Discovering the Diversity and Evolution of Danascelinae: A New Genus and Species from Eastern Asia and Insights into the Phylogenetic Placement of This Subfamily in Endomychidae (Coleoptera) †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Material–Preparation and Processing
2.2. Morphological Dataset Preparation and Analysis
3. Results
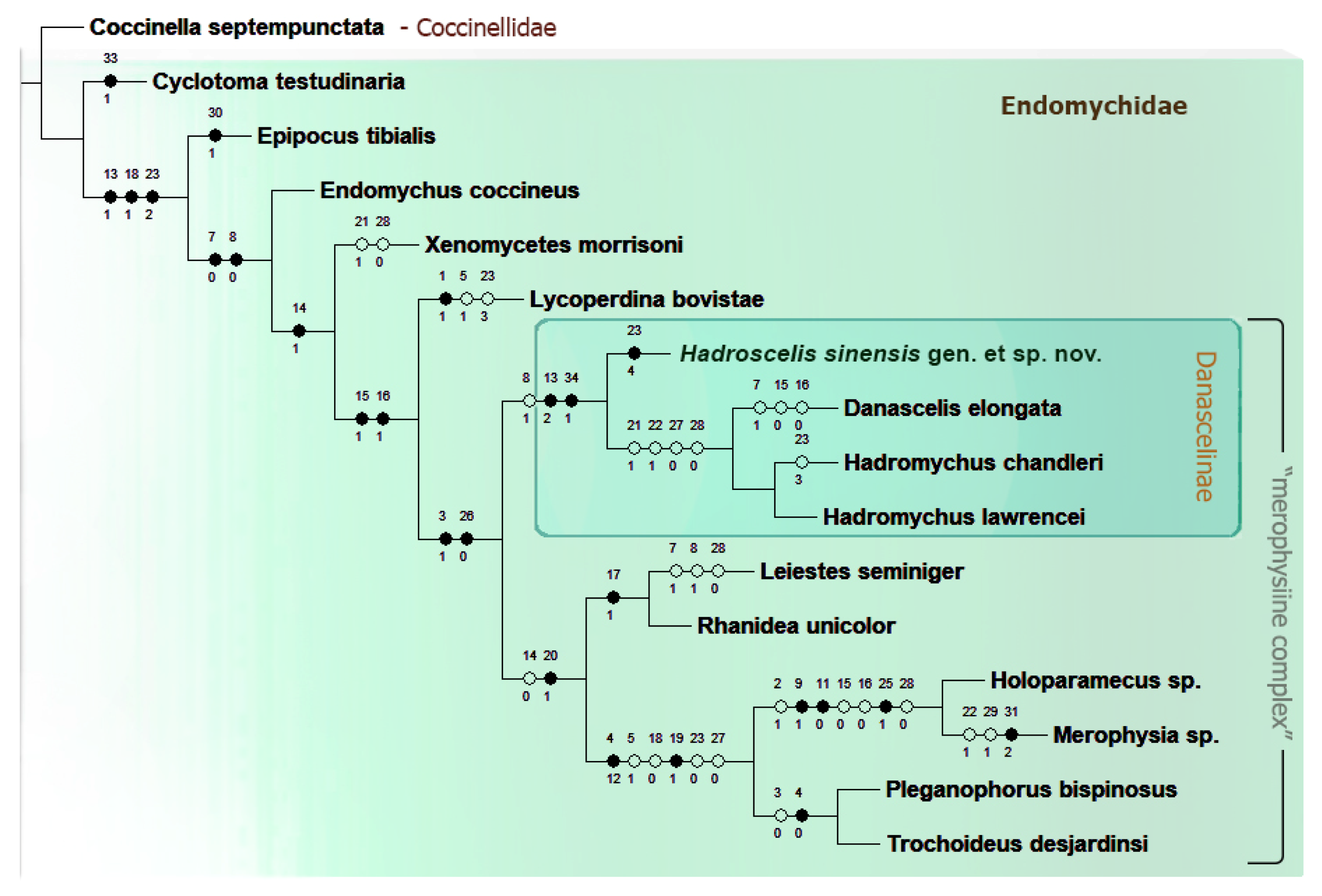
3.1. Phylogenetic Analysis of Morphological Dataset
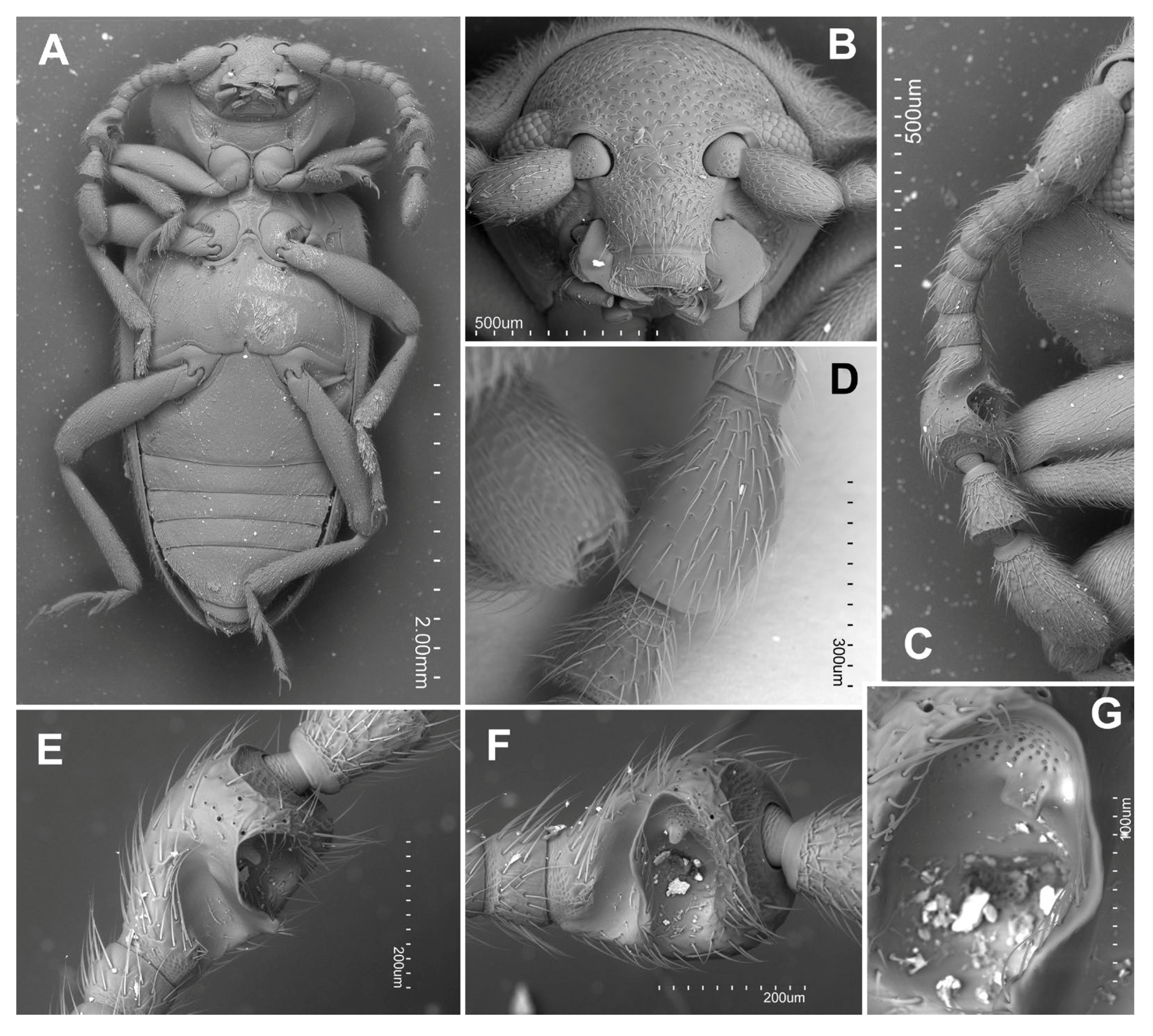
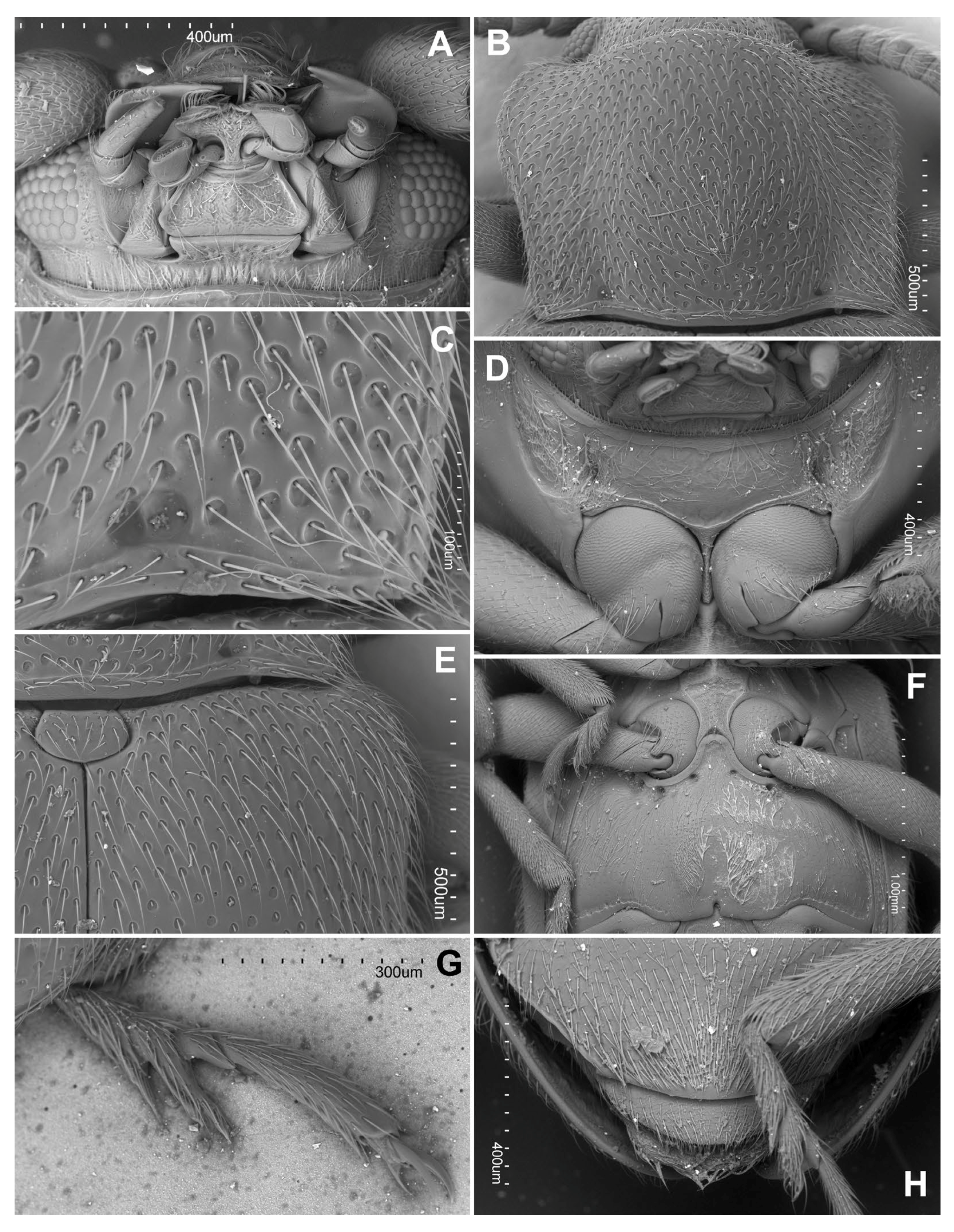
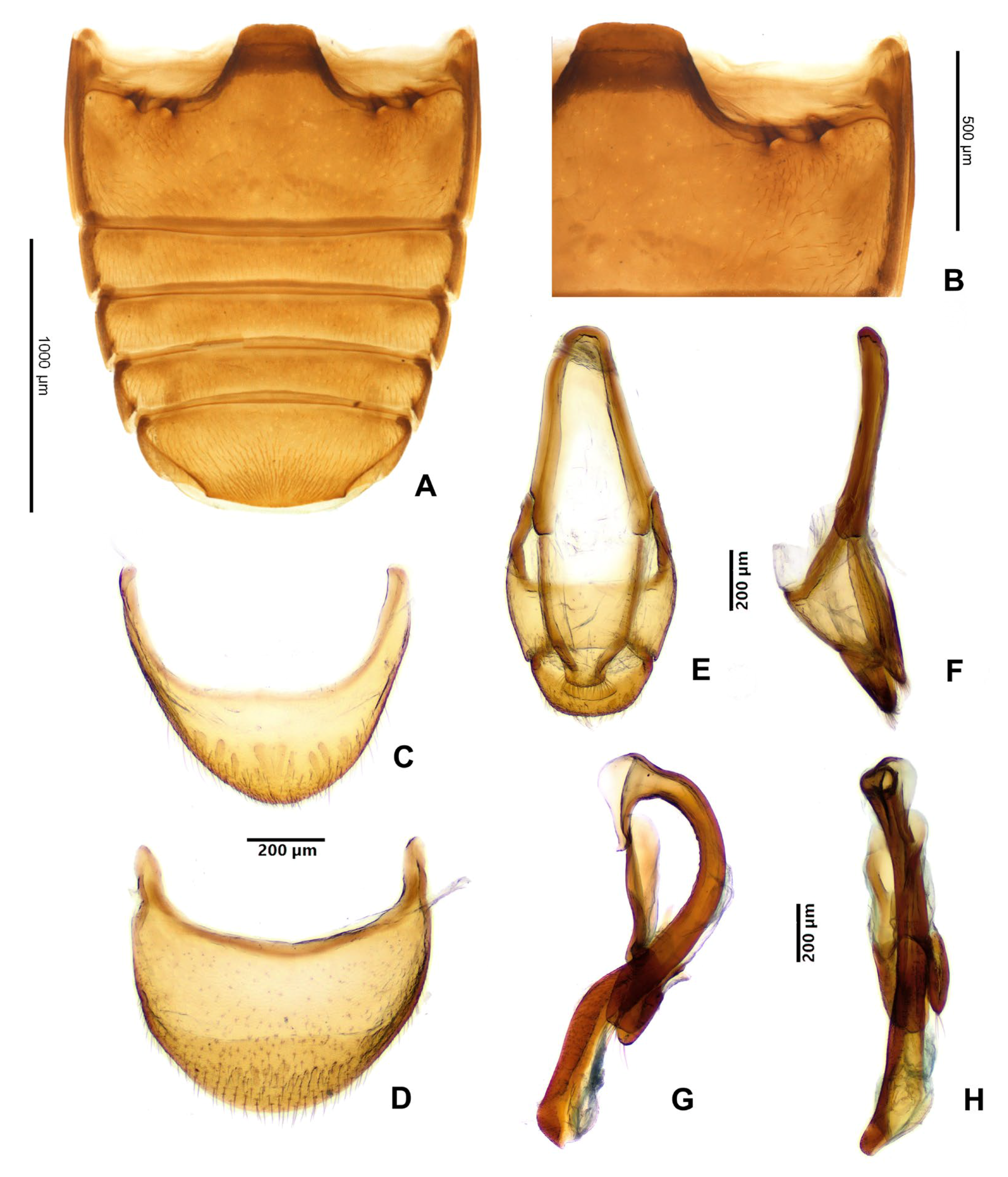
3.2. Taxonomy

4. Discussion
4.1. Taxonomic History of Danascelinae
4.2. Systematics–Current Research and the Present Position
4.3. Biogeography
5. Conclusions
- ▪
- The results of our phylogenetic analysis, indicated unequivocally that the new species described based on the specimen collected in Zhejiang, China constitutes a new monotypic genus belonging in the subfamily Danascelinae and as the most basal