Genetic Characterization and Mating Disruption in Spodoptera Species, a Case Study on Spodoptera frugiperda (Lepidoptera, Noctuidae): A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Search Strategy
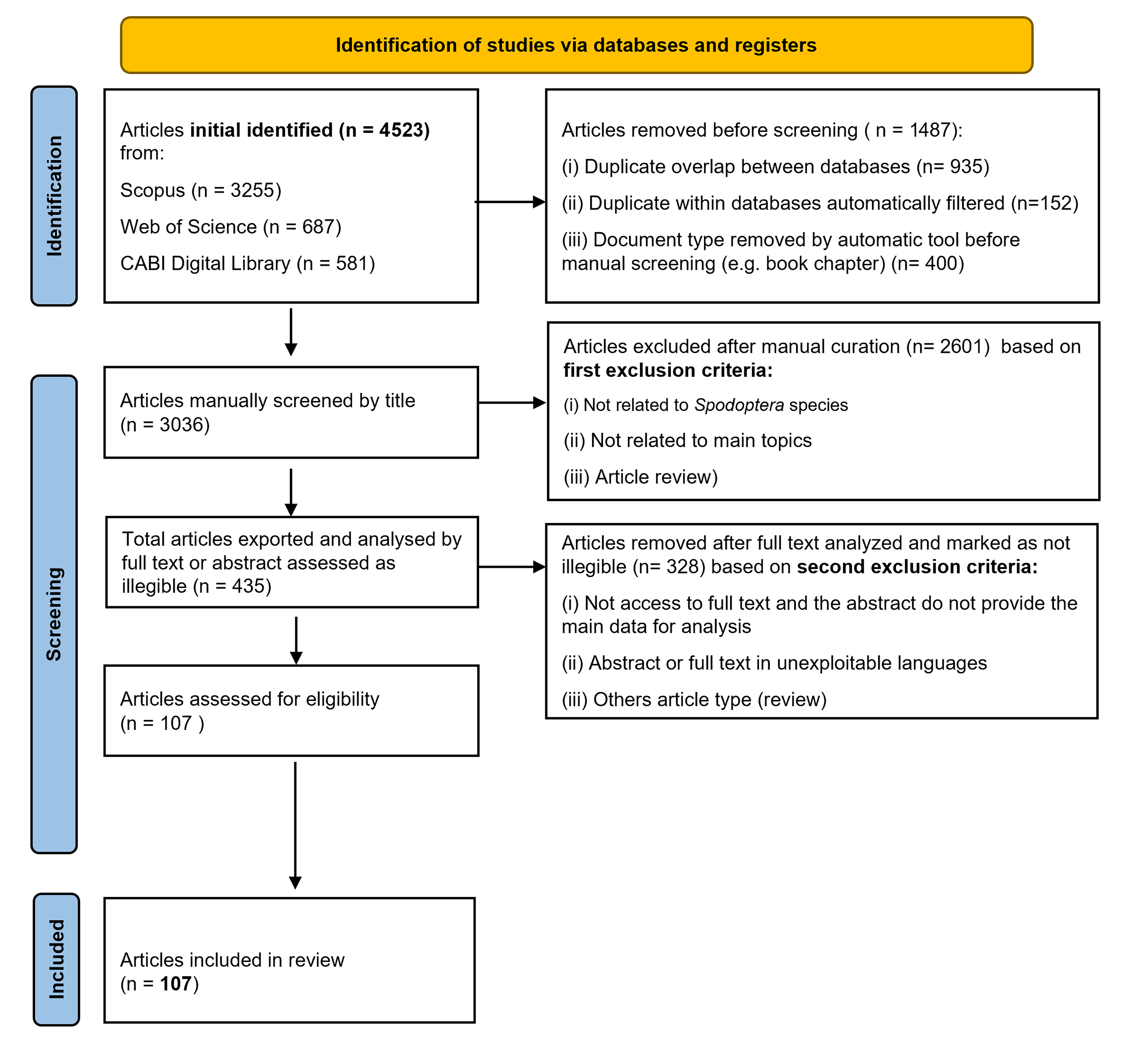
2.2. Screening and Data Curation Workflow: Inclusion and Exclusion Criteria of Relevant Studies
2.3. Data Extraction and Analysis
3. Results
3.1. Characterization of Included Sources
3.1.1. Number of Studies Identified and Included in This Review
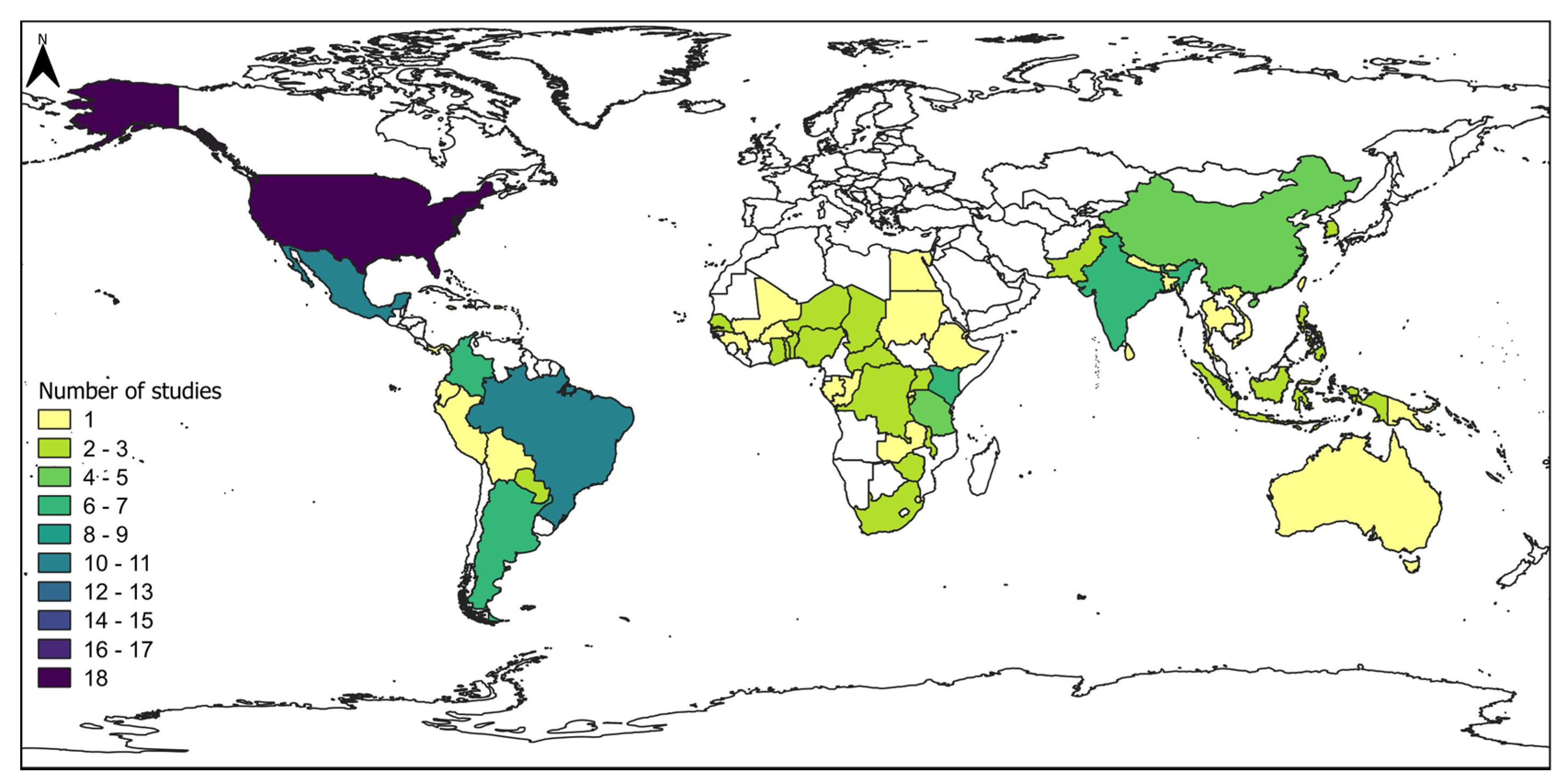
3.1.2. Geographical and Temporal Distribution of Studies
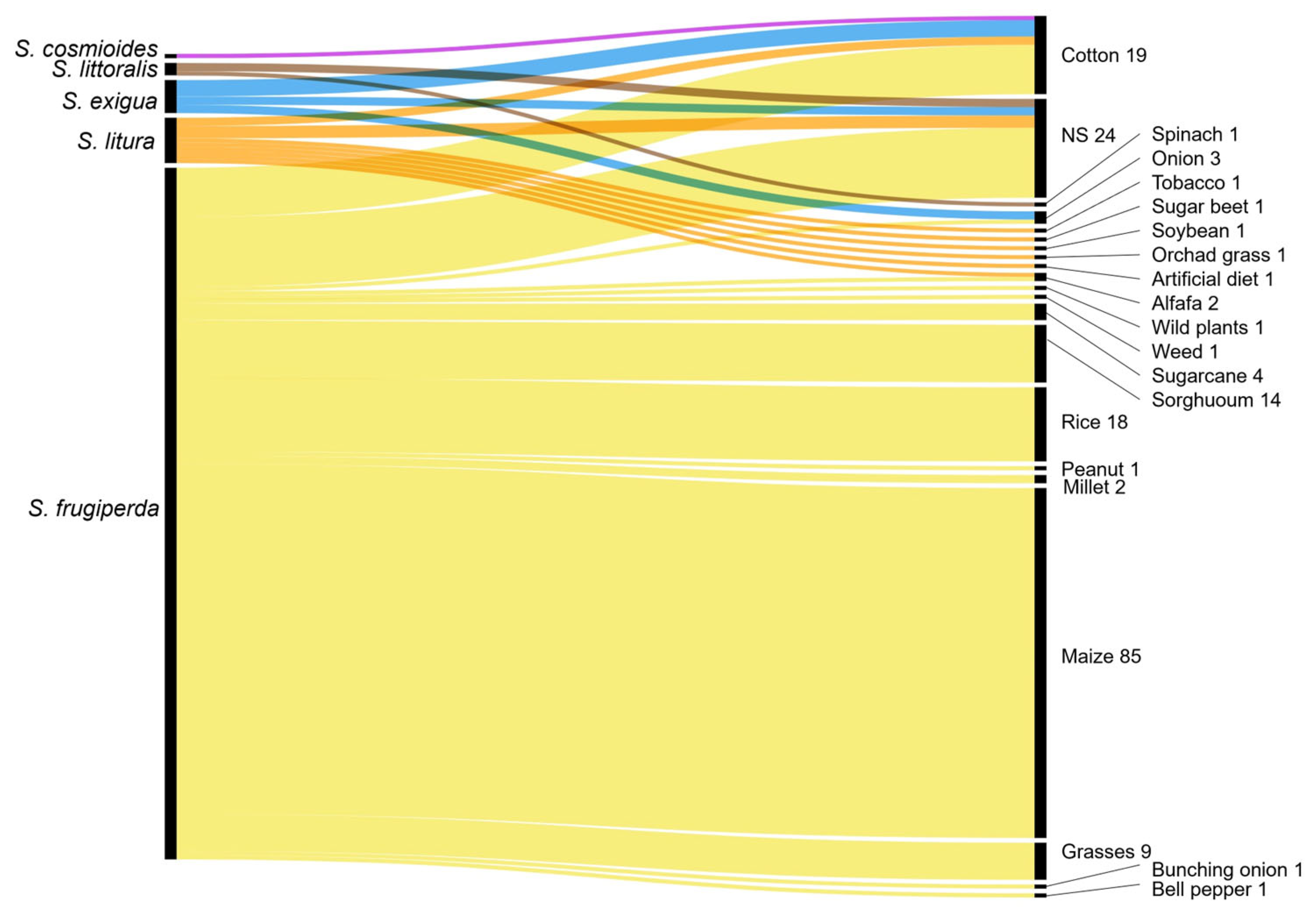
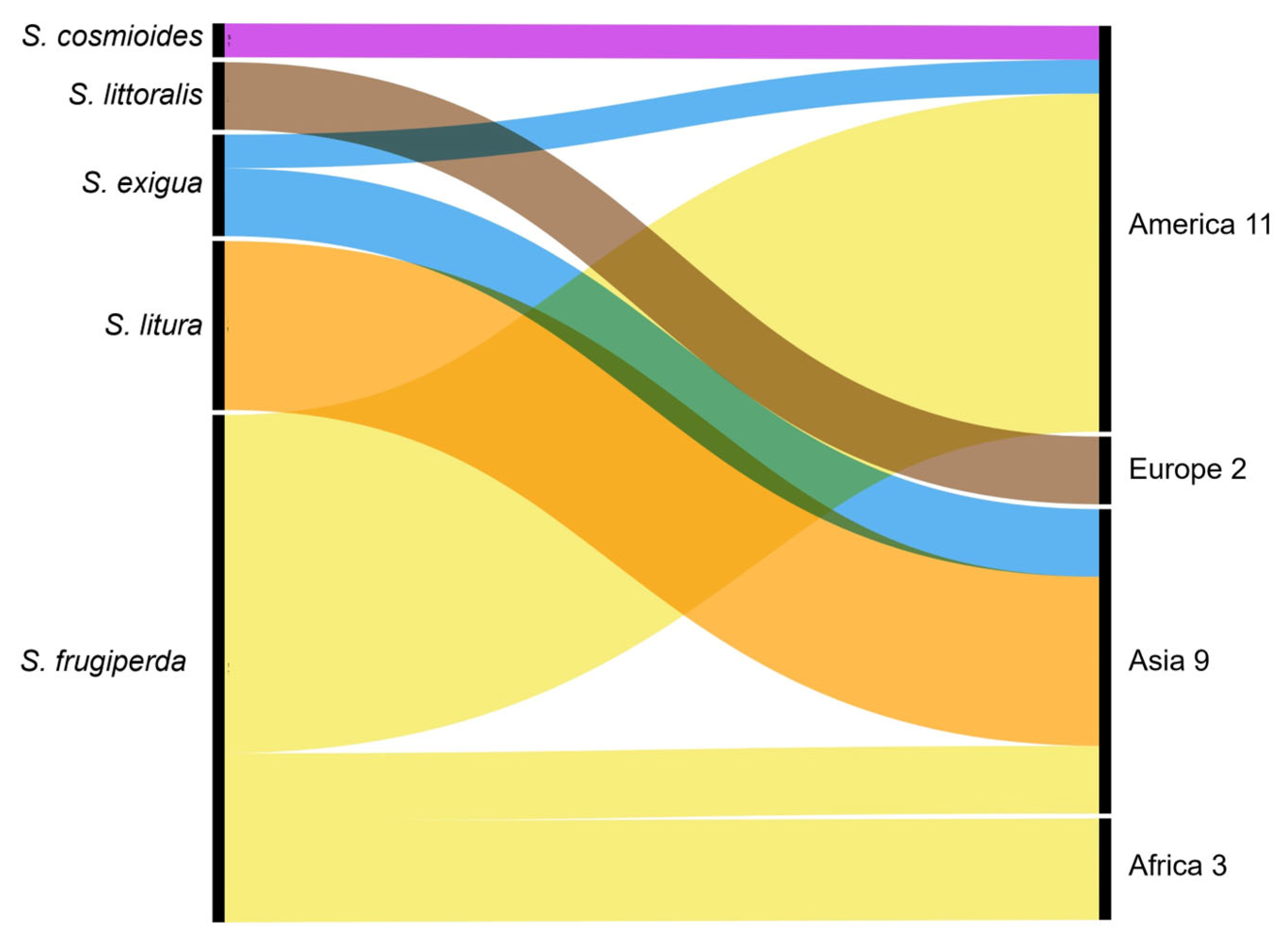
3.1.3. Spodoptera Species and Associated Host Crops
3.2. Data Analysis on Genetic Characterization and Pheromone-Based Mating Disruption in Spodoptera Species
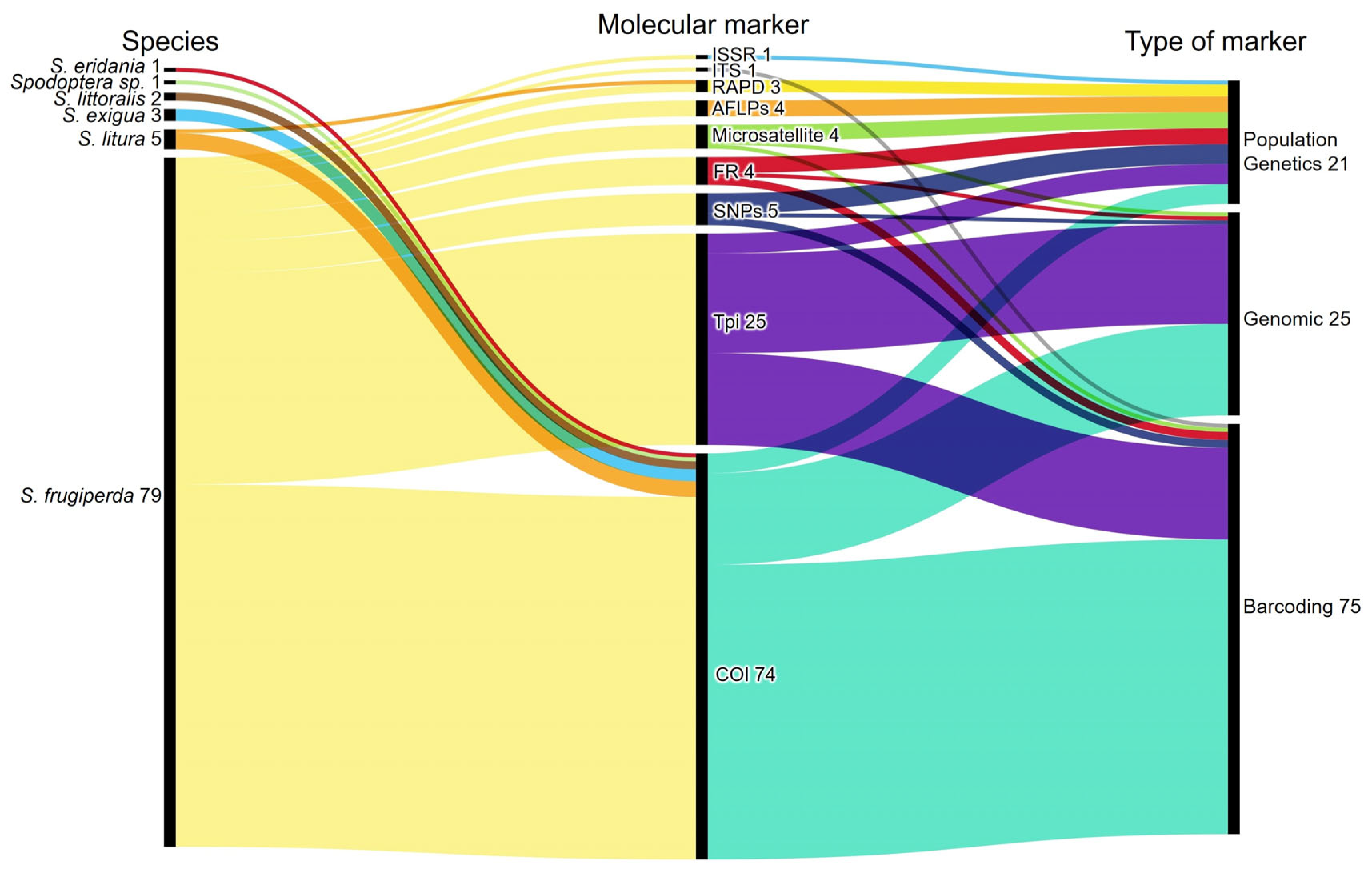
3.2.1. Genetic Characterization and Molecular Methodology Employed
3.2.2. Pheromone-Based Mating Disruption Strategies in Spodoptera Species
3.2.3. Effectiveness and Impact of Mating Disruption Method on Spodoptera Species
3.2.4. Impact of Genetic Variability, Pheromone Geographical Origin, and Composition on Mating Disruption Effectiveness
4. Discussion
4.1. Genetic Characterization of Spodoptera Frugiperda: Which Approach Should Be Used for Accurate Identification?
4.2. Pheromone-Based Control Strategies: Advances and Challenges for Mating Disruption Effectiveness for Spodoptera frugiperda
4.3. Integration of Genetic Studies with Pheromone-Based Approaches: Challenges for Spodoptera frugiperda Management
4.4. Gaps and Future Directions in Mating Disruption Research for Spodoptera frugiperda
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| COI | Cytochrome c Oxidase Subunit I |
| TPi | Triosephosphate Isomerase |
| SNPs | Single-Nucleotide Polymorphisms |
| SSRs | Simple Sequence Repeats |
| AFLPs | Amplified Fragment Length Polymorphisms |
| RAPD | Random Amplified Polymorphic DNA |
References
- Meagher, R.L.; Brambila, J.; Hung, E. Monitoring for exotic Spodoptera species (Lepidoptera: Noctuidae) in Florida. Fl. Entomol. 2008, 91, 517–522. [Google Scholar] [CrossRef]
