New Discoveries Supporting the Exceptional Species Diversity of Opostegidae in Central America and the Caribbean, Alerting on Misidentified Barcodes #
Simple Summary
Abstract
1. Introduction
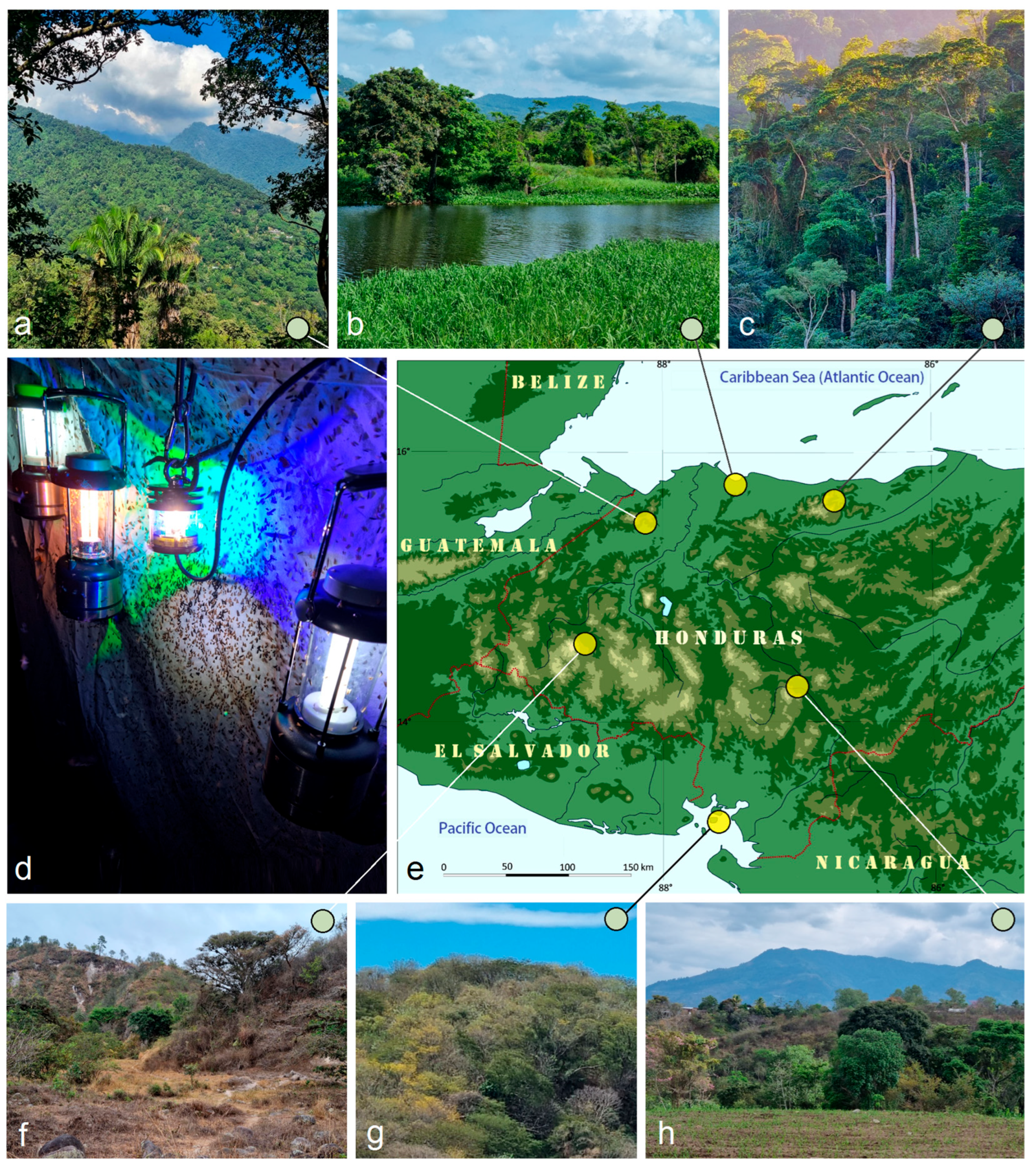
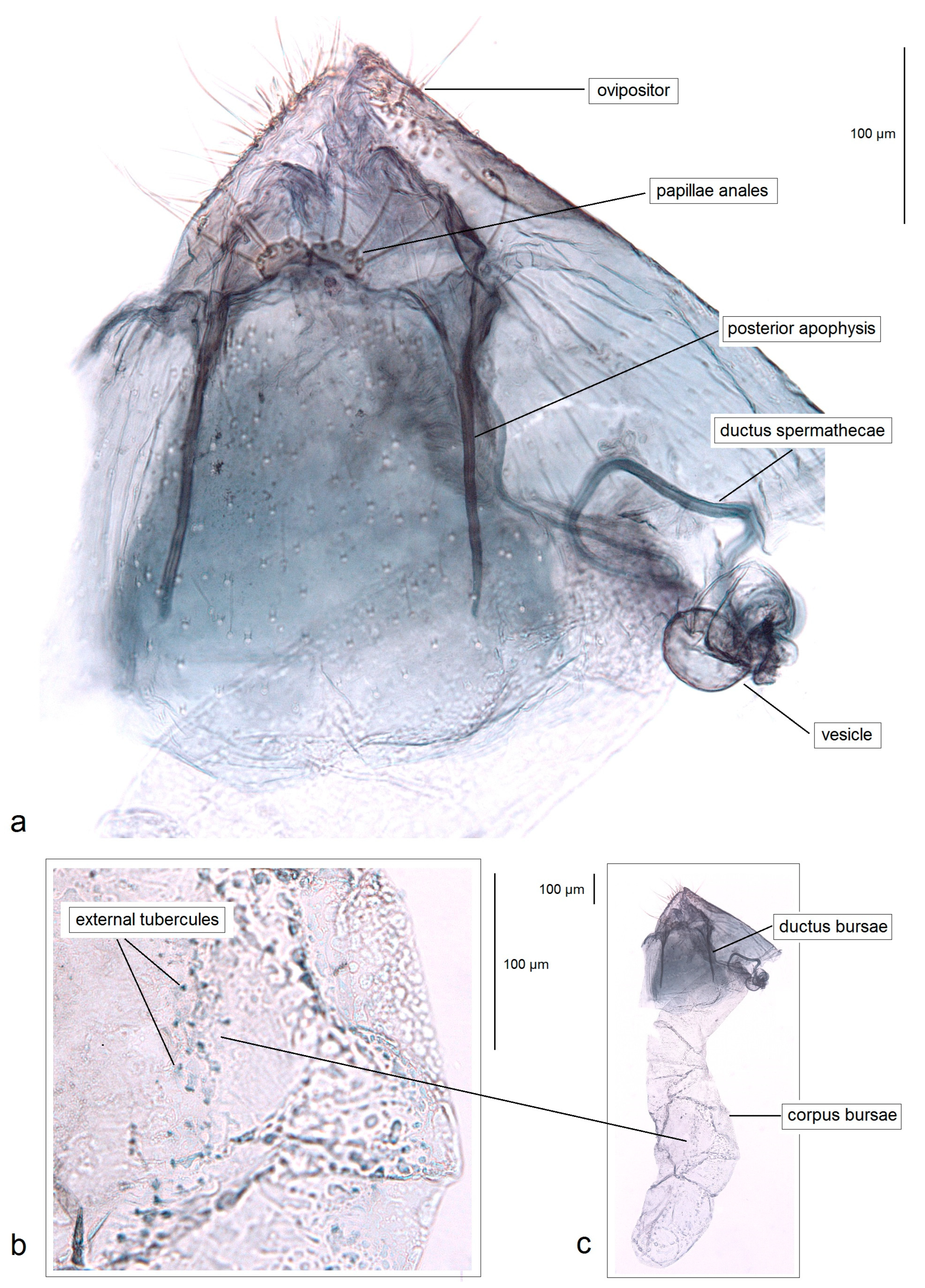
2. Materials and Methods