lineage sister to (Danascelis + Hadromychus); and the subfamily is recovered and assigned for the first time as a member of ‘merophysine complex’, as sister to (Leiestinae + (Pleganophorinae + Merophysiinae).
- ▪
- Danascelinae is recovered as sister-group to the subfamilies of the ‘merophysiine complex’ of Endomychidae based on characters such as second tarsomere not enclosing the third one and antennal insertions mostly invisible from above.
- ▪
- Hadroscelis sinensis gen. et sp. nov. is distinguished from other Danascelinae based on the type of basal impressions on the pronotum (one excavated pit and one shallow concavity on each side), the number of setose pores on the anterior margin of the metaventrite (four pores on each side), the type of trochantero-femoral attachment (heteromeroid) and the shape of the second tarsomeres (slightly lobed).
- ▪
- The Danascelinae might have split from other endomychids at least as far back as the Cretaceous and the split between the Palaearctic and Nearctic populations of the subfamily could have occurred in the early Cenozoic; and the currently known disjunct distribution might represent a relictual pattern or refugia.
- ▪
- Further research is needed to understand the biogeographic pattern of Danascelinae species and the processes that led to it.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of Morphological Characters Used in Cladistics Analyses
- Head with occipital file: absent (0); present (1).
- Head with antennal grooves: short (0); long (1) absent (2).
- Antennal sockets: well visible from above (0); not visible (mostly hidden) (1)
- Antenna: 4-5/7 (0); 8 (1); 9-10 (2); 11 (3).
- Antennal club: 3-segmented (0); 1 or 2-segmented (1).
- Fronto-clypeal suture: absent (0); present (1)
- Left mandible: with apical and subapical tooth (0); with apical tooth/teeth only (1)
- Right mandible: with apical and subapical tooth (0); with apical tooth only (1)
- Labial palp with palpomere 2: subcylindrical or transverse (0); oval, inflated (1).
- Tentorium with anterior arms: separate (0); meeting medially (1).
- Corpotentorium absent (0); present (1).
- Pronotum with basal and/or lateral sulci/modifications: absent (0); present (1).
- Pronotal base smooth (0); with single modification (pit/pore/concavity) laterally (1); with double modifications on each side (2).
- Prosternal precoxal pits: absent (0); present (1).
- Prosternal process: at least 0.2 times as wide as procoxa (0); very narrow with procoxae nearly contiguous (1).
- Prosternal process: reaching at least hind margin of procoxae (0); short, not prolonged to posterior margin of procoxa (1).
- Mesoventrite with intercoxal process bicarinate (boat-shaped): absent (0); present (1).
- Mesoventral postcoxal pits: absent (0); present (1).
- Mesocoxal cavity: open outwardly (0); narrowly closed outwardly (1)
- Mesotrochantin: at least partially exposed (0); concealed (1).