- Van De Vossenberg, B.T.L.H.; Van Der Straten, M.J. Development and validation of real-time PCR tests for the identification of four Spodoptera species: S. eridania, S. frugiperda, S. littoralis, and S. litura (Lepidoptera: Noctuidae). J. Econ. Entomol. 2014, 107, 1643–1654. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. Scientific Review of the Impact of Climate Change on Plant Pests; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Q.-L.; Zhang, H.-W.; Wu, K.-M. Spread of invasive migratory pest Spodoptera frugiperda and management practices throughout China. J. Integr. Agric. 2021, 20, 637–645. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Carnovale, D.; Ekesi, S.; Day, R.; Umina, P.A.; Meagher, R.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Tambo, J.A.; Kansiime, M.K.; Mugambi, I.; Agboyi, L.K.; Beseh, P.K.; Day, R. Economic impacts and management of fall armyworm (Spodoptera frugiperda) in smallholder agriculture: A panel data analysis for Ghana. CABI Agric. Biosci. 2023, 4, 38. [Google Scholar] [CrossRef]
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall Armyworm: Impacts and Implications for Africa Evidence Note Update, October 2018; CABI: Wallingford, UK, 2018; Available online: https://www.invasive-species.org/wp-content/uploads/sites/2/2019/02/FAW-Evidence-Note-October-2018.pdf (accessed on 14 October 2024).
- FAO, Food and Agriculture Organization of the United Nations. The Global Action for Fall Armyworm Control: A Resource Mobilization Guide; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Du Plessis, H.; Van Den Berg, J.; Ota, N.; Kriticos, D.J. Spodoptera frugiperda. In Pest Geography; CSIRO-InSTePP: Canberra, Australia; Durham, NC, USA, 2019; pp. 1–7. [Google Scholar]
- Huang, Y.; Dong, Y.; Huang, W.; Ren, B.; Deng, Q.; Shi, Y.; Bai, J.; Ren, Y.; Geng, Y.; Ma, H. Overwintering distribution of fall armyworm (Spodoptera frugiperda) in Yunnan, China, and influencing environmental factors. Insects 2020, 11, 805. [Google Scholar] [CrossRef]
- CABI. Fall Armyworm Portal. CABI Compendium Invasive Species. 2019. Available online: https://www.cabidigitallibrary.org/product/qi/portal/fallarmyworm (accessed on 16 September 2024).
- Zhang, L.; Liu, B.; Zheng, W.; Liu, C.; Zhang, D.; Zhao, S.; Li, Z.; Xu, P.; Wilson, K.; Withers, A.; et al. Genetic structure and insecticide resistance characteristics of fall armyworm populations invading China. Mol. Ecol. Resour. 2020, 20, 1682–1696. [Google Scholar] [CrossRef]
- Van den Berg, J.; Du Plessis, H. Chemical control and insecticide resistance in Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Econ. Entomol. 2022, 115, 1761–1771. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Huang, J.-M.; Ni, H.; Guo, D.; Yang, F.-X.; Wang, X.; Wu, S.-F.; Gao, C.-F. Susceptibility of fall armyworm, Spodoptera frugiperda (JE Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol. 2020, 168, 104623. [Google Scholar] [CrossRef]