3. Results
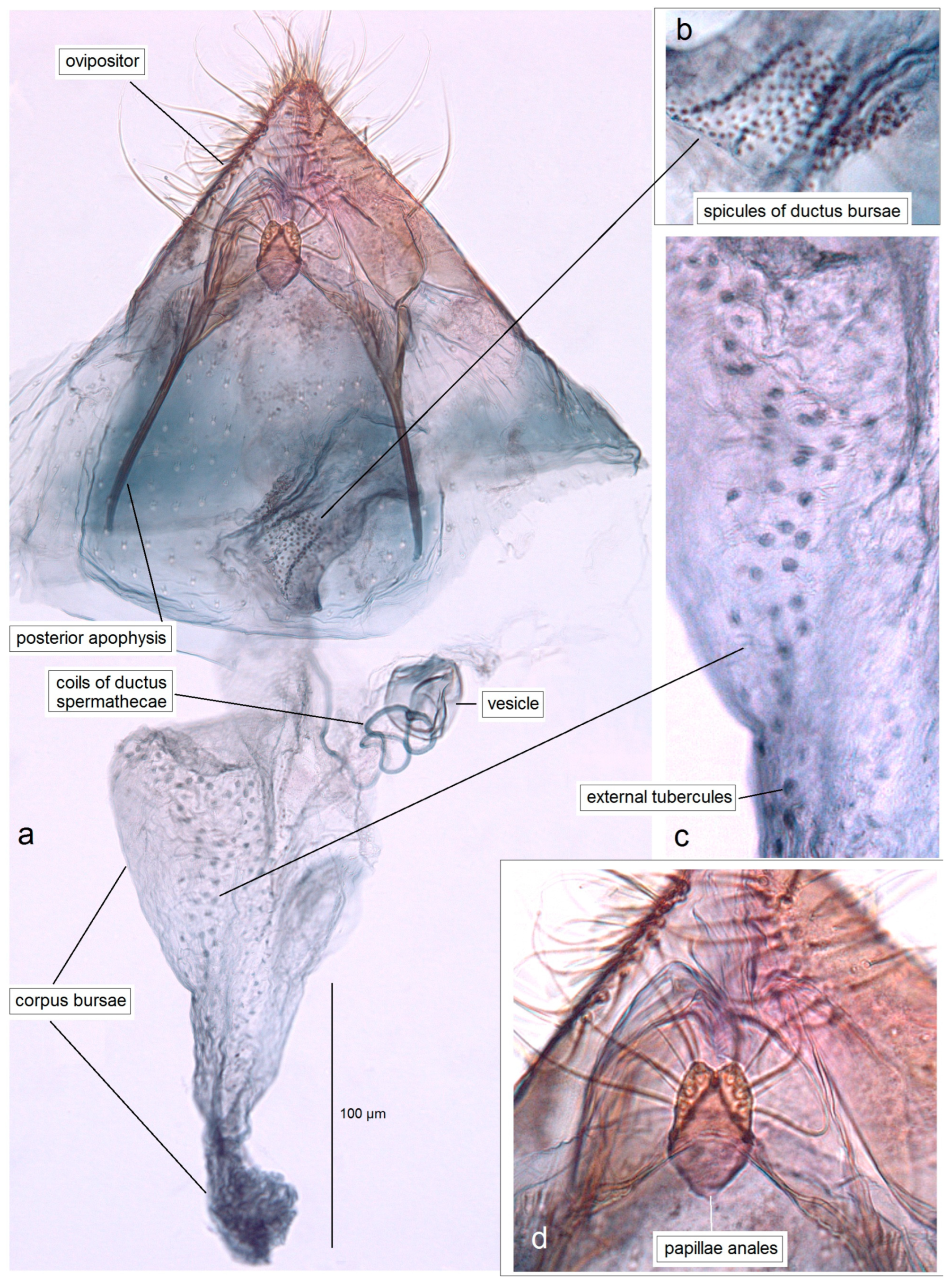
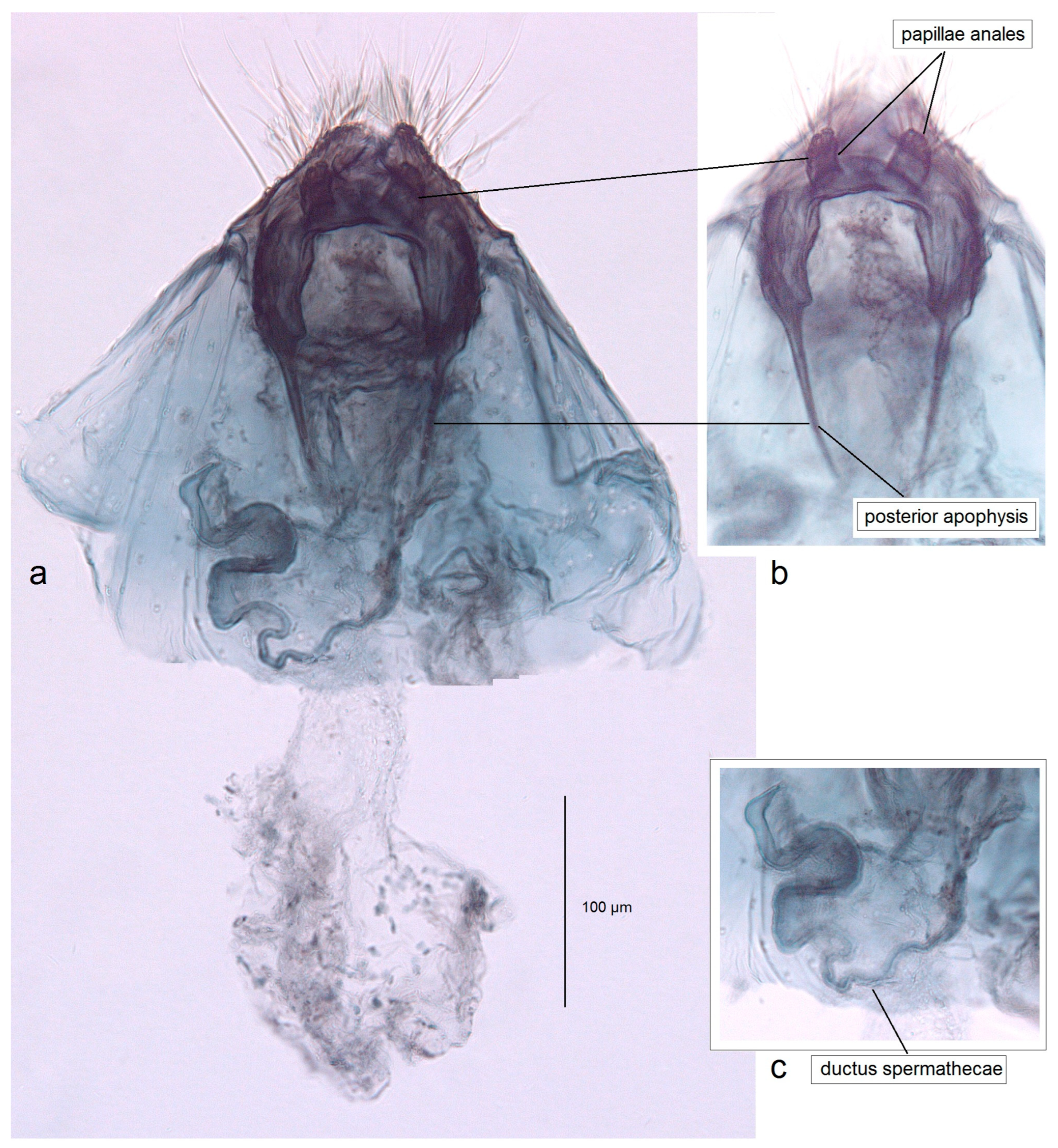
3.1. Re-Examination of Historical Material from the Collection of the NHMUK (London, UK)
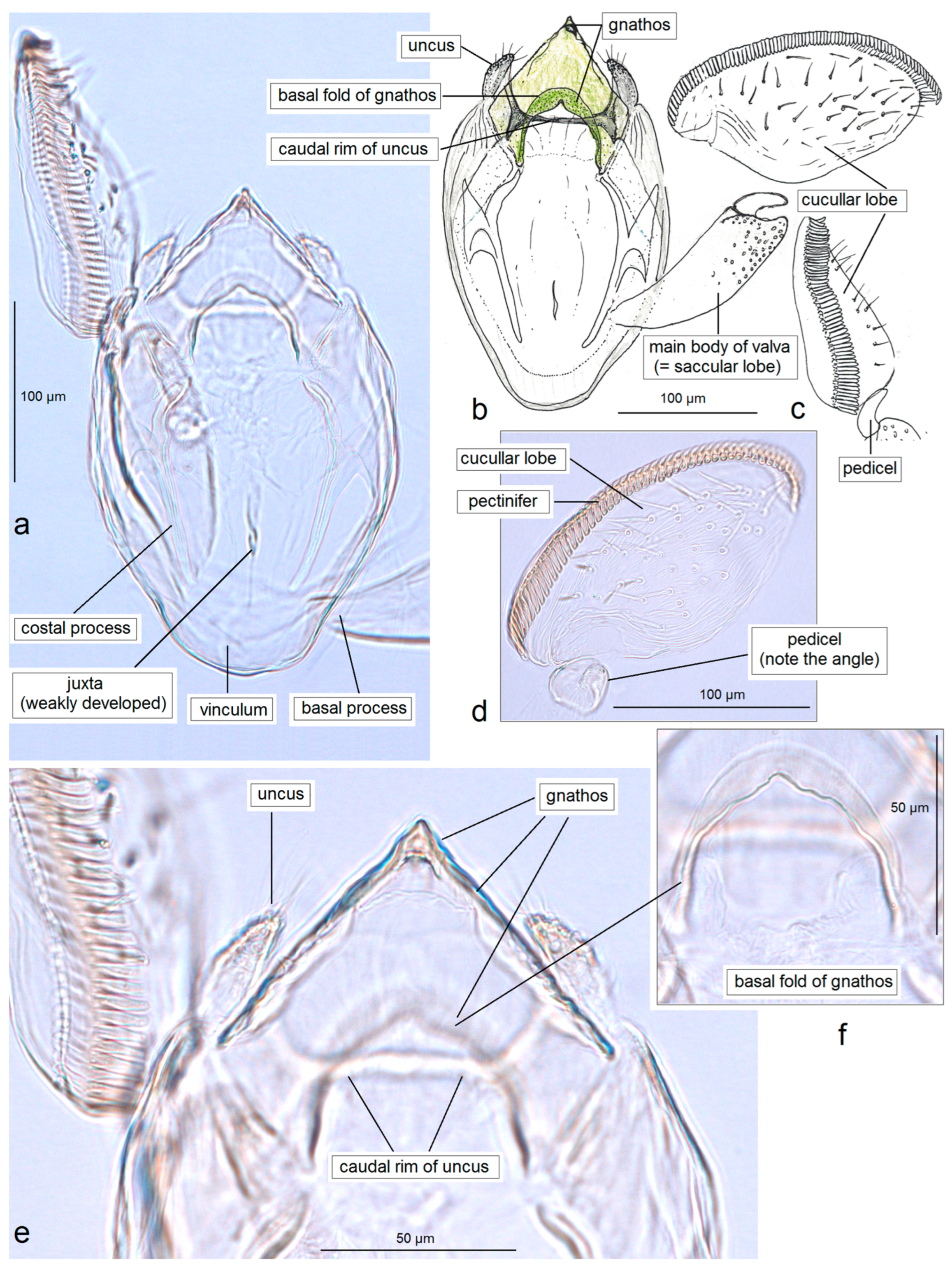
3.1.1. First Documentation of the True Pseudopostega saltatrix (Walsingham, 1897)
