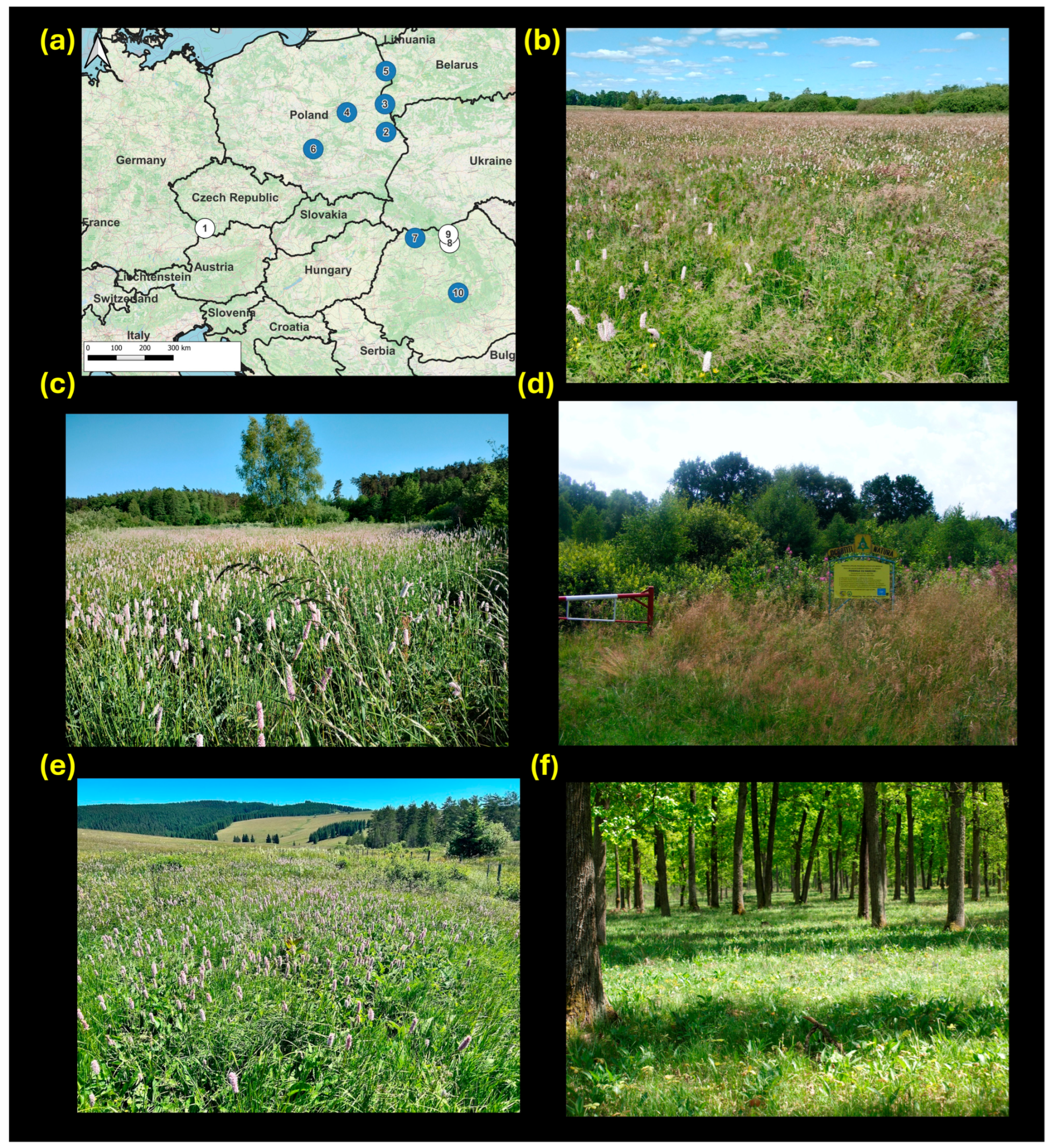
2.1. Study Species
2.2. Sampling and DNA Analysis
3. Results
4. Discussion
4.1. Within-Population Patterns
4.2. Conservation Biogeography in East-Central Europe
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Locality | Sample ID | Lhe03 | Lhe14 | LheB06 | LheE12 | LheF12 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Šumava | HEL076 | 262 | 270 | 259 | 259 | 0 | 0 | 446 | 446 | 398 | 402 |
| HEL077 | 262 | 262 | 0 | 0 | 0 | 0 | 446 | 446 | 402 | 422 | |
| HEL078 | 262 | 262 | 257 | 257 | 0 | 0 | 441 | 445 | 400 | 422 | |
| HEL079 | 262 | 270 | 0 | 0 | 0 | 0 | 445 | 445 | 400 | 422 | |
| HEL080 | 262 | 270 | 257 | 257 | 0 | 0 | 445 | 449 | 400 | 400 | |
| HEL081 | 262 | 262 | 0 | 0 | 0 | 0 | 446 | 449 | 400 | 400 | |
| HEL082 | 262 | 270 | 259 | 259 | 0 | 0 | 441 | 449 | 422 | 422 | |
| HEL083 | 262 | 270 | 0 | 0 | 0 | 0 | 449 | 449 | 422 | 422 | |
| HEL084 | 262 | 262 | 0 | 0 | 0 | 0 | 446 | 449 | 381 | 422 | |
| HEL085 | 262 | 270 | 0 | 0 | 0 | 0 | 441 | 446 | 400 | 422 | |
| HEL086 | 262 | 270 | 259 | 259 | 0 | 0 | 445 | 449 | 402 | 402 | |
| HEL087 | 262 | 262 | 259 | 259 | 0 | 0 | 445 | 446 | 400 | 422 | |
| Siedliszcze | HEL088 | 262 | 270 | 180 | 281 | 507 | 507 | 417 | 443 | 383 | 389 |
| HEL089 | 262 | 262 | 254 | 259 | 0 | 0 | 445 | 448 | 382 | 406 | |
| HEL090 | 270 | 270 | 290 | 290 | 483 | 483 | 440 | 443 | 413 | 435 | |
| HEL091 | 254 | 262 | 259 | 295 | 516 | 516 | 441 | 448 | 390 | 413 | |
| HEL092 | 262 | 262 | 285 | 315 | 522 | 522 | 443 | 443 | 389 | 423 | |
| HEL093 | 262 | 269 | 259 | 277 | 520 | 520 | 413 | 448 | 382 | 383 | |
| HEL094 | 262 | 270 | 275 | 297 | 477 | 522 | 443 | 448 | 389 | 390 | |
| HEL095 | 254 | 254 | 289 | 289 | 480 | 480 | 443 | 449 | 395 | 407 | |
| HEL096 | 254 | 262 | 279 | 281 | 0 | 0 | 443 | 445 | 383 | 420 | |
| HEL097 | 262 | 262 | 254 | 287 | 522 | 522 | 431 | 443 | 389 | 413 | |
| HEL098 | 262 | 270 | 267 | 295 | 0 | 0 | 437 | 445 | 401 | 431 | |
| HEL099 | 262 | 262 | 257 | 257 | 507 | 507 | 441 | 445 | 382 | 404 | |
| HEL100 | 262 | 262 | 299 | 301 | 522 | 522 | 413 | 441 | 413 | 420 | |
| HEL101 | 262 | 262 | 315 | 317 | 0 | 0 | 439 | 441 | 394 | 396 | |
| HEL102 | 262 | 262 | 180 | 180 | 507 | 507 | 441 | 448 | 394 | 435 | |
| Kijowiec | HEL111 | 264 | 270 | 259 | 259 | 483 | 483 | 443 | 443 | 396 | 433 |
| HEL112 | 264 | 264 | 316 | 316 | 483 | 507 | 435 | 445 | 384 | 384 | |
| HEL113 | 270 | 270 | 0 | 0 | 483 | 507 | 443 | 445 | 416 | 433 | |
| HEL114 | 264 | 264 | 316 | 316 | 483 | 507 | 443 | 443 | 396 | 396 | |
| HEL115 | 264 | 264 | 259 | 259 | 483 | 507 | 443 | 445 | 401 | 406 | |
| HEL116 | 264 | 264 | 316 | 316 | 0 | 0 | 417 | 443 | 374 | 396 | |
| HEL117 | 264 | 264 | 279 | 289 | 483 | 483 | 443 | 445 | 406 | 433 | |
| HEL118 | 262 | 270 | 306 | 306 | 483 | 483 | 443 | 445 | 396 | 396 | |
| HEL120 | 264 | 264 | 279 | 318 | 507 | 507 | 435 | 445 | 390 | 406 | |
| HEL121 | 262 | 262 | 291 | 316 | 507 | 507 | 443 | 443 | 396 | 396 | |
| HEL122 | 262 | 262 | 292 | 299 | 483 | 508 | 417 | 443 | 396 | 406 | |
| HEL123 | 264 | 264 | 299 | 318 | 483 | 483 | 443 | 445 | 381 | 396 | |
| HEL124 | 270 | 270 | 281 | 292 | 0 | 0 | 443 | 443 | 381 | 396 | |
| HEL125 | 254 | 270 | 275 | 281 | 483 | 483 | 443 | 443 | 396 | 396 | |
| Podbiel | HEL130 | 270 | 270 | 277 | 277 | 0 | 0 | 439 | 448 | 392 | 396 |
| HEL131 | 270 | 270 | 263 | 263 | 0 | 0 | 443 | 443 | 392 | 406 | |
| HEL132 | 269 | 270 | 289 | 303 | 0 | 0 | 439 | 443 | 392 | 396 | |
| HEL133 | 270 | 270 | 259 | 289 | 0 | 0 | 439 | 445 | 384 | 407 | |
| HEL134 | 269 | 269 | 275 | 275 | 0 | 0 | 439 | 439 | 406 | 407 | |
| HEL135 | 270 | 270 | 263 | 263 | 0 | 0 | 439 | 445 | 383 | 429 | |
| HEL136 | 270 | 270 | 275 | 275 | 0 | 0 | 443 | 443 | 407 | 407 | |
| HEL137 | 270 | 270 | 259 | 259 | 0 | 0 | 437 | 443 | 384 | 392 | |
| HEL138 | 262 | 262 | 259 | 259 | 0 | 0 | 439 | 443 | 392 | 427 | |
| HEL139 | 270 | 270 | 294 | 294 | 0 | 0 | 439 | 443 | 392 | 406 | |
| HEL145 | 270 | 270 | 277 | 277 | 503 | 513 | 445 | 456 | 407 | 385 | |
| HEL146 | 270 | 270 | 294 | 294 | 0 | 0 | 439 | 443 | 392 | 406 | |
| HEL147 | 270 | 270 | 259 | 271 | 0 | 0 | 439 | 439 | 385 | 407 | |
| HEL148 | 262 | 270 | 275 | 283 | 513 | 513 | 439 | 441 | 409 | 427 | |
| HEL149 | 262 | 270 | 259 | 259 | 503 | 503 | 439 | 439 | 392 | 407 | |
| Straszewo | HEL150 | 262 | 270 | 272 | 272 | 0 | 0 | 439 | 442 | 390 | 395 |
| HEL154 | 262 | 262 | 261 | 261 | 0 | 0 | 439 | 449 | 389 | 395 | |
| HEL155 | 262 | 270 | 265 | 265 | 0 | 0 | 441 | 445 | 390 | 389 | |
| HEL158 | 262 | 270 | 279 | 285 | 0 | 0 | 439 | 456 | 389 | 390 | |
| HEL159 | 262 | 270 | 277 | 277 | 0 | 0 | 439 | 445 | 383 | 383 | |