- Kumar, R.M.; Gadratagi, B.-G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Paredes-Sánchez, F.A.; Rivera, G.; Bocanegra-García, V.; Martínez-Padrón, H.Y.; Berrones-Morales, M.; Niño-García, N.; Herrera-Mayorga, V. Advances in control strategies against Spodoptera frugiperda. A review. Molecules 2021, 26, 5587. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Lucchi, A.; Thomson, D.; Ioriatti, C. Sex pheromone aerosol devices for mating disruption: Challenges for a brighter future. Insects 2019, 10, 308. [Google Scholar] [CrossRef]
- Holkenbrink, C.; Ding, B.-J.; Wang, H.-L.; Dam, M.I.; Petkevicius, K.; Kildegaard, K.R.; Wenning, L.; Sinkwitz, C.; Lorántfy, B.; Koutsoumpeli, E.; et al. Production of moth sex pheromones for pest control by yeast fermentation. Metab. Eng. 2020, 62, 312–321. [Google Scholar] [CrossRef]
- Mevada, R.R.; Sisodiya, D.B.; Parmarand, R.G.; Prajapati, D.R. Mating disruption: An ecological step towards sustainable pest management. J. Eco-Friendly Agric. 2023, 18, 144–150. [Google Scholar] [CrossRef]
- Muthukumar, M.; Kennedy, J.S. A review of the scientific literature on the use of reproductive pheromones in the management of Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Entomol. Sci. 2021, 56, 475–486. [Google Scholar] [CrossRef]
- Cruz-Esteban, S.; Rojas, J.C.; Malo, E.A. A pheromone lure for catching fall armyworm males (Lepidoptera: Noctuidae) in Mexico. Acta Zool. Mex. 2020, 36, 1–11. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. The Spodoptera frugiperda host strains: What they are and why they matter for understanding and controlling this global agricultural pest. J. Econ. Entomol. 2022, 115, 1729–1743. [Google Scholar] [CrossRef]
- Unbehend, M.; Hänniger, S.; Meagher, R.L.; Heckel, D.G.; Groot, A.T. Pheromonal divergence between two strains of Spodoptera frugiperda. J. Chem. Ecol. 2013, 39, 364–376. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L.; Nuessly, G.; Hall, D.G. Effects of fall armyworm (Lepidoptera: Noctuidae) interstrain mating in wild populations. Environ. Entomol. 2006, 35, 561–568. [Google Scholar] [CrossRef]