3.1.2. Description of Pseudopostega jamaicensis Stonis & Remeikis, sp. nov., a New Species Related to P. saltatrix (Walsingham)
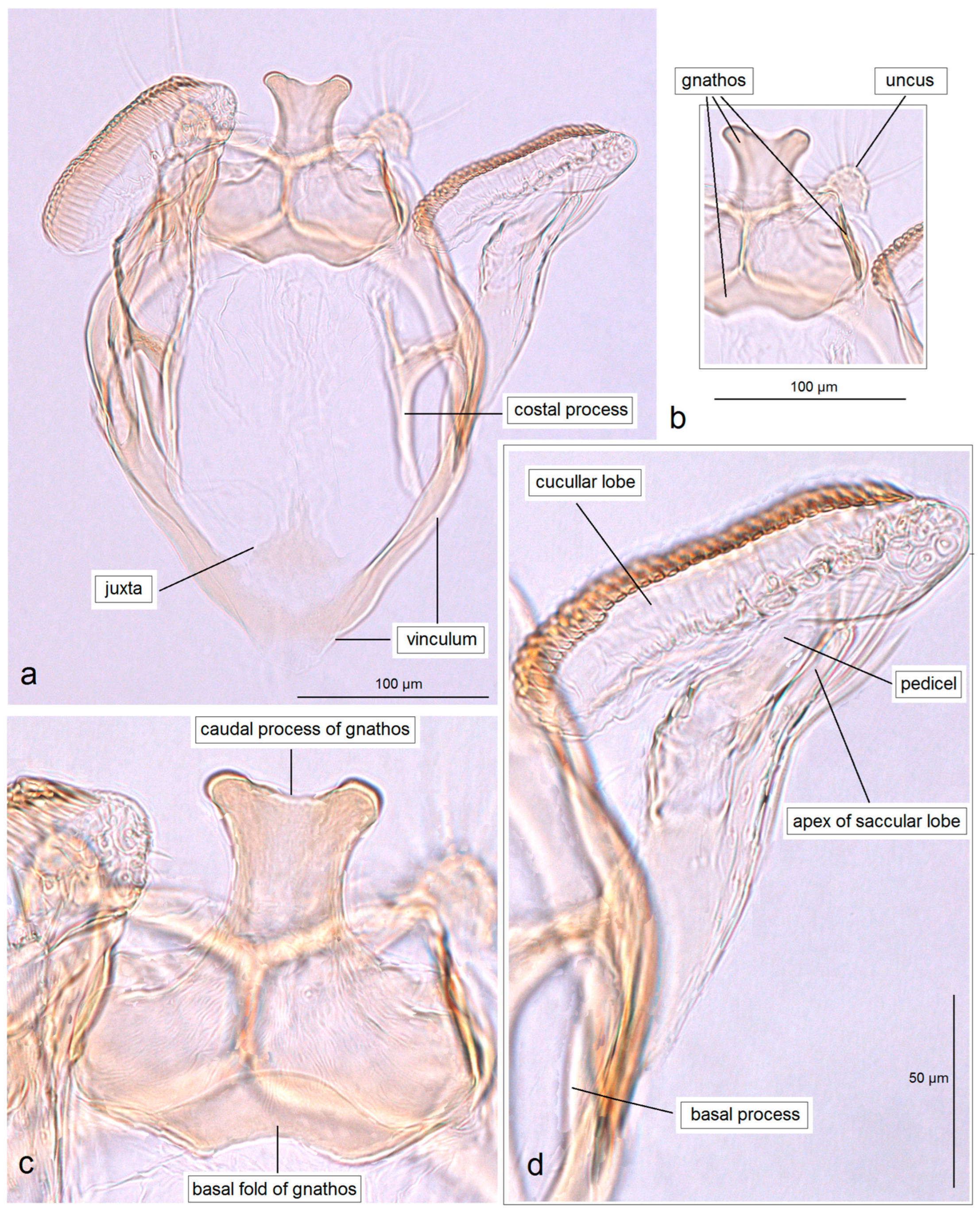
3.2. The First Attempt to Assess the Taxonomic Diversity of Opostegidae in Honduras, Resulting in the Discovery of New Species