- Elytral punctation: irregular (0); more or less seriate (1)
- Discrimen: present (0); absent (1)
- Metaventral postcoxal pits (anterior margin of metaventrite, adjacent to posterior margin of mesocoxal cavities): absent (0); one pair present (1); two pairs present (2); three pairs present (3); four pairs present (4).
- Hind wing: well developed (0); reduced or absent (1)
- Tarsal formula: 4-4-4 (0); 3-3-3 (1).
- Penultimate mesotarsomere: not or slightly reduced and not enclosed within lobe of antepenultimate tarsomere (0); highly reduced and partly or entirely enclosed within ventral lobe of antepenultimate tarsomere (1).
- Trochantero-femoral attachment: oblique (0); heteromeroid (1).
- Abdomen: with 5 freely articulated ventrites (0); with ventrite 6 visible (1)
- Abdominal ventrite 1: without postcoxal lines (0); with postcoxal lines (1).
- Sternite (IX) of male genital segment with lateral edges deeply, asymmetrically curved inwardly: absent (0); present (1).
- Tegmen: without penis guide (0); with penis guide (1); tegmen absent (2).
- Ovipositor without infundibulum (0); with infundibulum (1).
- Ovipositor with sperm duct attached: directly to spermatheca (0); to broad connection between spermatheca and accessory gland (1).
- Antennomere 9: simple (0); enlarged, bulbous (1).
- Antennomere 9: without concavities (0); with deep concavity(ies) (1).
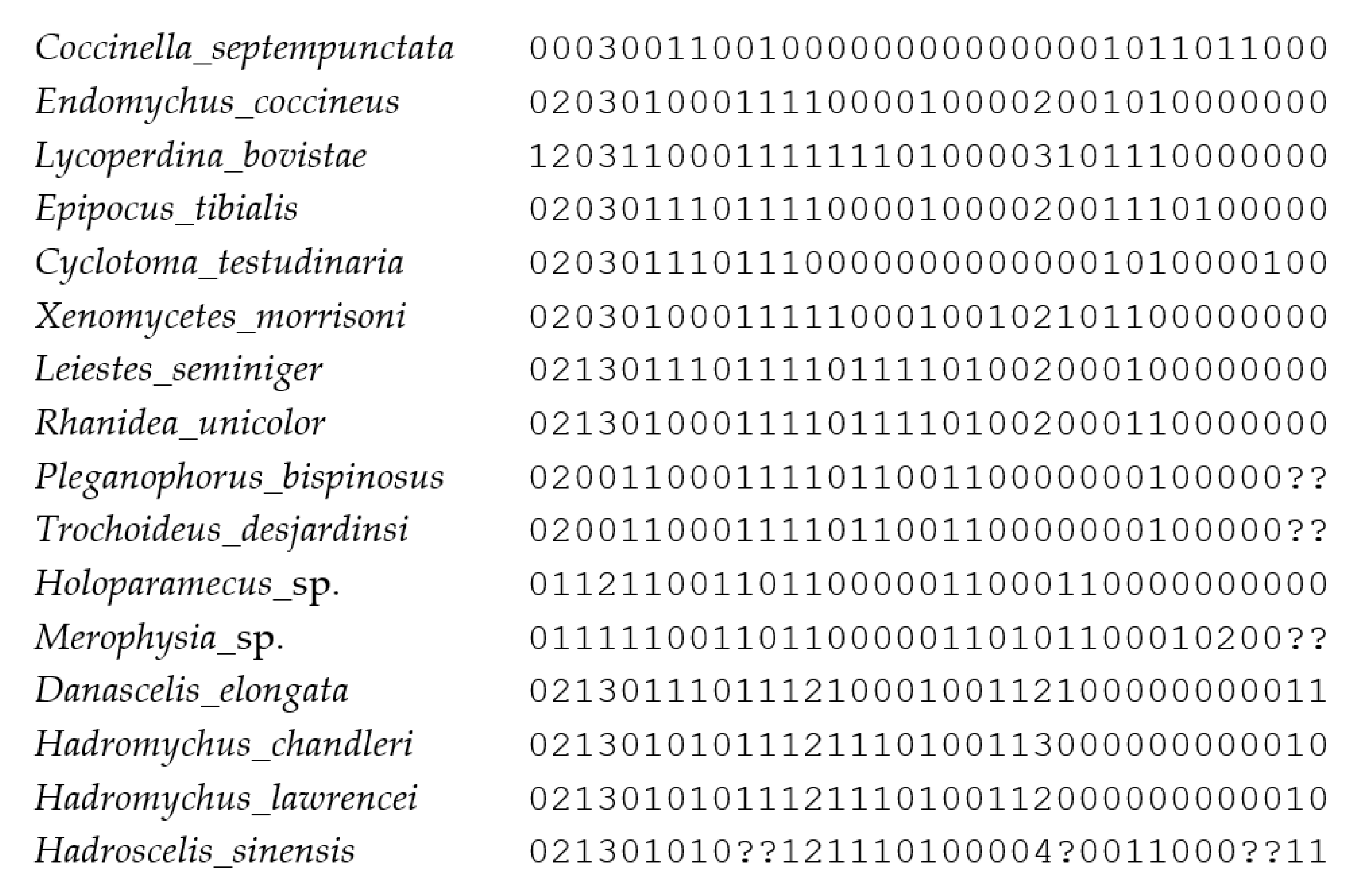
Appendix B. Morphological Matrix Scored for 16 Taxa and 35 Characters Used for Cladistics Analyses in TNT
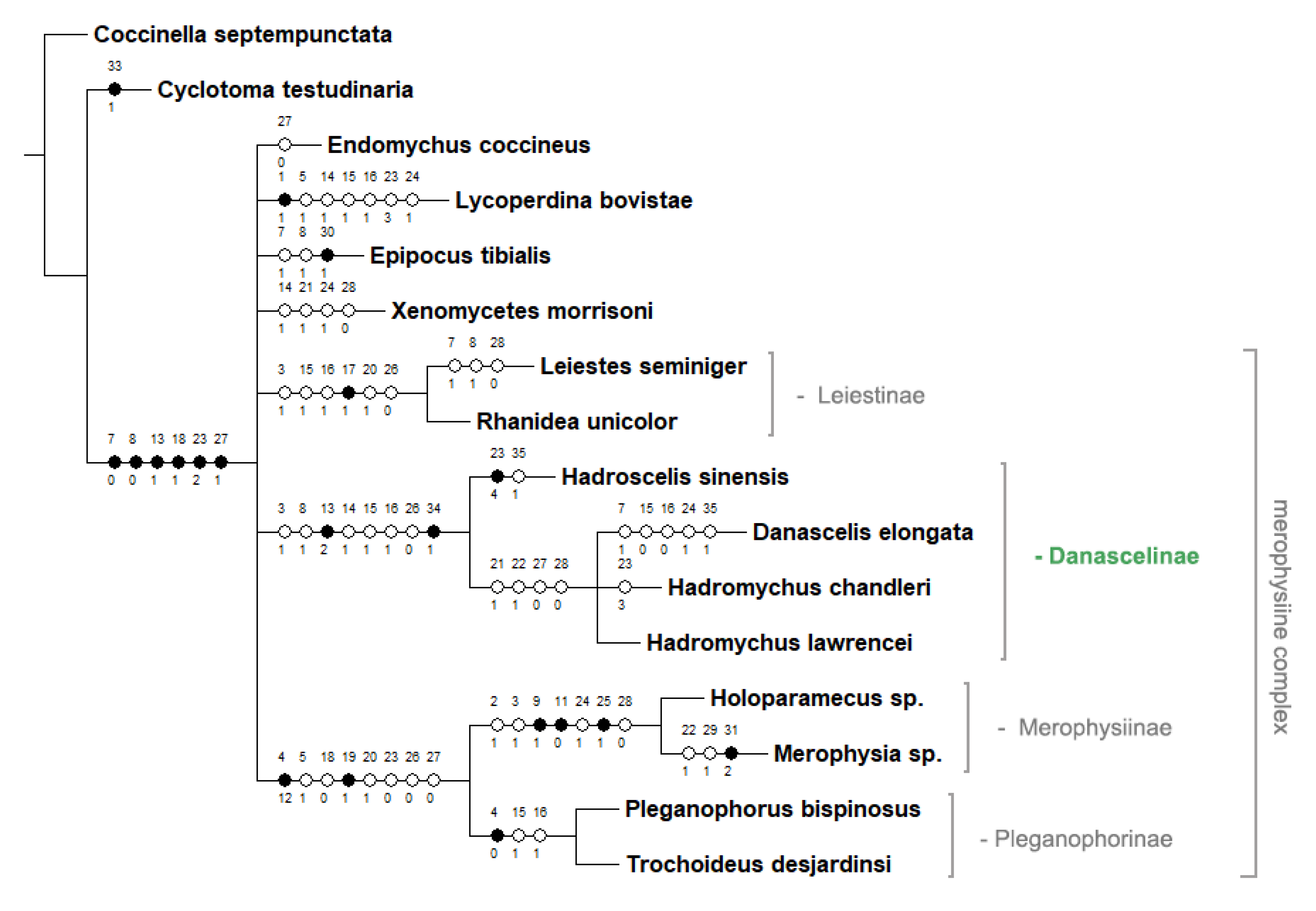
Appendix C. Strict Consensus Tree from 3 Most Parsimonious Trees Calculated in TNT Under Traditional Search Option
References
- Robertson, J.A.; Ślipiński, S.A.; Moulton, M.; Shockley, F.W.; Giorgi, A.; Lord, N.P.; Mckenna, D.D.; Tomaszewska, W.; Forrester, J.; Miller, K.B.; et al. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 2015, 40, 745–778. [Google Scholar] [CrossRef]
- Shockley, F.W.; Tomaszewska, K.W.; McHugh, J.V. An annotated checklist of the handsome fungus beetles of the world (Coleoptera: Cucujoidea: Endomychidae). Zootaxa 2009, 1999, 1–113. [Google Scholar] [CrossRef]
- Tomaszewska, W. Phylogeny and generic classification of the subfamily Lycoperdininae with re-analysis of the family Endomychidae (Coleoptera: Cucujoidea). Ann. Zool. 2005, 55 (Suppl. S1), 1–172. [Google Scholar]