- Pashley, D.P. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae). A sibling species complex? Ann. Entomol. Soc. Am. 1986, 79, 898–904. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Gabriela Murúa, M.; Hay-Roe, M.; Juárez, L.M.; Willink, E.; Meagher, R.L. Genetic characterization of fall armyworm (Lepidoptera: Noctuidae) host strains in Argentina. J. Econ. Entomol. 2012, 105, 418–428. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, F.; Zhang, D.; Zhang, S.; Yu, X.; Yang, M. Genetic diversity and fine-scale genetic structure of Spodoptera litura Fabricius (Lepidoptera: Noctuidae) in Southern China based on microsatellite markers. Animals 2023, 13, 560. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, M.-M.; Chen, C.; Wang, X.-Q. Genetic variation and phylogeographic structure of Spodoptera exigua in western China based on mitochondrial DNA and microsatellite markers. PLoS ONE 2020, 15, e0233133. [Google Scholar] [CrossRef]
- Tojo, S.; Hayakawa, Y.; Phaophan, P. Strains in the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae) with differing host ranges. Appl. Entomol. Zool. 2008, 43, 491–496. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 21954. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Koffi, D.; Agboka, K.; Tounou, K.A.; Banerjee, R.; Jurat-Fuentes, J.L.; Meagher, R.L. Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. PLoS ONE 2017, 12, e0181982. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Koffi, D.; Agboka, K.; Adjevi, A.K.M.; Du Plessis, H.; Berg, J.V.D.; Tepa-Yotto, G.T.; Winsou, J.K.; Meagher, R.L.; et al. Genetic studies of fall armyworm indicate a new introduction into Africa and identify limits to its migratory behavior. Sci. Rep. 2022, 12, 1941. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.; Catarino, S.; Mexia, A.; Borges da Silva, E.; Monteiro, F. List of articles retrieved from PRISMA guidelines, synthesizes and current knowledge on genetic characterization and pheromone-based control methods for Spodoptera species, with a particular focus on Spodoptera frugiperda. Figshare Dataset 2025. [Google Scholar] [CrossRef]
- Mendeley Reference Manager Desktop (v. 2.112.2); Elsevier: Amsterdam, The Netherlands, 2024; Available online: https://www.mendeley.com (accessed on 18 December 2024).
- Microsoft Corporation. Microsoft Excel (v.2403); Microsoft Corporation: Redmond, WA, USA, 2018; Available online: https://office.microsoft.com/excel (accessed on 10 March 2025).
- QGIS Development Team. QGIS Geographic Information System. Open-Source Geospatial Foundation Project. 2024. Available online: http://qgis.osgeo.org (accessed on 25 March 2025).