| HEL163 | 262 | 270 | 283 | 293 | 0 | 0 | 433 | 439 | 399 | 412 | |
| HEL166 | 262 | 270 | 277 | 283 | 0 | 0 | 374 | 435 | 389 | 399 | |
| HEL167 | 262 | 262 | 261 | 265 | 0 | 0 | 456 | 456 | 390 | 412 | |
| Bobry | HEL168 | 270 | 270 | 285 | 295 | 0 | 0 | 435 | 435 | 391 | 415 |
| HEL169 | 262 | 262 | 290 | 290 | 513 | 513 | 417 | 435 | 391 | 410 | |
| HEL170 | 262 | 270 | 259 | 295 | 0 | 0 | 448 | 448 | 383 | 391 | |
| HEL171 | 262 | 262 | 295 | 295 | 0 | 0 | 417 | 448 | 392 | 408 | |
| HEL172 | 262 | 270 | 180 | 277 | 506 | 506 | 439 | 445 | 383 | 391 | |
| HEL173 | 262 | 262 | 291 | 291 | 513 | 514 | 417 | 435 | 406 | 408 | |
| HEL174 | 270 | 270 | 277 | 277 | 510 | 510 | 435 | 435 | 410 | 410 | |
| HEL175 | 262 | 262 | 290 | 290 | 508 | 508 | 374 | 448 | 408 | 410 | |
| HEL176 | 262 | 270 | 277 | 277 | 481 | 481 | 374 | 438 | 392 | 407 | |
| HEL177 | 262 | 262 | 261 | 261 | 0 | 0 | 445 | 445 | 383 | 408 | |
| HEL178 | 262 | 262 | 277 | 277 | 514 | 514 | 435 | 438 | 408 | 408 | |
| HEL179 | 270 | 270 | 291 | 291 | 513 | 513 | 415 | 435 | 395 | 407 | |
| HEL180 | 270 | 270 | 291 | 291 | 513 | 513 | 415 | 435 | 391 | 395 | |
| HEL181 | 270 | 270 | 180 | 180 | 0 | 0 | 435 | 438 | 391 | 408 | |
| HEL182 | 262 | 262 | 287 | 287 | 513 | 513 | 435 | 438 | 408 | 410 | |
| Lăpușel | HEL012 | 262 | 262 | 281 | 295 | 481 | 511 | 442 | 443 | 383 | 389 |
| HEL013 | 262 | 262 | 253 | 283 | 481 | 481 | 442 | 442 | 400 | 403 | |
| HEL014 | 262 | 262 | 281 | 283 | 481 | 481 | 439 | 442 | 383 | 383 | |
| HEL015 | 262 | 262 | 281 | 281 | 481 | 481 | 442 | 442 | 400 | 403 | |
| HEL016 | 262 | 262 | 283 | 283 | 481 | 483 | 442 | 443 | 386 | 405 | |
| HEL017 | 262 | 262 | 261 | 261 | 0 | 0 | 448 | 448 | 400 | 400 | |
| HEL018 | 262 | 262 | 281 | 281 | 0 | 0 | 443 | 448 | 384 | 384 | |
| HEL183 | 262 | 262 | 281 | 295 | 481 | 495 | 417 | 443 | 400 | 413 | |
| HEL185 | 262 | 262 | 286 | 286 | 481 | 481 | 427 | 448 | 403 | 416 | |
| HEL186 | 262 | 262 | 259 | 259 | 481 | 481 | 439 | 443 | 391 | 400 | |
| HEL187 | 262 | 262 | 283 | 283 | 0 | 0 | 442 | 443 | 389 | 389 | |
| HEL188 | 262 | 262 | 283 | 283 | 511 | 511 | 417 | 442 | 386 | 405 | |
| HEL189 | 0 | 0 | 281 | 295 | 495 | 495 | 439 | 448 | 400 | 403 | |
| HEL190 | 262 | 262 | 257 | 286 | 0 | 0 | 442 | 443 | 400 | 403 | |
| Coșna | HEL192 | 262 | 262 | 293 | 293 | 0 | 0 | 435 | 443 | 395 | 395 |
| HEL193 | 262 | 262 | 293 | 293 | 508 | 508 | 433 | 443 | 395 | 395 | |
| HEL194 | 262 | 262 | 291 | 293 | 483 | 483 | 433 | 443 | 395 | 395 | |
| HEL195 | 262 | 262 | 291 | 293 | 513 | 513 | 433 | 443 | 379 | 395 | |
| HEL196 | 262 | 262 | 283 | 293 | 508 | 508 | 443 | 443 | 395 | 406 | |
| HEL197 | 262 | 262 | 291 | 293 | 513 | 513 | 433 | 435 | 395 | 395 | |
| HEL198 | 262 | 262 | 293 | 299 | 508 | 508 | 443 | 443 | 395 | 395 | |
| HEL199 | 262 | 262 | 291 | 299 | 508 | 508 | 435 | 443 | 393 | 406 | |
| HEL200 | 262 | 262 | 299 | 299 | 483 | 513 | 443 | 443 | 379 | 379 | |
| HEL201 | 262 | 262 | 291 | 293 | 483 | 483 | 435 | 439 | 393 | 395 | |
| HEL202 | 262 | 262 | 291 | 299 | 508 | 508 | 443 | 443 | 395 | 395 | |
| HEL203 | 262 | 262 | 299 | 299 | 513 | 513 | 433 | 439 | 379 | 395 | |
| HEL204 | 262 | 262 | 293 | 299 | 508 | 513 | 435 | 443 | 393 | 395 | |
| HEL205 | 262 | 262 | 291 | 299 | 0 | 0 | 435 | 435 | 395 | 395 | |
| HEL206 | 262 | 262 | 299 | 299 | 0 | 0 | 439 | 443 | 393 | 395 | |
| HEL207 | 262 | 262 | 293 | 299 | 513 | 513 | 443 | 443 | 395 | 395 | |
| HEL208 | 262 | 262 | 293 | 299 | 508 | 513 | 435 | 443 | 378 | 393 | |
| HEL209 | 262 | 262 | 291 | 299 | 508 | 508 | 443 | 443 | 393 | 395 | |
| Moldova Sulița | HEL057 | 262 | 262 | 291 | 291 | 505 | 505 | 439 | 442 | 379 | 406 |
| HEL058 | 262 | 262 | 286 | 286 | 483 | 507 | 433 | 442 | 389 | 406 | |
| HEL059 | 262 | 270 | 277 | 277 | 507 | 508 | 443 | 445 | 401 | 407 | |
| HEL060 | 262 | 262 | 0 | 0 | 507 | 507 | 443 | 443 | 389 | 410 | |
| HEL061 | 262 | 262 | 291 | 291 | 508 | 508 | 443 | 449 | 389 | 389 | |
| HEL062 | 262 | 262 | 277 | 285 | 508 | 508 | 433 | 439 | 406 | 406 | |
| HEL063 | 262 | 262 | 293 | 293 | 481 | 485 | 439 | 443 | 392 | 396 | |
| HEL064 | 262 | 262 | 277 | 285 | 480 | 508 | 442 | 449 | 389 | 396 | |
| HEL065 | 262 | 262 | 291 | 291 | 505 | 505 | 433 | 443 | 389 | 407 | |
| HEL066 | 262 | 262 | 180 | 180 | 485 | 508 | 442 | 445 | 406 | 407 | |
| HEL067 | 262 | 262 | 180 | 285 | 505 | 505 | 439 | 443 | 389 | 406 | |
| HEL068 | 262 | 262 | 301 | 342 | 481 | 485 | 433 | 439 | 389 | 389 | |
| HEL069 | 262 | 262 | 0 | 0 | 485 | 514 | 433 | 442 | 389 | 389 | |
| HEL070 | 262 | 262 | 291 | 308 | 505 | 507 | 443 | 443 | 407 | 410 | |
| HEL071 | 262 | 262 | 0 | 0 | 481 | 481 | 433 | 445 | 401 | 411 | |
| HEL072 | 0 | 0 | 293 | 295 | 481 | 505 | 433 | 443 | 386 | 410 | |