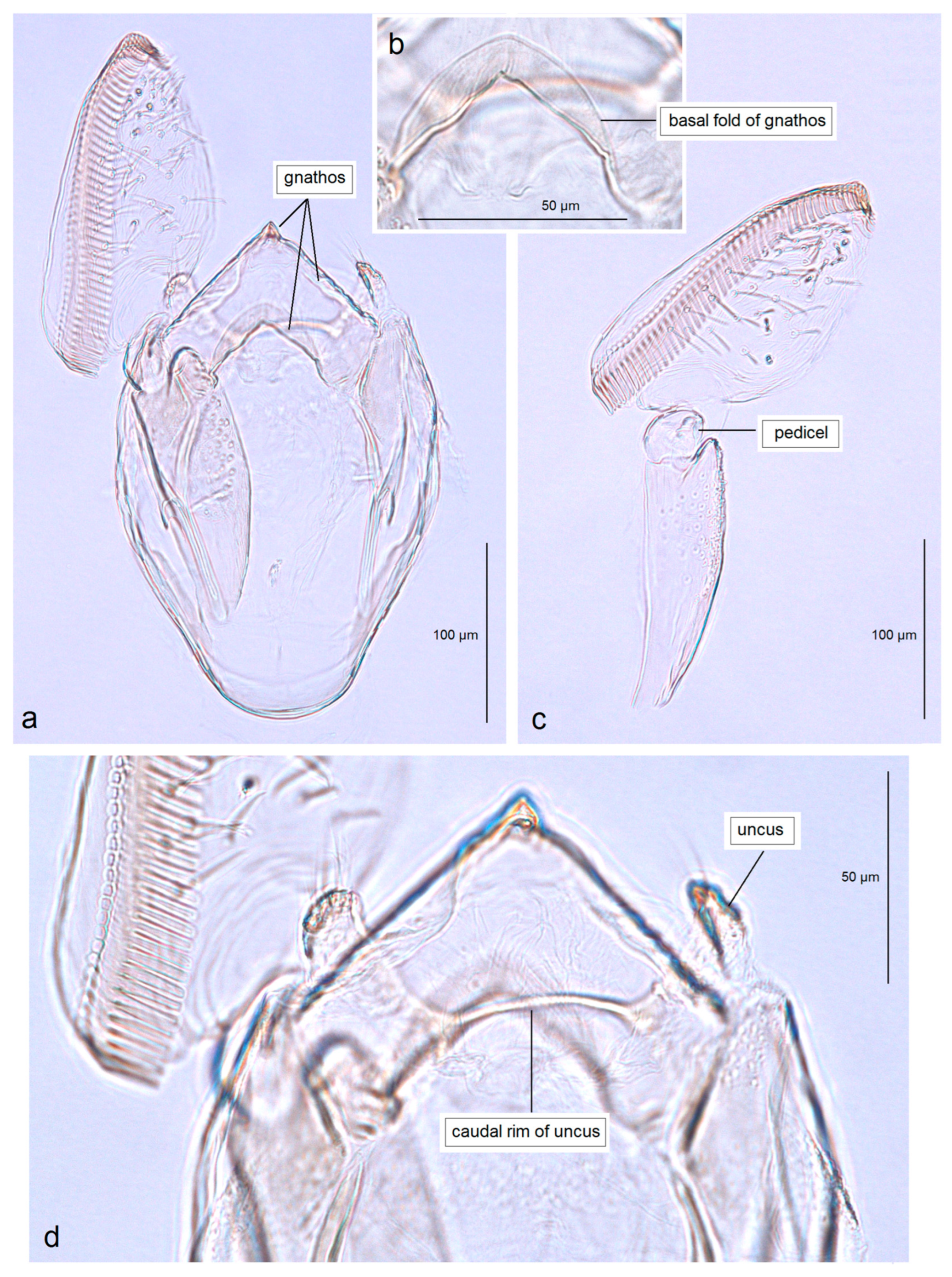
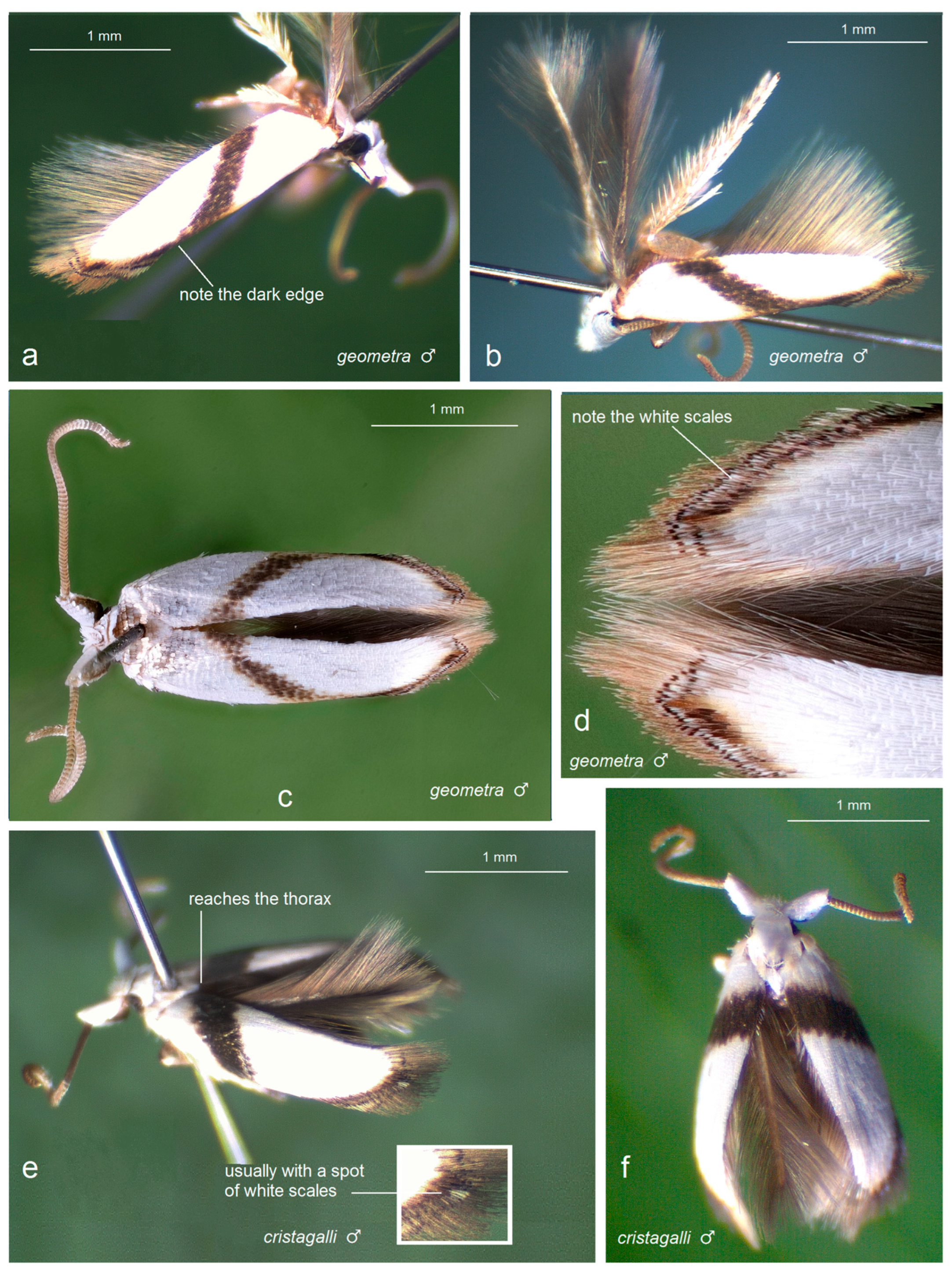
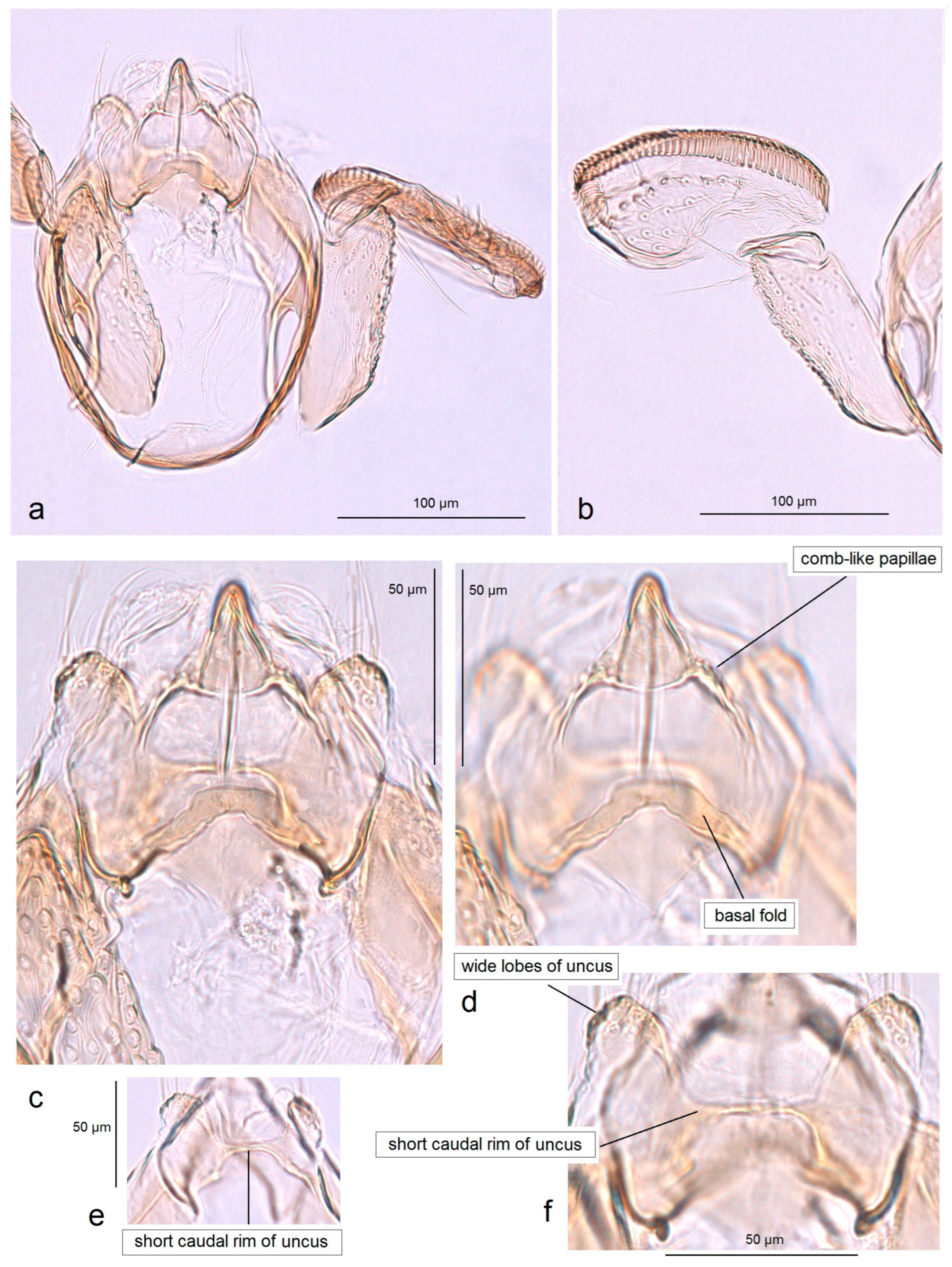
3.2.1. Pseudopostega geometra Stonis & Remeikis, sp. nov.
3.2.2. Pseudopostega cristagalli Stonis & Remeikis, sp. nov.