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Aazzi, M. RAWGraphs: A visualisation platform to create open outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI, Conference CHITALY, Cagliari, Italy, 18–20 September 2017; Association for Computing Machinery: New York, NY, USA, 2017; Volume 28, pp. 1–5. [Google Scholar] [CrossRef]
- Meagher, R.L.; Nagoshi, R.N. Attraction of fall armyworm males (Lepidoptera: Noctuidae) to host strain females. Environ. Entomol. 2013, 42, 751–757. [Google Scholar] [CrossRef][Green Version]
- Saldamando-Benjumea, C.I.; Estrada-Piedrahíta, K.; Velásquez-Vélez, M.I.; Bailey, R.I. Assortative mating and lack of temporality between corn and rice strains of Spodoptera frugiperda (Lepidoptera: Noctuidae) from Central Colombia. J. Insect Behav. 2014, 27, 555–566. [Google Scholar] [CrossRef]
- Haenniger, S.; Goergen, G.; Akinbuluma, M.D.; Kunert, M.; Heckel, D.G.; Unbehend, M. Sexual communication of Spodoptera frugiperda from West Africa: Adaptation of an invasive species and implications for pest management. Sci. Rep. 2020, 10, 2892. [Google Scholar] [CrossRef]
- Rupali, J.S.; Ramya, N.; Sagar, D.; Padala, V.K.; Madhuri, E.V.; Subramanian, S. Reproductive behaviour in different aged adults of fall armyworm, Spodoptera frugiperda (J. E. Smith). Curr. Sci. 2023, 125, 309–316. [Google Scholar] [CrossRef]
- Tabata, J.; Nakano, R.; Yasui, H.; Nakamura, K.; Takehara, K.; Matsuda, H.; Ikenoue, Y.; Kusuhata, Y.; Kinjo, K.; Nakama, K.; et al. Sex pheromone of the fall armyworm, Spodoptera frugiperda: Identification of a trace component that enhances attractiveness and specificity. Entomol. Exp. Appl. 2023, 171, 535–541. [Google Scholar] [CrossRef]
- Sisay, B.; Tamiru, A.; Subramanian, S.; Weldon, C.W.; Khamis, F.; Green, K.K.; Anderson, P.; Torto, B. Pheromonal variation and mating between two mitotypes of fall armyworm (Spodoptera frugiperda) in Africa. Sci. Rep. 2024, 14, 3848. [Google Scholar] [CrossRef]
- Akinbuluma, M.D.; Van Schaijk, R.A.H.; Roessingh, P.; Groot, A.T. Region-specific variation in the electrophysiological responses of Spodoptera frugiperda (Lepidoptera: Noctuidae) to synthetic sex pheromone compounds. J. Chem. Ecol. 2024, 50, 631–642. [Google Scholar] [CrossRef]
- Patil, S.; Nayyar, N.; Gracy, G.; Patil, J.; Kesavan, S.; Gopalsamy, S.; Aravindram, K.; Rajagopal, R.; Gopal, A.; Munikrishnappa, V.K.T.; et al. Biological characterization of the predominant strains of fall armyworm in India with regards to biocontrol agents and pheromone. Curr. Sci. 2024, 127, 475–482. [Google Scholar] [CrossRef]