| HEL073 | 262 | 262 | 287 | 291 | 481 | 481 | 442 | 445 | 389 | 389 | |
| HEL074 | 262 | 262 | 180 | 295 | 507 | 515 | 433 | 445 | 406 | 406 | |
| Vad | HEL019 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 440 | 391 | 398 |
| HEL020 | 262 | 262 | 281 | 281 | 505 | 505 | 440 | 440 | 398 | 407 | |
| HEL021 | 262 | 262 | 0 | 0 | 505 | 505 | 445 | 445 | 393 | 393 | |
| HEL022 | 262 | 262 | 0 | 0 | 505 | 505 | 439 | 445 | 396 | 407 | |
| HEL023 | 262 | 262 | 0 | 0 | 0 | 0 | 445 | 445 | 393 | 393 | |
| HEL024 | 262 | 262 | 0 | 0 | 0 | 0 | 445 | 445 | 391 | 393 | |
| HEL025 | 262 | 262 | 281 | 281 | 505 | 505 | 439 | 445 | 407 | 407 | |
| HEL026 | 262 | 262 | 0 | 0 | 0 | 0 | 445 | 445 | 391 | 393 | |
| HEL027 | 262 | 262 | 0 | 0 | 480 | 480 | 443 | 445 | 393 | 400 | |
| HEL028 | 262 | 262 | 0 | 0 | 505 | 505 | 439 | 439 | 393 | 396 | |
| HEL029 | 262 | 262 | 315 | 315 | 0 | 0 | 439 | 445 | 393 | 400 | |
| HEL030 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 445 | 393 | 398 | |
| HEL031 | 262 | 262 | 281 | 281 | 0 | 0 | 445 | 445 | 393 | 393 | |
| HEL032 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 443 | 398 | 400 | |
| HEL033 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 445 | 393 | 407 | |
| HEL034 | 262 | 262 | 0 | 0 | 505 | 505 | 445 | 445 | 391 | 393 | |
| HEL035 | 262 | 262 | 281 | 281 | 508 | 508 | 439 | 445 | 393 | 398 | |
| HEL036 | 262 | 262 | 0 | 0 | 0 | 0 | 440 | 445 | 391 | 398 | |
| HEL037 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 445 | 393 | 393 | |
| HEL038 | 262 | 262 | 0 | 0 | 505 | 505 | 440 | 440 | 391 | 400 | |
| HEL039 | 262 | 262 | 0 | 0 | 0 | 0 | 443 | 445 | 393 | 398 | |
| HEL040 | 262 | 262 | 268 | 268 | 0 | 0 | 443 | 445 | 400 | 407 | |
| HEL041 | 262 | 262 | 281 | 281 | 508 | 508 | 445 | 445 | 398 | 398 | |
| HEL042 | 262 | 262 | 0 | 0 | 505 | 505 | 439 | 445 | 391 | 393 | |
| HEL043 | 262 | 262 | 230 | 230 | 0 | 0 | 445 | 445 | 393 | 398 | |
| HEL044 | 262 | 262 | 0 | 0 | 0 | 0 | 445 | 443 | 396 | 407 | |
| HEL045 | 262 | 262 | 0 | 0 | 0 | 0 | 445 | 445 | 393 | 393 | |
| HEL046 | 262 | 262 | 0 | 0 | 480 | 508 | 443 | 445 | 391 | 407 | |
| HEL047 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 445 | 393 | 407 | |
| HEL048 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 445 | 407 | 407 | |
| HEL049 | 262 | 262 | 0 | 0 | 0 | 0 | 439 | 445 | 398 | 407 | |
| HEL050 | 262 | 262 | 0 | 0 | 0 | 0 | 445 | 445 | 393 | 393 |
References
- Hewitt, G.M. Genetic consequences of climatic oscillations in the Quaternary. Philos. Trans. R. Soc. Lond. B 2004, 359, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Front. Zool. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Maresova, J.; Suchackova Bartonova, A.; Konvicka, M.; Høye, T.T.; Gilg, O.; Kresse, J.C.; Shapoval, N.A.; Yakovlev, R.V.; Faltynek Fric, Z. The story of endurance: Biogeography and the evolutionary history of four Holarctic butterflies with different habitat requirements. J. Biogeogr. 2021, 48, 590–602. [Google Scholar] [CrossRef]
- Markova, A.K.; Van Kolfschoten, T.; Bohncke, S.; Kosintsev, P.A.; Mol, J.; Puzachenko, A.Y.; Simakova, A.N.; Smirnov, N.G.; Verpoorte, A.; Golovachev, I.B. Evolution of European Ecosystems During Pleistocene–Holocene Transition (24–8 Kyr BP); GEOS: Moscow, Russia, 2008; ISBN 978-5-87317-456-0. [Google Scholar]
- Maresova, J.; Habel, J.C.; Neve, G.; Sielezniew, M.; Bartonova, A.; Kostro-Ambroziak, A.; Fric, Z.F. Cross-continental phylogeography of two Holarctic Nymphalid butterflies, Boloria eunomia and Boloria selene. PLoS ONE 2019, 14, e0214483. [Google Scholar] [CrossRef]
- Bartonova, A.; Konvicka, M.; Korb, S.; Kramp, K.; Schmitt, T.; Faltynek Fric, Z. Range dynamics of Palaearctic steppe species under glacial cycles: The phylogeography of Proterebia afra (Lepidoptera: Nymphalidae: Satyrinae). Biol. J. Linn. Soc. Lond. 2018, 125, 867–884. [Google Scholar] [CrossRef]
- Zinovyev, E. Sub-fossil beetle assemblages associated with the “mammoth fauna” in the Late Pleistocene localities of the Ural Mountains and West Siberia. ZooKeys 2011, 100, 149–169. [Google Scholar] [CrossRef]
- Ruzicka, M. The Pleistocene glaciation of Czechia. In Quaternary Glaciations—Extent and Chronology. Part I: Europe. Developments in Quaternary Science; Ehlers, J., Gibbard, P.L., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2004; Volume 1, pp. 27–34. ISBN 978-0-444-51462-2. [Google Scholar]
- Kuhlemann, J.; Dobre, F.; Urdea, P.; Krumrei, I.; Gachev, E.; Kubik, P.; Rahn, M. Last glacial maximum glaciation of the central south Carpathian range (Romania). Austrian J. Earth Sci. 2013, 106, 50–62. [Google Scholar]