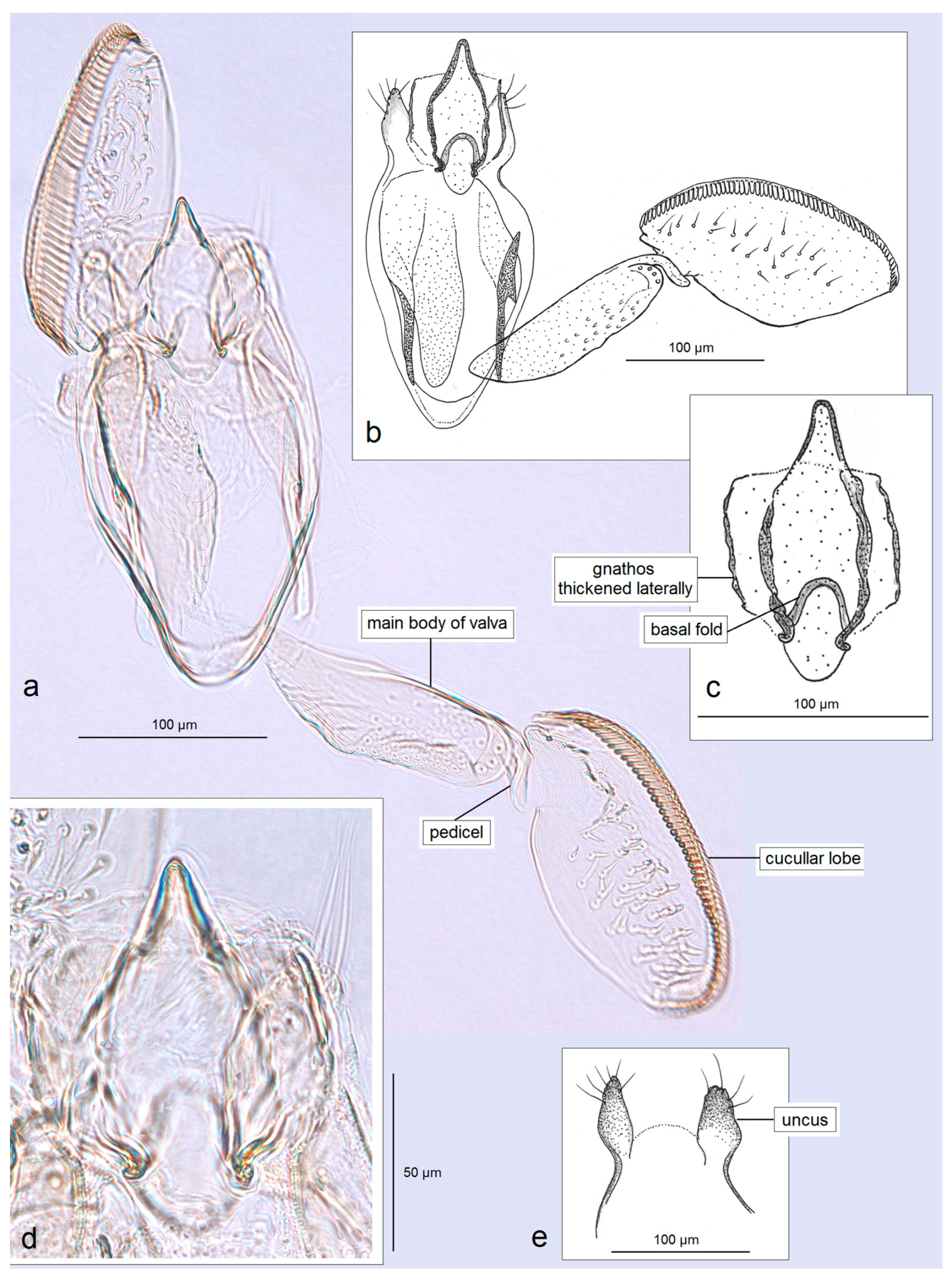
3.2.3. Pseudopostega bestiola Stonis & Remeikis, sp. nov.
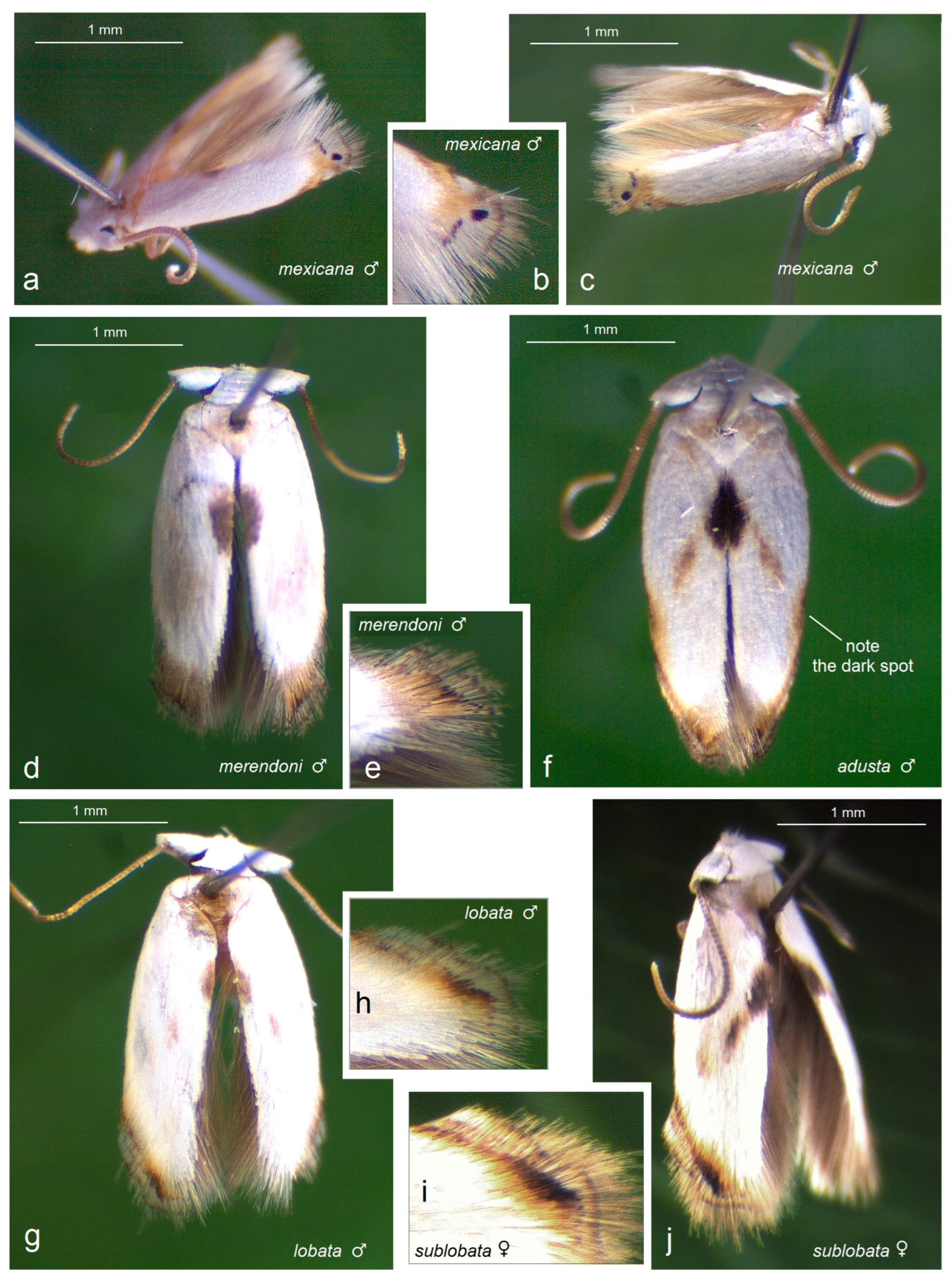
3.2.4. Pseudopostega merendoni Stonis & Remeikis, sp. nov.