- Rojas, J.C.; Roblero, E.; Malò, E.A. Assessment of meso-dispensers for mating disruption of fall armyworm in maize. Int. J. Pest Manag. 2024, 19, 1–11. [Google Scholar] [CrossRef]
- Tumlinson, J.H.; Mitchell, E.R.; Teal, P.E.A.; Heath, R.R.; Mengelkoch, L.J. Sex pheromone of fall armyworm, Spodoptera frugiperda (J.E. Smith)—Identification of components critical to attraction in the field. J. Chem. Ecol. 1986, 12, 1909–1926. [Google Scholar] [CrossRef]
- Unbehend, M.; Hänniger, S.; Vásquez, G.M.; Juárez, M.L.; Reisig, D.; McNeil, J.N.; Meagher, R.L.; Jenkins, D.A.; Heckel, D.G.; Groot, A.T. Geographic variation in sexual attraction of Spodoptera frugiperda corn- and rice-strain males to pheromone lures. PLoS ONE 2014, 9, e89255. [Google Scholar] [CrossRef] [PubMed]
- Blassioli-Moraes, M.C.; Borges, M.; Viana, A.R.; Laumann, R.A.; Miranda, J.E.; Magalhães, D.M.; Birkett, M.A. Identification and field evaluation of the sex pheromone of a Brazilian population of Spodoptera cosmioides. Pesqui. Agropecu. Bras. 2016, 51, 545–554. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Mayer, M.S. Spodoptera exigua: Mating disruption, measurement of airborne concentration of pheromone, and use of specialist receptor cell responses for comparison to female pheromone emission. J. Environ. Sci. Health B 2001, 36, 467–488. [Google Scholar] [CrossRef]
- Wakamura, S.; Kozai, S.; Inoue, H.; Takai, M.; Yamashita, I.; Kawahara, S.; Kawamur, M. Control of the beet armyworm, Spodoptera exigua (Huebner) (Lepidoptera: Noctuidae), using synthetic sex pheromone. I. Effect of communication disruption in welsh onion fields. Appl. Entomol. Zool. 1989, 24, 387–397. [Google Scholar] [CrossRef]
- Wakamura, S.; Takai, N. Communication disruption for control of beet armyworm, Spodoptera exigua (Hubner), with synthetic sex pheromone. JARQ 1995, 29, 125–130. Available online: https://www.jircas.go.jp/sites/default/files/publication/jarq/29-2-125-130_0.pdf (accessed on 4 December 2024).
- Lanzoni, A.; Bazzocchi, G.G.; Reggiori, F.; Rama, F.; Sannino, L.; Maini, S.; Burgio, G. Spodoptera littoralis male capture suppression in processing spinach using two kinds of synthetic sex-pheromone dispensers. Bull. Insectol. 2012, 65, 311–318. [Google Scholar]
- Chen, Y.; Chen, X.; Chen, Y.; Wei, H.; Lin, S.; Tian, H.; Lin, T. Preparation, characterisation, and controlled release of sex pheromone-loaded MPEG-PCL diblock copolymer micelles for Spodoptera litura (Lepidoptera: Noctuidae). PLoS ONE 2018, 13, e0203062. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K. A functional difference of the individual components of Spodoptera litura (F) (Lepidoptera, Noctuidae) sex-pheromone in the attraction of flying male moths. Appl. Entomol. Zool. 1981, 16, 63–70. [Google Scholar] [CrossRef]