- Jurickova, L.; Horackova, J.; Lozek, V. Direct evidence of central European forest refugia during the last glacial period based on mollusc fossils. Quat. Res. 2014, 82, 222–228. [Google Scholar] [CrossRef]
- Hosek, J.; Pokorny, P.; Storch, D.; Kvacek, J.; Havig, J.; Novak, J.; Hajkova, P.; Jamrichova, E.; Brengman, L.; Radomersky, T.; et al. Hot spring oases in the periglacial desert as the Last Glacial Maximum refugia for temperate trees in Central Europe. Sci. Adv. 2024, 10, eado6611. [Google Scholar] [CrossRef]
- Puzachenko, A.Y.; Markova, A.K. Evolution of mammal species composition and species richness during the Late Pleistocene-Holocene transition in Europe: A general view at the regional scale. Quat. Int. 2019, 530, 88–106. [Google Scholar] [CrossRef]
- Suchackova Bartonova, A.; Konvicka, M.; Maresova, J.; Blahova, D.; Cip, D.; Skala, P.; Andres, M.; Hula, V.; Dolek, M.; Geyer, A.; et al. Extremely endangered butterflies of scattered central European dry grasslands under current habitat alteration. Insect Syst. Divers. 2021, 5, 6. [Google Scholar] [CrossRef]
- Willner, W.; Moser, D.; Plenk, K.; Aćić, S.; Demina, O.N.; Höhn, M.; Kuzemko, A.; Roleček, J.; Vassilev, K.; Vynokurov, D.; et al. Long-term continuity of steppe grasslands in eastern Central Europe: Evidence from species distribution patterns and chloroplast haplotypes. J. Biogeogr. 2021, 48, 3104–3117. [Google Scholar] [CrossRef]
- Spitzer, K.; Bezděk, A.; Jaroš, J. Ecological succession of a relict Central European peat bog and variability of its insect biodiversity. J. Insect Conserv. 1999, 3, 97–106. [Google Scholar] [CrossRef]
- Kramp, K.; Cizek, O.; Madeira, P.M.; Ramos, A.A.; Konvicka, M.; Castilho, R.; Schmitt, T. Genetic implications of phylogeographical patterns in the conservation of the boreal wetland butterfly Colias palaeno (Pieridae). Biol. J. Linn. Soc. 2016, 119, 1068–1081. [Google Scholar] [CrossRef]
- Sommer, R.S.; Thiele, V.; Sushko, G.; Sielezniew, M.; Kolligs, D.; Dapkus, D. The distribution pattern of mire specialist butterflies in raised bogs of the northern lowlands of Central Europe. Nota Lepidopterol. 2022, 45, 41–52. [Google Scholar] [CrossRef]
- Feurdean, A.; Ruprecht, E.; Molnár, Z.; Hutchinson, S.M.; Hickler, T. Biodiversity-rich European grasslands: Ancient, forgotten ecosystems. Biol. Conserv. 2018, 228, 224–232. [Google Scholar] [CrossRef]
- Craioveanu, C.; Sitar, C.; Rákosy, L. Mobility, behaviour and phenology of the Violet Copper Lycaena helle in North-Western Romania. In Jewels in the Mist: A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Habel, J.C., Meyer, M., Schmitt, T., Eds.; Pensoft: Sofia, Bulgaria, 2014; pp. 91–105. ISBN 978-954-642-721-2. [Google Scholar]
- Ion, C.M.; Manu, M.; Stanescu, M.; Maican, S.; Helepciuc, F.E.; Morosanu, A.M.; Stefanut, M.M.; Tamas, G.; Birsan, C.C.; Nicoara, R.G.; et al. The rediscovery of Lycaena helle (Lepidoptera: Lycaenidae) in Dorna depression (Romania), 125 years after its first mention. Sci. Pap. Ser. D Anim. Sci. 2023, 66, 612–620. [Google Scholar]
- Popovic, M.; Duric, M.; Franeta, F.; van Deijk, J.R.; Vermeer, R. First records of Lycaena helle ([Denis & Schiffermüller], 1775) for the Balkan Peninsula (Lepidoptera: Lycaenidae). Shilap Rev. Lepidopt. 2014, 42, 287–294. [Google Scholar] [CrossRef]
- Langourov, M.; Raeburn, H. A new locality of the violet copper Lycaena helle ([Denis et Schiffermüller], 1775) on the Balkan Peninsula. Hist. Nat. Bulg. 2022, 44, 41–44. [Google Scholar] [CrossRef]
- Beneš, J.; Konvička, M.; Dvořák, J.; Fric, Z.; Havelda, Z.; Pavlíčko, A.; Vrabec, V.; Weidenhoffer, Z. Motýli České republiky: Rozšíření a ochrana I, II [Butterflies of the Czech Republic: Distribution and Conservation]; SOM: Praha, Czech Republic, 2002; ISBN 80-903212-0-8. [Google Scholar]
- Reinhardt, R.; Harpke, A.; Caspari, S.; Dolek, M.; Kühn, E.; Musche, M.; Trusch, R.; Wiemers, M.; Settele, J. Verbreitungsatlas der Tagfalter und Widderchen Deutschlands; Verlag Eugen Ulmer: Stuttgart, Germany, 2020; ISBN 978-3-8186-0557-5. [Google Scholar]
- Goffart, P.; Schtickzelle, N.; Turlure, C. Conservation and management of the habitats of two relict butterflies in the Belgian Ardenne: Proclossiana eunomia and Lycaena helle. In Relict Species: Phylogeography and Conservation Biology; Habel, J.C., Schmitt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 357–370. ISBN 978-3-540-92161-5. [Google Scholar]
- Goffart, P.; Cavelier, E.; Lighezzolo, P.; Rauw, A.; Lafontaine, D. Restoration and management of habitat networks for Lycaena helle in Belgium. In Jewels in the Mist: A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Habel, J.C., Meyer, M., Schmitt, T., Eds.; Pensoft: Sofia, Bulgaria, 2014; pp. 197–216. ISBN 978-954-642-721-2. [Google Scholar]