- Tomaszewska, K.W. Larvae of Xenomycetes with description of mature larva of X. morrisoni Horn, 1880 (Coleoptera: Endomychidae). Genus 2004, 15, 163–171. [Google Scholar]
- Tomaszewska, W. Morphology, phylogeny and classification of adult Endomychidae (Coleoptera: Cucujoidea). Ann. Zool. 2000, 50, 449–558. [Google Scholar]
- Tomaszewska, K.W. A new genus and species of Epipocinae (Coleoptera: Endomychidae) from Pakistan. Rev. Suisse Zool. 1999, 106, 277–284. [Google Scholar] [CrossRef]
- Bousquet, Y.; Leschen, R.A.B. Description of a new genus and species of Endomychidae (Coleoptera: Cucujoidea) from Northeastern North America. Coleopt. Bull. 2002, 56, 291–298. [Google Scholar] [CrossRef]
- Arriaga-Varela, E.; Szawaryn, K.; Ivie, M.A.; Tomaszewska, W. The Nearctic Danascelinae (Coleoptera: Endomychidae) with description of a new species from Western North America. Ann. Zool. 2025, 75, 369–380. [Google Scholar] [CrossRef]
- Tomaszewska, W. Endomychidae Leach, 1815. In Handbook of Zoology, Vol. 2, Coleoptera; Leschen, R.A.B., Beutel, R.G., Lawrence, J.F., Eds.; Walter de Gruyter GmbH: Berlin, Germany; Co. KG: New York, NY, USA, 2010; pp. 442–454. [Google Scholar]
- Lawrence, J.F.; Ślipiński, A.; Seago, A.; Thayer, M.; Newton, A.; Marvaldi, A. Phylogeny of the Coleoptera based on adult and larval morphology. Ann. Zool. 2011, 61, 1–217. [Google Scholar] [CrossRef]
- Tomaszewska, W.; Szawaryn, K.; Arriaga-Varela, E. First Member of ‘Higher Endomychidae’ (Coleoptera: Coccinelloidea) from the Mid-Cretaceous Amber of Myanmar and New Insights into the Time of Origin of the Handsome Fungus Beetles. Insects 2022, 13, 690. [Google Scholar] [CrossRef]
- Tomaszewska, W.; Szawaryn, K.; Arriaga-Varela, E. ‘Where is my family?’ Molecular and morphological data reveal the phylogenetic position and diversity of the enigmatic handsome fungus beetle genus Anamycetaea Strohecker, 1975 (Coleoptera, Coccinelloidea). Invert. Syst. 2023, 37, 231–253. [Google Scholar] [CrossRef]
- Arriaga-Varela, E.; Tomaszewska, W.; Szawaryn, K.; Robertson, J.; Seidel, M.; Ślipiński, A.; Fikáček, M. The resurrection of Cerasommatidiidae, an enigmatic group of coccinelloid beetles (Coleoptera: Coccinelloidea) based on molecular and morphological evidence. Zool. J. Linn. Soc. 2023, 197, 1078–1115. [Google Scholar] [CrossRef]