- Kawasaki, K.; Miyashita, K. Mating suppression by individual components of sex-pheromone of Spodoptera litura F (Lepidoptera-Noctuidae) under field conditions. Appl. Entomol. Zool. 1976, 11, 320–326. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.-L.; Van den Berg, J. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Levy, H.C.; Garcia-Maruniak, A.; Maruniak, J.E. Strain identification of Spodoptera frugiperda (Lepidoptera: Noctuidae) Insects and cell line: PCR-RFLP of Cytochrome Oxidase C subunit I gene. Fl. Entomol. 2002, 85, 186–190. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Brambila, J.; Meagher, R.L. Use of DNA barcodes to identify invasive armyworm Spodoptera species in Florida. J. Insect Sci. 2011, 11, 154. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef]
- Nagoshi, R.N. Observations of genetic differentiation between the fall armyworm host strains. PLoS ONE 2022, 7, e0277510. [Google Scholar] [CrossRef]
- Fleischer, S.J.; Harding, C.L.; Blom, P.E.; White, J.; Grehan, J. Spodoptera frugiperda pheromone lures to avoid nontarget captures of Leucania phragmatidicola. J. Econ. Entomol. 2005, 98, 66–71. [Google Scholar] [CrossRef]
- Andrade, R.; Rodriguez, C.; Oehlschlager, A.C. Optimization of a pheromone lure for Spodoptera frugiperda (Smith) in Central America. J. Braz. Chem. Soc. 2000, 11, 609–613. [Google Scholar] [CrossRef]
- Saveer, A.M.; Hatano, E.; Wada-Katsumata, A.; Meagher, R.L.; Schala, C. Nonanal, a new fall armyworm sex pheromone component, significantly increases the efficacy of pheromone lures. Pest Manag. Sci. 2023, 79, 2831–2839. [Google Scholar] [CrossRef] [PubMed]
| Main Search Topic | Basic Search Keywords | Topic-Specific Search Keywords |
|---|---|---|
| (1) Genetic characterization and strain identification in Spodoptera species | “Spodoptera”/ “Spodoptera frugiperda”/“fall armyworm” | “genetic characterization” /“molecular identification” /“strain identification” /“genetic marker” |
| (2) Sexual pheromones and mating disruption in Spodoptera species | “sex * pheromone”/“mating disruption” | |
| (3) The effectiveness of sexual pheromones for mating disruption concerning genetic characterization and strain identification in Spodoptera species | “strain identification” /“genetic characterization” /“sex * pheromone”/“mating disruption” |