- Scherer, G.; Löffler, F.; Fartmann, T. Abandonment of traditional land use and climate change threaten the survival of an endangered relict butterfly species. Insect Conserv. Divers. 2021, 14, 556–567. [Google Scholar] [CrossRef]
- Habel, J.C.; Roedder, D.; Schmitt, T.; Nève, G. Global warming will affect the genetic diversity and uniqueness of Lycaena helle populations. Glob. Change Biol. 2011, 17, 194–205. [Google Scholar] [CrossRef]
- Kebaili, C.; Sherpa, S.; Guéguen, M.; Renaud, J.; Rioux, D.; Després, R. Comparative genetic and demographic responses to climate change in three peatland butterflies in the Jura massif. Biol. Conserv. 2023, 287, 110332. [Google Scholar] [CrossRef]
- Fischer, K.; Beinlich, B.; Plachter, H. Population structure, mobility and habitat preferences of the violet copper Lycaena helle (Lepidoptera: Lycaenidae) in Western Germany: Implications for conservation. J. Insect Conserv. 1999, 3, 43–52. [Google Scholar] [CrossRef]
- Kašpar, A. Chrysophanus amphidamas Esp., nový motýl pro Moravu [Chrysophanus amphidamas Esp., new butterfly for Moravia]. Časopis Vlasteneckého Spolku Muzejního Olomouc 1939, 52, 175–178. [Google Scholar]
- Plazio, E.; Nowicki, P. Inter-sexual and inter-generation differences in dispersal of a bivoltine butterfly. Sci. Rep. 2021, 11, 10950. [Google Scholar] [CrossRef]
- Habel, J.C.; Schmitt, T.; Meyer, M.; Finger, A.; Roedder, D.; Assmann, T.; Zachos, F.E. Biogeography meets conservation: The genetic structure of the endangered lycaenid butterfly Lycaena helle (Denis & Schiffermüller, 1775). Biol. J. Linn. Soc. Lond. 2010, 101, 155–168. [Google Scholar] [CrossRef]
- Habel, J.C.; Meyer, M.; Schmitt, T. Jewels in the Mist, A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Pensoft: Sofia, Bulgaria, 2014; ISBN 978-954-642-721-2. [Google Scholar]
- Modin, H.; Öckinger, E. Mobility, habitat selection and population connectivity of the butterfly Lycaena helle in central Sweden. J. Insect Conserv. 2020, 24, 821–831. [Google Scholar] [CrossRef]
- Bauerfeind, S.S.; Theisen, A.; Fischer, K. Patch occupancy in the endangered butterfly Lycaena helle in a fragmented landscape: Effects of habitat quality, patch size and isolation. J. Insect Conserv. 2009, 13, 271–277. [Google Scholar] [CrossRef]
- Turlure, C.; Van Dyck, H.; Goffart, P.; Schtickzelle, N. Resource-based habitat use in Lycaena helle: Significance of a functional, ecological niche-oriented approach. In Jewels in the Mist, A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Habel, J.C., Meyer, M., Schmitt, T., Eds.; Pensoft: Sofia, Bulgaria, 2014; pp. 67–85. ISBN 978-954-642-721-2. [Google Scholar]
- Nabielec, J.; Nowicki, P. Drivers of local densities of endangered Lycaena helle butterflies in a fragmented landscape. Popul. Ecol. 2015, 57, 649–656. [Google Scholar] [CrossRef]
- Peškařová, T.; Pavlíčko, A.; Kuras, T.; Fric, Z.F.; Konvička, M. Population status of the highly endangered Lycaena helle (Papilionoidea, Lycaenidae) in the Šumava Mts. two decades after establishment. Nota Lepidopterol. 2024, 47, 171–186. [Google Scholar] [CrossRef]
- Finger, A.; Schmitt, T.; Zachos, F.E.; Meyer, M.; Assmann, T.; Habel, J.C. The genetic status of the violet copper Lycaena helle—A relict of the cold past in times of global warming. Ecography 2009, 32, 382–390. [Google Scholar] [CrossRef]
- Habel, J.C.; Finger, A.; Schmitt, T.; Nève, G. Survival of the endangered butterfly Lycaena helle in a fragmented environment: Genetic analyses over 15 years. J. Zool. Sys Evol. Res. 2010, 19, 25–31. [Google Scholar] [CrossRef]
- Sucháčková Bartoňová, A.; Škopek, P.; Konvička, M.; Beneš, J.; Spitzer, L.; Sbaraglia, C.; Vrabec, V.; Papp Marešová, J.; Konvičková, H.; Faltýnek Fric, Z. Czech Republic butterfly barcoding reveals that distribution of genetic lineages depends on species traits. J. Biogeogr. 2024, 51, 1575–1586. [Google Scholar] [CrossRef]
- Kayser, M. How to manage habitats of the endangered lycaenid butterfly Lycaena helle (Denis & Schiffermüller, 1775) (Insecta, Lepidoptera). Bull. Soc. Nat. Luxemb. 2014, 115, 241–249. [Google Scholar]
- Descimon, H.; Bachelard, P. Results of two introductions of Lycaena helle in France. In Jewels in the Mist: A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Habel, J.C., Meyer, M., Schmitt, T., Eds.; Pensoft: Sofia, Bulgaria, 2014; pp. 185–196. ISBN 978-954-642-721-2. [Google Scholar]
- Habel, J.C.; Finger, A.; Meyer, M.; Schmitt, T.; Assmann, T. Polymorphic microsatellite loci in the endangered butterfly Lycaena helle (Lepidoptera: Lycaenidae). Eur. J. Entomol. 2008, 105, 361–362. [Google Scholar] [CrossRef]
- Ryrholm, N. The Violet Copper Lycaena helle at its northern distribution range. In Jewels in the Mist: A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Habel, J.C., Meyer, M., Schmitt, T., Eds.; Pensoft: Sofia, Bulgaria, 2014; pp. 197–216. ISBN 978-954-642-721-2. [Google Scholar]