3.2.5. Pseudopostega mexicana Remeikis & Stonis, 2009
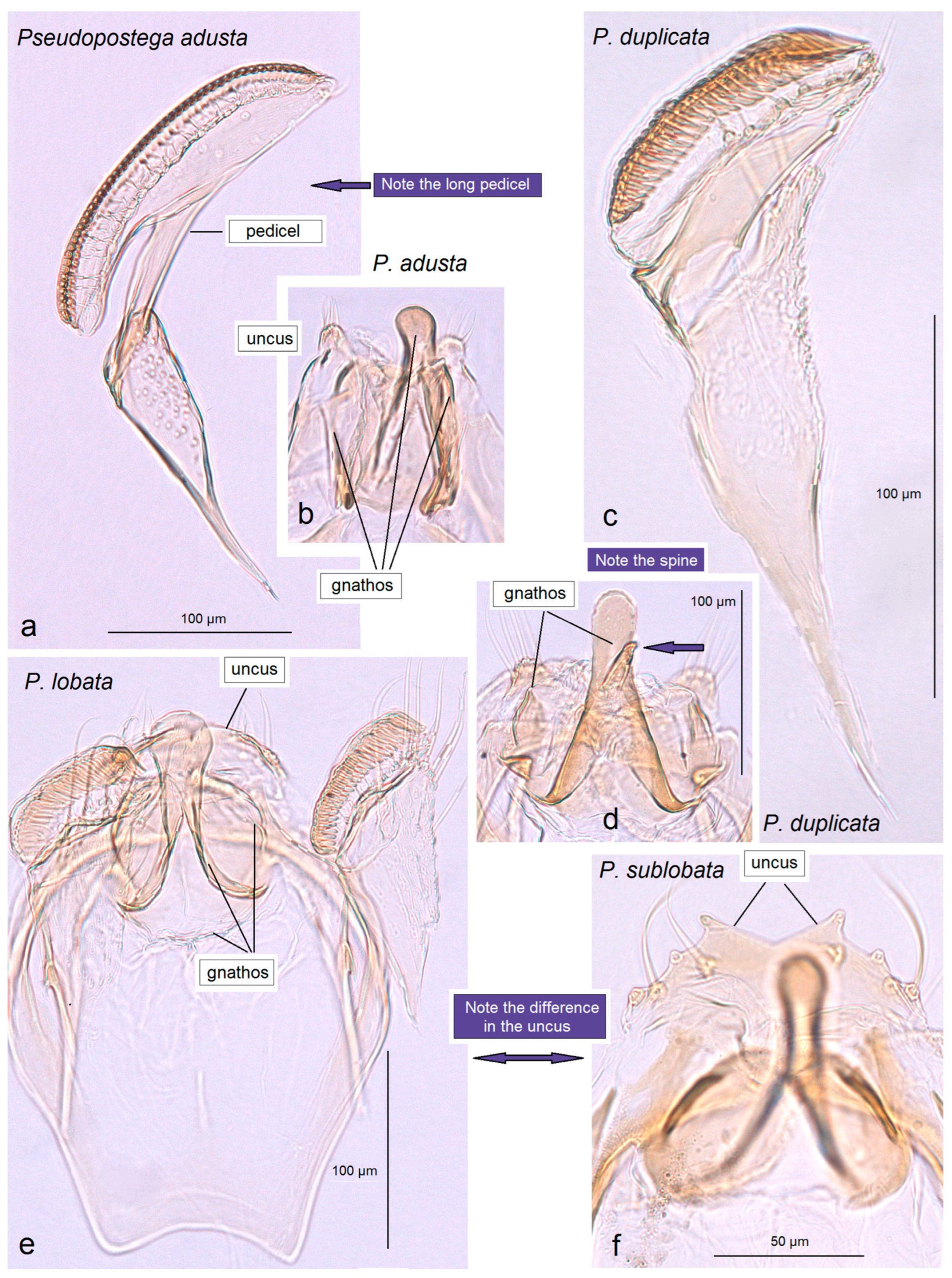
3.2.6. Pseudopostega adusta (Walsingham, 1897)
3.2.7. Pseudopostega lobata Davis & Stonis, 2007
3.2.8. Pseudopostega sublobata Davis & Stonis, 2007