| Spodoptera Species | Pheromone Type/ Active Components (Proportion) | Effectiveness/Capture Rate (%) | References |
|---|---|---|---|
| S. frugiperda | Single: Z9-14:Ac/Z9-12:Ac | Yes/NS | [23,43,45,50] |
| Single: Z11-16:Ac/Z7-12:Ac/E7-12:Ac | Yes/NS | [23,43,45,46,47] | |
| Blend: Z9-14:Ac + Z9-12:Ac (10:1) | N/S/NS | [52] | |
| Blend: Z9-14:Ac (77.49%) + Z11-16:Ac (11.58%) | Yes/87% | [49] | |
| Blend: Z9-14:Ac (87%) + Z11-16:Ac (12.5%) + Z7-12:Ac (0.5%) | NS/NS | [49] | |
| Blend: Z9-14:Ac (30%) + Z7-12:Ac | Yes/NS | [41] | |
| Blend: Z9-14:Ac (30%) + Z11-16:Ac + Z7-12:Ac | Yes/NS | [41] | |
| Blend: Z9-14:Ac (90%) + Z7-12:Ac + E7-12:Ac + Z9–12:Ac + Z11–16:Ac | NS/NS | [41] | |
| Corn blend: Z9-12:Ac (1%) + Z7-12:Ac (2%) + Z11-16:Ac (13%); Rice blend: Z9-12:Ac (2%) + Z7-12:Ac (4%) + Z11-16:Ac (8%) | Yes/80% | [25] | |
| S. exigua | Blend: Z9-12-14:Ac + Z9-14:OH (7:3) | Yes/93–97.8% | [53,54,55] |
| S. littoralis | Blend: Z9-11:Ac + Z9-12:Ac (95:5) | Yes/98.9% | [56] |
| S. cosmioides | Blend: Z9-14:Ac + Z9-12:Ac (10:1) | NS/NS | [53] |
| S. litura | Single: Z9-11:Ac; Single: Z9-12-14:Ac | NS/NS | [57,58,59] |
| Single: Z9-14:OH | No/NS | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, C.; Catarino, S.; Mexia, A.; da Silva, E.B.; Monteiro, F. Genetic Characterization and Mating Disruption in Spodoptera Species, a Case Study on Spodoptera frugiperda (Lepidoptera, Noctuidae): A Systematic Review. Insects 2025, 16, 1176. https://doi.org/10.3390/insects16111176
Tavares C, Catarino S, Mexia A, da Silva EB, Monteiro F. Genetic Characterization and Mating Disruption in Spodoptera Species, a Case Study on Spodoptera frugiperda (Lepidoptera, Noctuidae): A Systematic Review. Insects. 2025; 16(11):1176. https://doi.org/10.3390/insects16111176
Chicago/Turabian StyleTavares, Carla, Sílvia Catarino, António Mexia, Elsa Borges da Silva, and Filipa Monteiro. 2025. "Genetic Characterization and Mating Disruption in Spodoptera Species, a Case Study on Spodoptera frugiperda (Lepidoptera, Noctuidae): A Systematic Review" Insects 16, no. 11: 1176. https://doi.org/10.3390/insects16111176
APA StyleTavares, C., Catarino, S., Mexia, A., da Silva, E. B., & Monteiro, F. (2025). Genetic Characterization and Mating Disruption in Spodoptera Species, a Case Study on Spodoptera frugiperda (Lepidoptera, Noctuidae): A Systematic Review. Insects, 16(11), 1176. https://doi.org/10.3390/insects16111176