- Krahulec, F.; Blažková, D.; Balátová-Tuláčková, E.; Štursa, J.; Pecháčková, S.; Fabšicová, M. Meadows in the Krkonoše Mts—Plant communities and their dynamics. Opera Corcontica 1997, 33, 3–250. [Google Scholar]
- Blaik, T. Występowanie, stan populacji i siedliska czerwończyka fioletka Lycaena helle (Denis et Schiffermüller, 1775) (Lepidoptera: Lyceanidae) w województwie opolskim. Przyroda Sudetów 2014, 17, 135–146. [Google Scholar]
- Sielezniew, M.; Dziekańska, I. Czerwończyk fioletek Lycaena helle. In Monitoring Gatunków Zwierząt. Przewodnik Metodyczny. Część II; Makomaska-Juchiewicz, M., Baran, P., Eds.; GIOŚ: Warszawa, Poland, 2012; pp. 124–141. [Google Scholar]
- Krzywicki, M. Fauna Papilionoidea i Hesperioidea (Lepidoptera) Puszczy Białowieskiej. Ann. Zool. 1967, 25, 1–213. [Google Scholar]
- Chapuis, M.P.; Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Rousset, F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. J. Heredity 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Belkhir, K.; Borsa, P.; Chikhi, L.; Raufaste, N.; Bonhomme, F. GENETIX 4.05, Logiciel sous Windows TM pour la Génétique des Populations; Laboratoire Génome, Populations, Interactions, CNRS UMR 5171, Université de Montpellier II: Montpellier, France, 2004. [Google Scholar]
- Goudet, J. Fstat (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Res. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Wen, W. Documentation for STRUCTURE Software, Version 2. 2003. Available online: https://web.stanford.edu/group/pritchardlab/structure.html (accessed on 10 May 2025).
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Klimczuk, P. Butterflies (Lepidoptera: Hesperioidea, Papilionoidea) of the Knyszyńska Forest (Puszczy Knyszyńskiej) and adjacent woodland areas of Białystok. Nat. J. 2011, 44, 197–217. [Google Scholar]
- Sielezniew, M. Raport z inwentaryzacji czerwończyka fioletka na terenie obszaru Natura 2000 “Ostoja Knyszyńska”; Regionalna Dyrekcja Ochrony Środowiska w Białymstoku: Białystok, Poland, 2018; Unpublished Report. [Google Scholar]
- Bergman, K.O. Habitat utilization by Lopinga achine (Nymphalidae: Satyrinae) larvae and ovipositing females: Implications for conservation. Biol. Conserv. 1999, 88, 69–74. [Google Scholar] [CrossRef]
- Dolek, M.; Kőrösi, Á.; Freese-Hager, A. Successful maintenance of Lepidoptera by government-funded management of coppiced forests. J. Nat. Conserv. 2018, 43, 75–84. [Google Scholar] [CrossRef]
- Sielezniew, M.; Nowicki, P. Adult demography of an isolated population of the threatened butterfly Scarce Heath Coenonympha hero and its conservation implications. J. Insect Conserv. 2017, 21, 737–742. [Google Scholar] [CrossRef]
- Vlasanek, P.; Hauck, D.; Konvicka, M. Adult sex ratio in the Parnassius mnemosyne butterfly: Effects of survival, migration, and weather. Isr. J. Ecol. Evol. 2009, 55, 233–252. [Google Scholar] [CrossRef]
- Pearce, E.A.; Mazier, F.; Davison, C.W.; Baines, O.; Czyżewski, S.; Fyfe, R.; Bińka, K.; Boreham, S.; de Beaulieu, J.L.; Gao, C.; et al. Beyond the closed-forest paradigm: Cross-scale vegetation structure in temperate Europe before the late-Quaternary megafauna extinctions. Earth Hist. Biodiv. 2025, 3, 100022. [Google Scholar] [CrossRef]
- Svenning, J.C. A review of natural vegetation openness in north-western Europe. Biol. Conserv. 2002, 104, 133–148. [Google Scholar] [CrossRef]
- Buckley, P. Coppice restoration and conservation: A European perspective. J. For. Res. 2020, 25, 125–133. [Google Scholar] [CrossRef]
- Székely, L.; Görbe, R. The lepidoptera fauna of “Dumbrava Vaduluipoienile cu narcise” forest (Șercaia, Brașov county, Romania). Brukenthal Acta Musei 2019, 14, 597–620. [Google Scholar]
- Turlure, C.; Van Dyck, H.; Schtickzelle, N.; Baguette, M. Resource-based habitat definition, niche overlap and conservation of two sympatric glacial relict butterflies. Oikos 2009, 118, 950–960. [Google Scholar] [CrossRef]
- Corduneanu, C.; Corduneanu, G.; Rákosy, L.; Dincă, V. First records of the bog fritillary Boloria eunomia (Esper, 1800) in the Romanian fauna (Lepidoptera, Nymphalidae): Peripheral populations in habitats of conservation concern. J. Insect Conserv. 2025, 29, 26. [Google Scholar] [CrossRef]
- Fischer, K.; Schubert, E.; Limberg, J. Caught in a trap: How to preserve a post-glacial relict species in secondary habitats? In Relict Species: Phylogeography and Conservation Biology; Habel, J.C., Schmitt, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 217–229. ISBN 978-3-540-92161-5. [Google Scholar]