3.2.9. Pseudopostega duplicata Davis & Stonis, 2007
3.2.10. Pseudopostega ocellata Stonis & Remeikis, sp. nov.
3.2.11. Pseudopostega pumila (Walsingham, 1914)
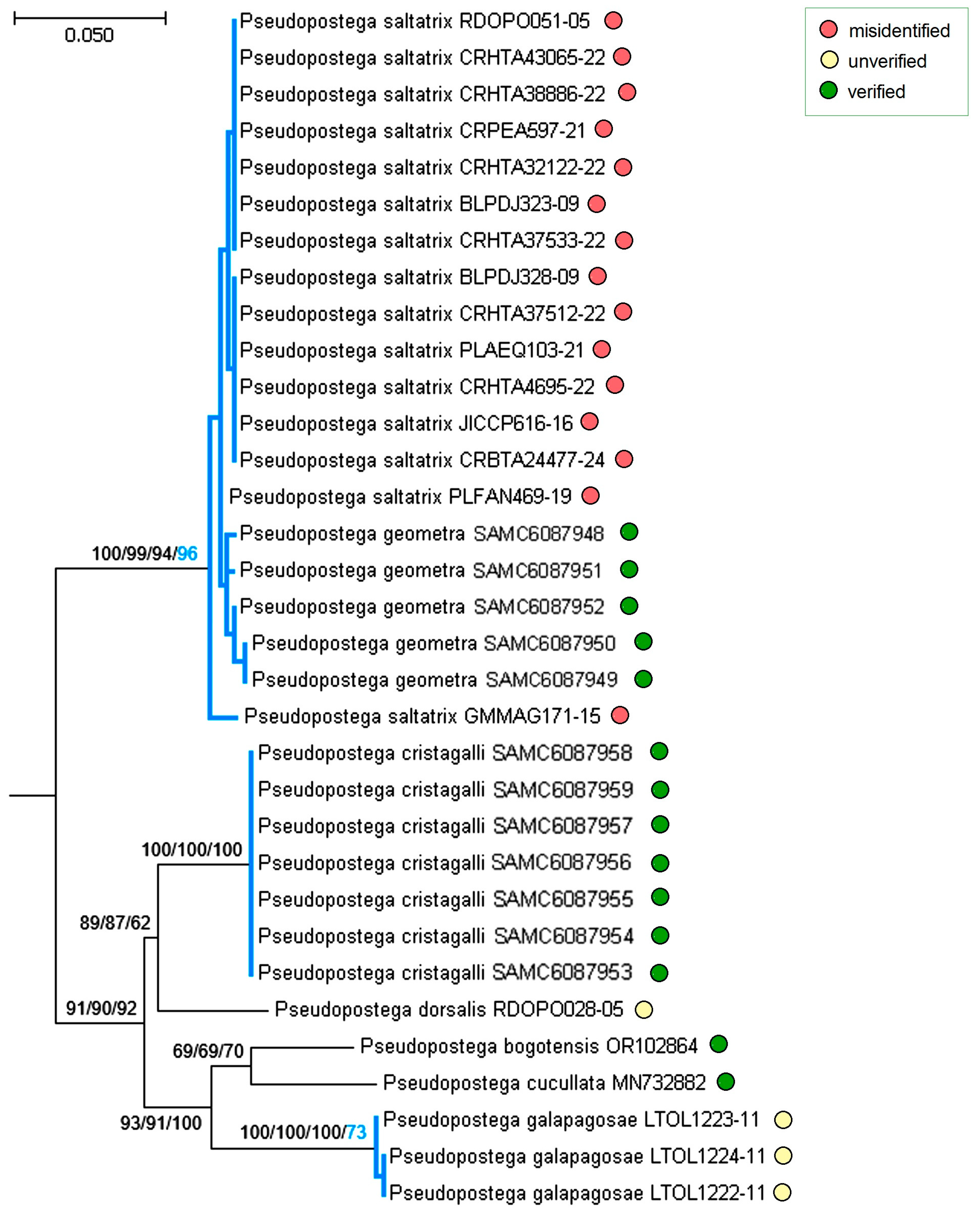
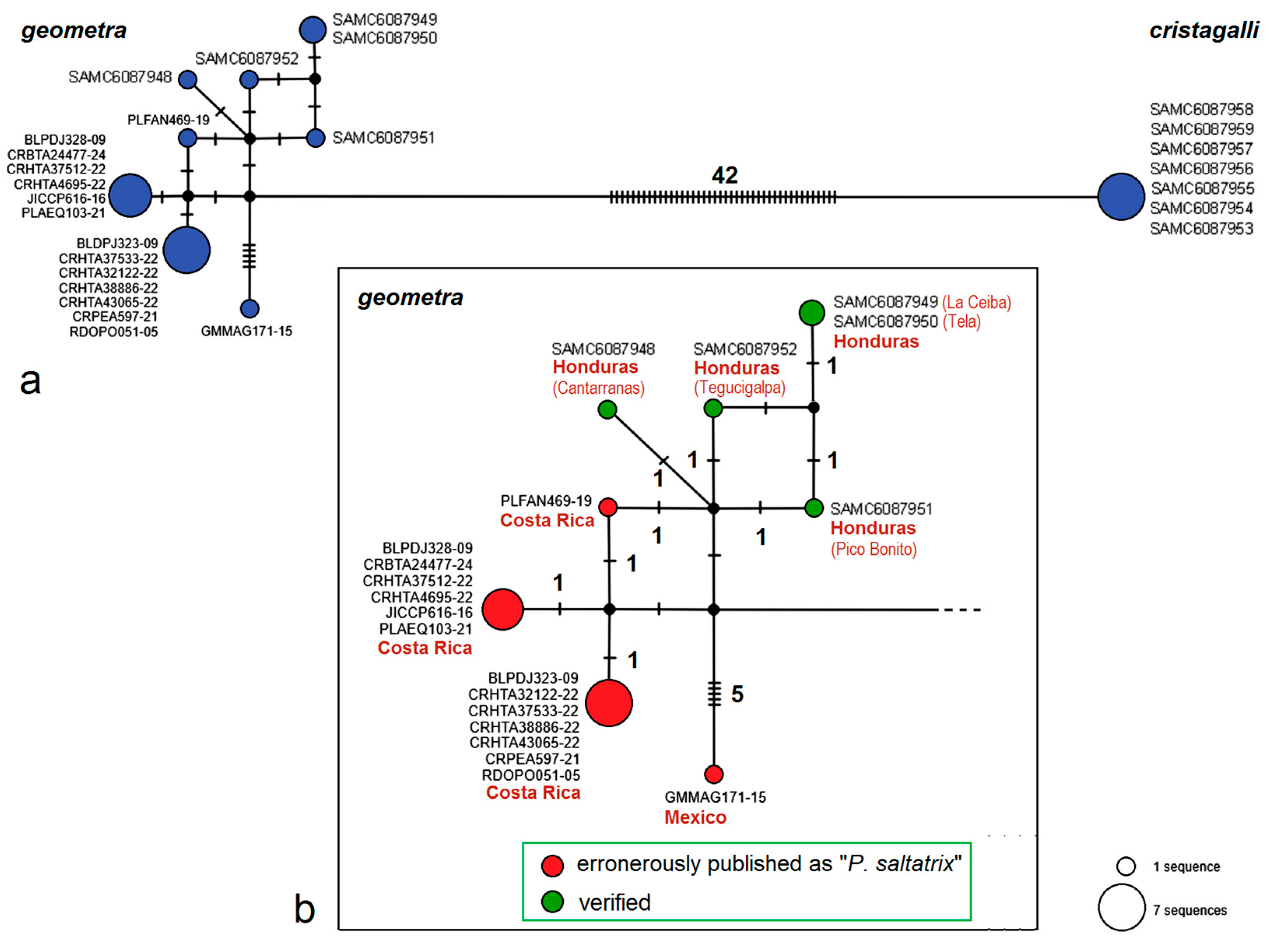