- Arriaga-Varela, E.; Szawaryn, K.; Zhou, Y.-L.; Bruthansova, J.; Li, Y.-D.; Tomaszewska, W. Early evolution of Anamorphidae (Coleoptera: Coccinelloidea): The oldest known anamorphid beetles from Upper Cretaceous amber of northern Myanmar and the first report of potential glandular pores in the family. Cladistics 2024, 40, 411–429. [Google Scholar] [CrossRef]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- Nixon, K.C. WinClada; Ithaca: New York, NY, USA, 2002. [Google Scholar]
- Toussaint, E.A.F.; Seidel, M.; Arriaga-Varela, E.; Hájek, J.; Král, D.; Sekerka, L.; Short, A.E.Z.; Fikáček, M. The peril of dating beetles. Syst. Entomol. 2017, 42, 1–10. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Che, L.H.; Li, Y.; Liang, D.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 1e11. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tihelka, E.; Giacomelli, M.; Lawrence, J.F.; Ślipiński, A.; Kundrata, R.; Yamamoto, S.; Thayer, M.K.; Newton, A.F.; Leschen, R.A.B.; et al. Integrated phylogenomics and fossil data illuminate the evolution of beetles. R. Soc. Open Sci. 2022, 9, 211771. [Google Scholar] [CrossRef]
- Hsiao, Y.; Tomaszewska, W. Diversity dynamics of handsome fungus beetles (Coleoptera: Coccinelloidea: Endomychidae) during the Meso-Cenozoic period, with description of the second species of ‘Higher Endomychidae’ from the mid-Cretaceous amber of northern Myanmar. PalZ 2025, 99, 139–150. [Google Scholar] [CrossRef]
- Alekseev, V.; Tomaszewska, W. A new handsome fungus beetle of the subfamily Endomychinae (Coccinelloidea: Endomychidae) from Baltic amber of the Sambian Peninsula. Zootaxa 2025, 5692, 577–584. [Google Scholar] [CrossRef]
- Shockley, F.W.; Alekseev, V.I. Glesirhanis bercioi, a new genus and species from Baltic amber (Coleoptera: Endomychidae: Leiestinae) with a checklist and nomenclatural notes regarding fossil Endomychidae. Zootaxa 2014, 3755, 391–400. [Google Scholar] [CrossRef]
- Alekseev, V.I.; Tomaszewska, W. New handsome fungus beetles (Coleoptera: Coccinelloidea: Anamorphidae, Endomychidae) from European amber of the upper Eocene. Palaeontol. Electron. 2018, 21.1.6A, 1–23. [Google Scholar] [CrossRef]
- Tomaszewska, W.; Ślipiński, A.; Bai, M.; Zhang, W.; Ren, D. The oldest representatives of Endomychidae (Coleoptera: Coccinelloidea) from the Cretaceous Burmese amber. Cretac. Res. 2018, 91, 287–298. [Google Scholar] [CrossRef]