- Jangjoo, M.; Matter, S.F.; Roland, J.; Keyghobadi, N. Connectivity rescues genetic diversity after a demographic bottleneck in a butterfly population network. Proc. Natl. Acad. Sci. USA 2016, 113, 10914–10919. [Google Scholar] [CrossRef] [PubMed]
- Vandewoestijne, S.; Schtickzelle, N.; Baguette, M. Positive correlation between genetic diversity and fitness in a large, well-connected metapopulation. BMC Biol. 2008, 6, 46. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Maes, D.; Kelager, A.; Wynhoff, I.; WallisDeVries, M.F.; Nash, D.R.; Oostermeijer, J.G.B.; Van Dyck, H.; Mergeay, J. Gene flow and effective population sizes of the butterfly Maculinea alcon in a highly fragmented, anthropogenic landscape. Biol. Conserv. 2017, 209, 89–97. [Google Scholar] [CrossRef]
- Hanski, I.; Schulz, T.; Wong, S.C.; Ahola, V.; Ruokolainen, A.; Ojanen, S.P. Ecological and genetic basis of metapopulation persistence of the Glanville fritillary butterfly in fragmented landscapes. Nat. Commun. 2017, 8, 14504. [Google Scholar] [CrossRef]
- Gabryszuk, M.; Barszczewski, J.; Wróbel, B. Characteristics of grasslands and their use in Poland. J. Water Land. Develop 2021, 51, 243–249. [Google Scholar] [CrossRef]
- Kulik, M.; Urban, D.; Grzywaczewski, G.; Bochniak, A.; Grzywna, A.; Sender, J. Half a century of wetland degradation: The present state and trends of changes in Western Polesie-Long-term wetland degradation. Glob. Ecol. Conserv. 2024, 56, e03324. [Google Scholar] [CrossRef]
- Medyńska-Gulij, B.; Szoszkiewicz, K.; Cybulski, P.; Wielebski, Ł. Permanent areas and changes in forests, grasslands, and wetlands in the North European Plain since the eighteenth century—A case study of the Kościan Plain in Poland. Sci. Rep. 2024, 14, 10305. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Seitz, A. Intraspecific allozymatic differentiation reveals the glacial refugia and the postglacial expansions of European Erebia medusa (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 2001, 74, 429–458. [Google Scholar] [CrossRef]
- Schmitt, T.; Gießl, A.; Seitz, A. Postglacial colonisation of western Central Europe by Polyommatus coridon (Poda 1761) (Lepidoptera: Lycaenidae): Evidence from population genetics. Heredity 2002, 88, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Patricelli, D.; Sielezniew, M.; Ponikwicka-Tyszko, D.; Ratkiewicz, M.; Bonelli, S.; Barbero, F.; Witek, M.; Buś, M.M.; Rutkowski, R.; Balletto, E. Contrasting genetic structure of rear edge and continuous range populations of a parasitic butterfly infected by Wolbachia. BMC Evol. Biol. 2013, 13, 14. [Google Scholar] [CrossRef]
- Cassel, A.; Tammaru, T. Allozyme variability in central, peripheral and isolated populations of the scarce heath (Coenonympha hero: Lepidoptera, Nymphalidae); implications for conservation. Conserv. Genet. 2003, 4, 83–93. [Google Scholar] [CrossRef]
- Altermatt, F. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. B Biol. 2010, 277, 1281–1287. [Google Scholar] [CrossRef]
- Wepprich, T.; Henry, E.; Haddad, N.M. Voltinism shifts in response to climate warming generally benefit populations of multivoltine butterflies. Ecol. Lett. 2025, 28, e70018. [Google Scholar] [CrossRef]
- Aguilar-Gómez, D.; Yuan, L.; Zhang, Y.; Ochoa, A.; Culver, M.; Fitak, R.R.; Onorato, D.; Lohmueller, K.E.; Nielsen, R. Genetic rescue of Florida panthers reduced homozygosity but did not swamp ancestral genotypes. Proc. Natl. Acad. Sci. USA 2025, 122, e2410945122. [Google Scholar] [CrossRef] [PubMed]
- Srbek-Araujo, A.C.; Haag, T.; Chiarello, A.G.; Salzano, F.M.; Eizirik, E. Worrisome isolation: Noninvasive genetic analyses shed light on the critical status of a remnant jaguar population. J. Mammal. 2018, 99, 397–407. [Google Scholar] [CrossRef]
- Vera, F.W.M. Grazing Ecology and Forest History; CABI Publishing: Wallingford, UK, 2000; ISBN 978-0851994420. [Google Scholar]
- Williams, M. Dark ages and dark areas: Global deforestation in the deep past. J. Hist. Geogr. 2000, 26, 28–46. [Google Scholar] [CrossRef]
- Youri, M.; Habel, J.C.; Van Dyck, H.; Titeux, N. Losing genetic uniqueness under global change: The Violet Copper (Lycaena helle) in Europe. In Jewels in the Mist: A Synopsis on the Endangered Violet Copper Butterfly Lycaena helle; Habel, J.C., Meyer, M., Schmitt, T., Eds.; Pensoft: Sofia, Bulgaria, 2014; pp. 165–184. ISBN 978-954-642-721-2. [Google Scholar]

| ID | Site | Area | Con. | Alt. | Volt. | N | HE ± SE | HO ± SE | FIS | A | AR | AP | HWE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CZ | 1 | Šumava | 3 | 1 | 860 | U | 12 | 0.60 ± 0.163 | 0.46 ± 0.406 | 0.248 | 3.3 | n.a. d | 3 | 0.16 |
| AT | 1a a | Mariazell | 2 | 1 | 900 | U | 30 | 0.77 ± n.a. | 0.54 ± n.a. | n.a. | 11.4 | 5.0 | n.a. | – |
| PL | 2 | Siedliszcze | 3 | 3 | 200 | B | 15 | 0.83 ± 0.179 | 0.63 ± 0.382 | 0.247 | 11.4 | 4.4 | 17 | *** |
| PL | 3 | Kijowiec | 1 | 1 | 150 | B | 14 | 0.68 ± 0.152 | 0.49 ± 0.181 | 0.286 | 6.2 | 3.2 | 7 | *** |
| PL | 4 | Podbiel | 3 | 2 | 100 | B | 15 | 0.69 ± 0.198 | 0.48 ± 0.311 | 0.319 | 6.2 | 3.3 | 9 | *** |
| PL | 5 | Straszewo | 2 | 2 | 150 | B | 8 | 0.79 ± 0.194 | 0.75 ± 0.468 | 0.054 | 6.3 | n.a. d | 4 | 0.13 |
| PL | 6 | Bobry | 2 | 2 | 210 | B | 15 | 0.77 ± 0.152 | 0.40 ± 0.353 | 0.465 | 6.8 | 3.7 | 6 | *** |
| RO | 7 | Lăpușel | 3 | 2 | 180 | B | 14 | 0.61 ± 0.363 | 0.45 ± 0.319 | 0.278 | 5.8 | 3.2 | 6 | *** |
| RO | 8 | Coșna | 1 | 2 | 950 | U | 18 | 0.50 ± 0.286 | 0.41 ± 0.299 | 0.200 | 3.4 | 2.4 | 1 | 0.02 |
| RO | 9 | Moldova Sulița | 3 | 1 | 1230 | U | 16 | 0.69 ± 0.350 | 0.54 ± 0.311 | 0.221 | 7.2 | 3.6 | 5 | *** |
| RO | 10 | Vad | 2 | 1 | 500 | B | 32 | 0.50 ± 0.293 | 0.28 ± 0.349 | 0.454 | 3.6 | 2.5 | 3 | *** |
| RO | 10a b | Vad spring | 16 | 0.41 ± 0.298 | 0.25 ± 0.354 | 0.423 | 3.0 | 0.12 | ||||||
| RO | 10b b | Vad summer | 16 | 0.55 ± 0.316 | 0.32 ± 0.350 | 0.463 | 3.4 | 0.04 | ||||||
| Sum, or mean ± SD c | Σ 189 | 0.67 ± 0.113 | 0.49 ± 0.129 | 0.277 ± 0.1199 | 6.1 ± 2.42 | 3.3 ± 1.49 | 6.0 ± 4.55 | |||||||
| Area | Connectivity | |||||
|---|---|---|---|---|---|---|
| Genetic Parameter | r | t | p | r | t | p |
| HE | 0.36 | 1.03 | 0.34 | 0.49 | 1.49 | 0.18 |
| HO | 0.23 | 0.64 | 0.54 | 0.42 | 1.24 | 0.25 |
| Fis | 0.02 | 0.06 | 0.96 | −0.23 | −0.61 | 0.56 |
| A | 0.56 | 1.77 | 0.12 | 0.54 | 1.69 | 0.14 |
| AR | 0.54 | 1.59 | 0.16 | 0.50 | 1.43 | 0.20 |
| AP | 0.52 | 1.62 | 0.15 | 0.63 | 2.12 | 0.07 # |
| Šumava | Siedliszcze | Kijowiec | Podbiel | Straszewo | Bobry | Lăpușel | Coșna | Moldova Sulița | Mean per Site ± SD | |
|---|---|---|---|---|---|---|---|---|---|---|
| Šumava | 0.239 ± 0.0682 | |||||||||
| Siedliszcze | 0.169 *** | 0.127 ± 0.0512 | ||||||||
| Kijowiec | 0.315 *** | 0.127 *** | 0.245 ± 0.0687 | |||||||
| Podbiel | 0.227 *** | 0.154 *** | 0.222 *** | 0.231 ± 0.0828 | ||||||
| Straszewo | 0.156 *** | 0.099 *** | 0.261 *** | 0.115 *** | 0.182 ± 0.0719 | |||||
| Bobry | 0.200 *** | 0.067 *** | 0.194 *** | 0.144 *** | 0.104 *** | 0.160 ± 0.0587 | ||||
| Lăpușel | 0.240 *** | 0.083 *** | 0.241 *** | 0.263 *** | 0.173 *** | 0.143 *** | 0.191 ± 0.0697 | |||
| Coșna | 0.369 *** | 0.170 *** | 0.286 *** | 0.351 *** | 0.292 *** | 0.201 *** | 0.226 *** | 0.266 ± 0.0786 | ||
| Moldova Sulița | 0.260 *** | 0.066 *** | 0.201 *** | 0.261 *** | 0.189 *** | 0.126 *** | 0.097 *** | 0.163 *** | 0.175 ± 0.0687 | |
| Vad | 0.212 *** | 0.208 *** | 0.355 *** | 0.339 *** | 0.251 *** | 0.258 *** | 0.257 *** | 0.335 *** | 0.214 *** | 0.270 ± 0.0582 |
| Analysis (Number of Groups) | Variance | FST | FIS | FIT |
|---|---|---|---|---|
| All populations (n = 10) | 2.032 | 0.446 | 0.515 | 1.071 |
| % variation, significance tests | 21.9%, 9df *** | 25.4%, 151df *** | 52.7%, 161df *** | |
| Among countries (n = 3) | 2.081 | 0.107 | 0.423 | 0.485 |
| % variation, significance tests | 10.7%, 2df *** | 37.8%, 158df *** | 51.5%, 161df *** | |
| Within Poland (n = 5) | 2.162 | 0.150 | 0.322 | 0.424 |
| % variation, significance tests | 15.0%, 4df *** | 27.3%, 62df *** | 57.6%, 67df *** | |
| Within Romania (n = 4) | 1.855 | 0.230 | 0.334 | 0.487 |
| % variation, significance tests | 23.0%, 3df *** | 25.7%, 78df *** | 51.3%, 82df *** | |
| Vad among generations (n = 2) | 1.184 | −0.012 | 0.432 | 0.426 |
| % variation, significance tests | 0.0%, 1df | 43.2%, 30df *** | 56.8%, 32df *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitar, C.; Sielezniew, M.; Malkiewicz, A.; Fric, Z.F.; Konvička, M.; Konvickova, H. Eastern Arc of Glacial Relict Species—Population Genetics of Violet Copper Lycaena helle Butterfly in East-Central Europe. Insects 2025, 16, 1202. https://doi.org/10.3390/insects16121202
Sitar C, Sielezniew M, Malkiewicz A, Fric ZF, Konvička M, Konvickova H. Eastern Arc of Glacial Relict Species—Population Genetics of Violet Copper Lycaena helle Butterfly in East-Central Europe. Insects. 2025; 16(12):1202. https://doi.org/10.3390/insects16121202
Chicago/Turabian StyleSitar, Cristian, Marcin Sielezniew, Adam Malkiewicz, Zdenek Faltynek Fric, Martin Konvička, and Hana Konvickova. 2025. "Eastern Arc of Glacial Relict Species—Population Genetics of Violet Copper Lycaena helle Butterfly in East-Central Europe" Insects 16, no. 12: 1202. https://doi.org/10.3390/insects16121202
APA StyleSitar, C., Sielezniew, M., Malkiewicz, A., Fric, Z. F., Konvička, M., & Konvickova, H. (2025). Eastern Arc of Glacial Relict Species—Population Genetics of Violet Copper Lycaena helle Butterfly in East-Central Europe. Insects, 16(12), 1202. https://doi.org/10.3390/insects16121202