- Reike, H.P.; Alekseev, V.I.; Gröhn, C.; Arlt, T.; Manke, I. First extinct species of the genus Holoparamecus (Coleoptera: Merophysiidae: Holoparamecinae) from Eocene amber deposits. Stud. Rep.-Taxon. Ser. 2020, 16, 241–255. [Google Scholar]
- Li, Y.D.; Tomaszewska, W.; Huang, D.Y.; Cai, C.Y. Rhomeocalpsua torosa gen. et sp. nov., a unique lineage of Endomychidae from mid-Cretaceous Burmese amber (Coleoptera: Coccinelloidea). Palaeoentomol. 2022, 5, 146–154. [Google Scholar] [CrossRef]
- Li, Y.-D.; Huang, D.-Y.; Cai, C.-Y. A new species of Burmalestes Tomaszewska & Ślipiński from mid-Cretaceous Kachin amber (Coleoptera: Endomychidae). Zootaxa 2023, 5396, 105–111. [Google Scholar] [CrossRef]
- Arriaga-Varela, E.; Szawaryn, K.; Shaw, J.J.; Bai, M.; Ren, D.; Tomaszewska, W. Remarkable diversity of the handsome fungus beetles genus Cretaparamecus (Coleoptera: Endomychidae: Merophysiinae) from mid-Cretaceous amber of northern Myanmar. Cretac. Res. 2023, 151, 105664. [Google Scholar] [CrossRef]
- McIntyre, S.R.N.; Lineweaver, C.H.; Groves, C.P.; Chopra, A. Global biogeography since Pangaea. Proc. R. Soc. B 2017, 284, 20170716. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, W.; Arriaga-Varela, E. Discovering the Diversity and Evolution of Danascelinae: A New Genus and Species from Eastern Asia and Insights into the Phylogenetic Placement of This Subfamily in Endomychidae (Coleoptera). Insects 2025, 16, 1178. https://doi.org/10.3390/insects16111178
Tomaszewska W, Arriaga-Varela E. Discovering the Diversity and Evolution of Danascelinae: A New Genus and Species from Eastern Asia and Insights into the Phylogenetic Placement of This Subfamily in Endomychidae (Coleoptera). Insects. 2025; 16(11):1178. https://doi.org/10.3390/insects16111178
Chicago/Turabian StyleTomaszewska, Wioletta, and Emmanuel Arriaga-Varela. 2025. "Discovering the Diversity and Evolution of Danascelinae: A New Genus and Species from Eastern Asia and Insights into the Phylogenetic Placement of This Subfamily in Endomychidae (Coleoptera)" Insects 16, no. 11: 1178. https://doi.org/10.3390/insects16111178
APA StyleTomaszewska, W., & Arriaga-Varela, E. (2025). Discovering the Diversity and Evolution of Danascelinae: A New Genus and Species from Eastern Asia and Insights into the Phylogenetic Placement of This Subfamily in Endomychidae (Coleoptera). Insects, 16(11), 1178. https://doi.org/10.3390/insects16111178