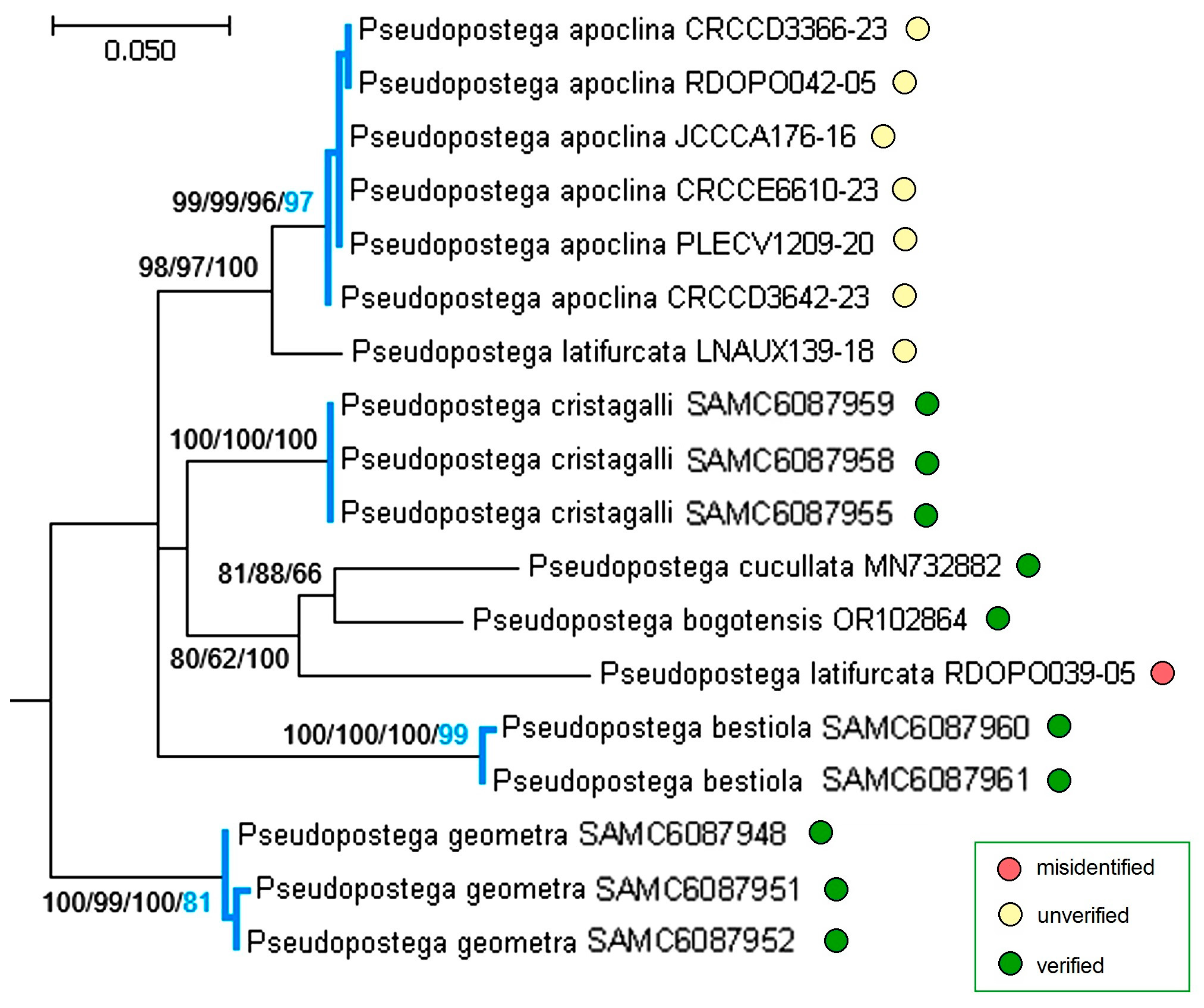
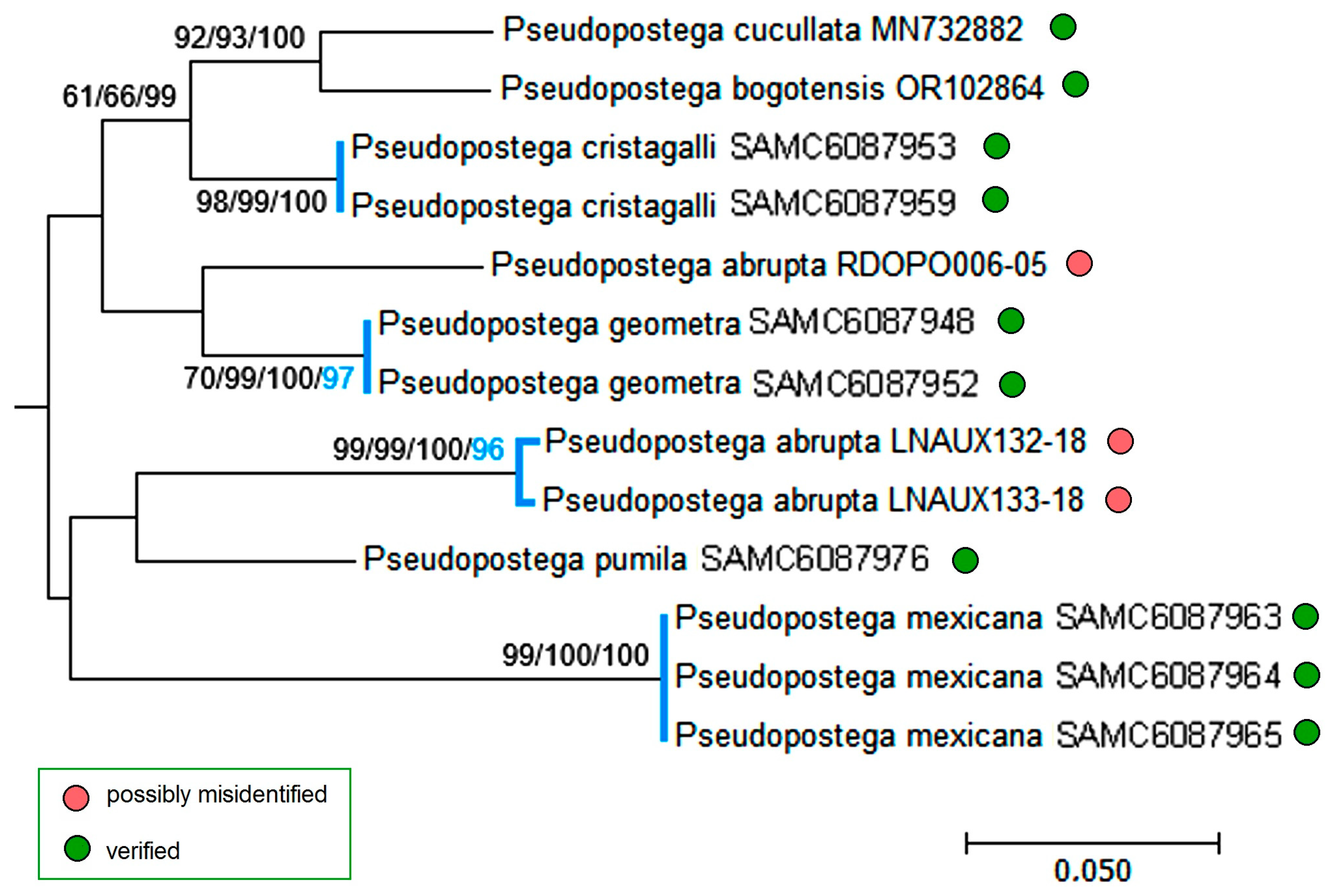
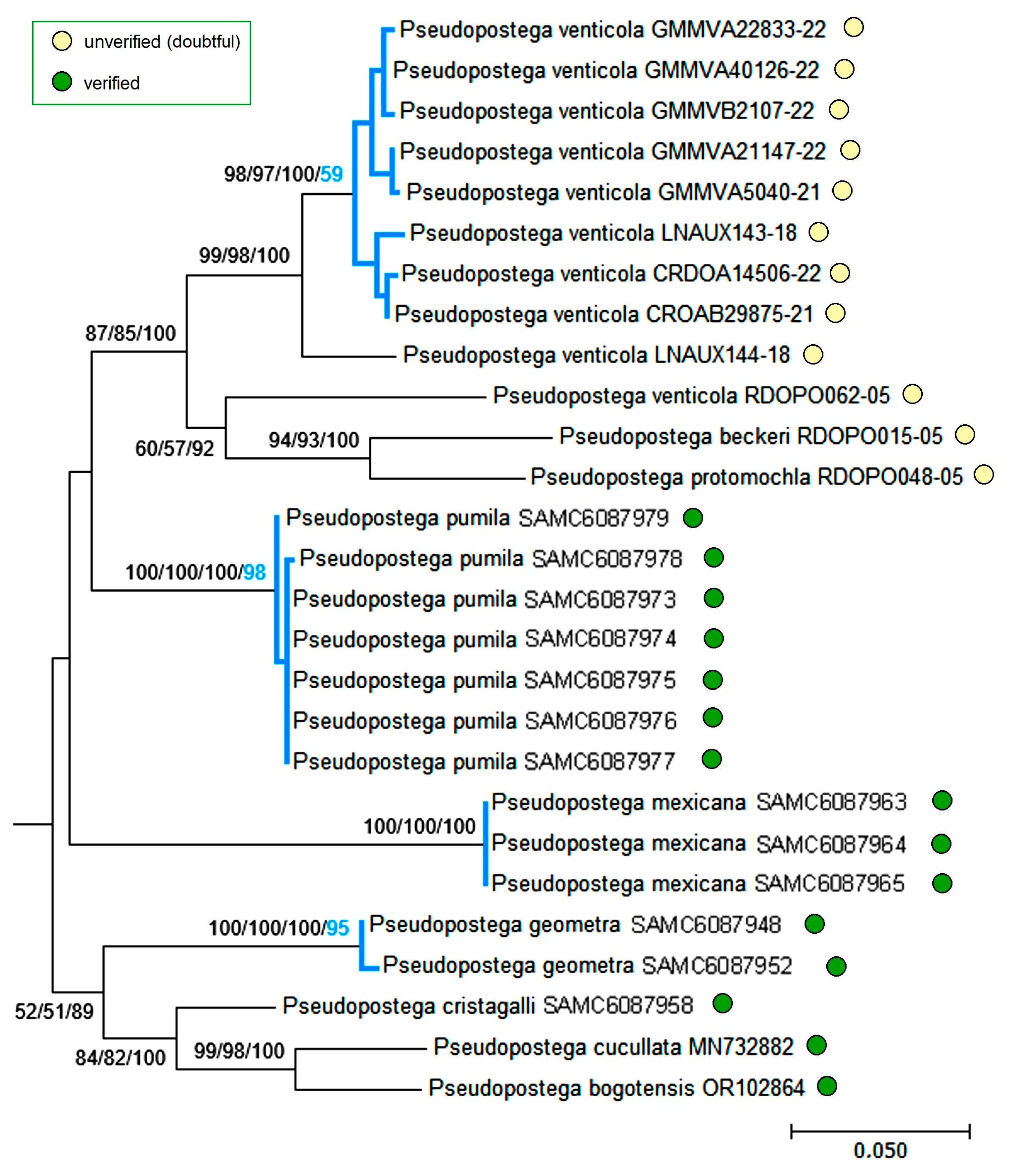
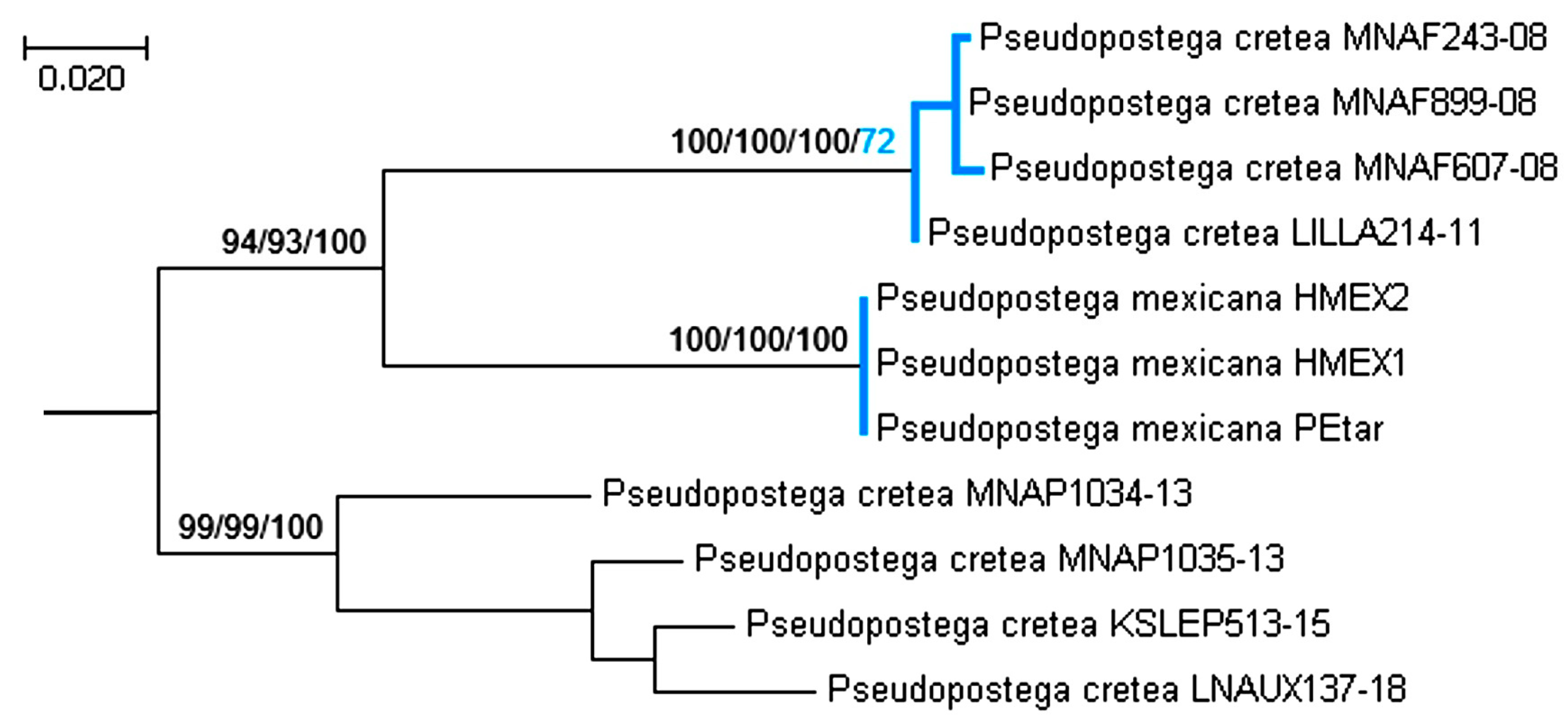
3.3. Molecular Considerations
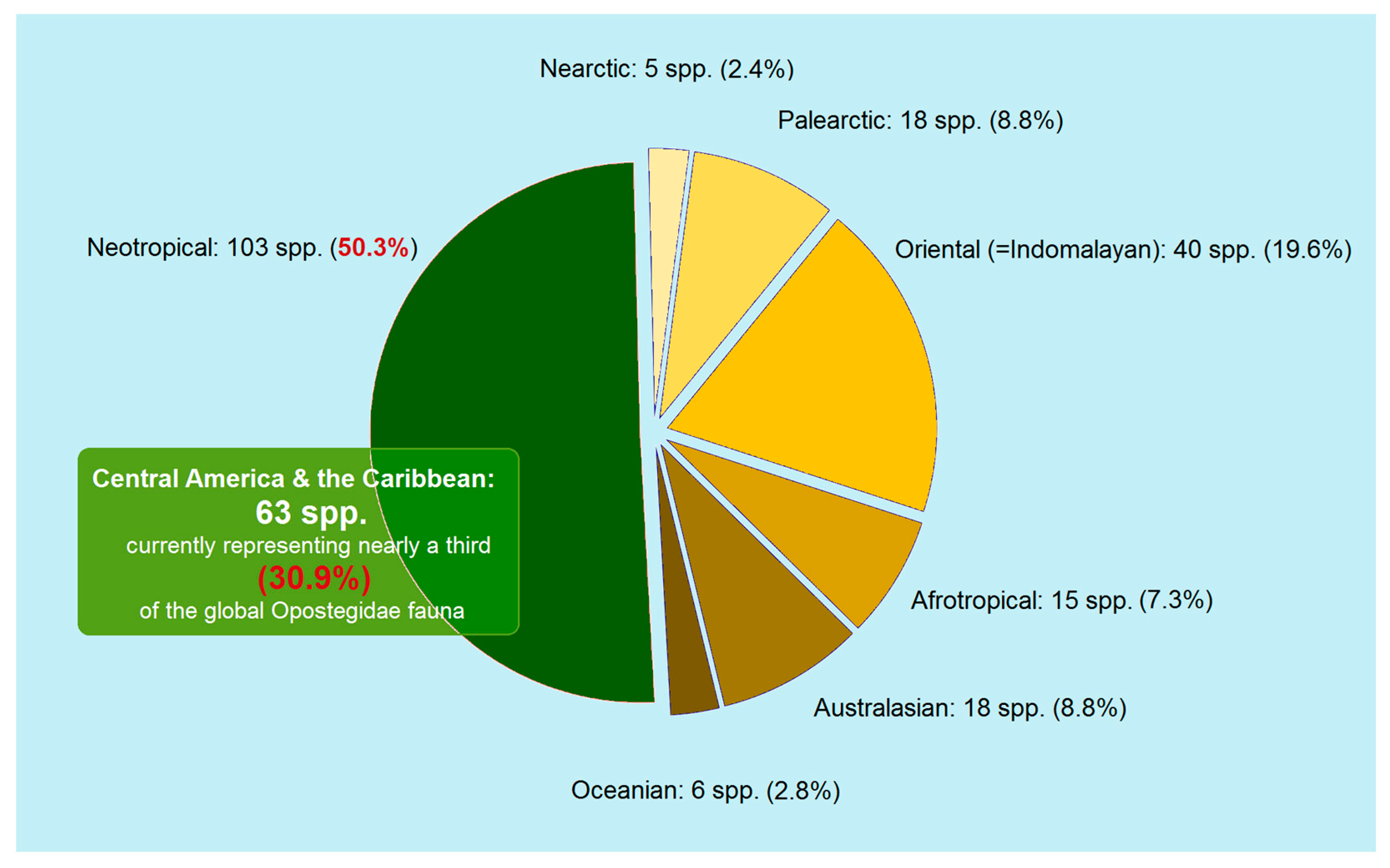
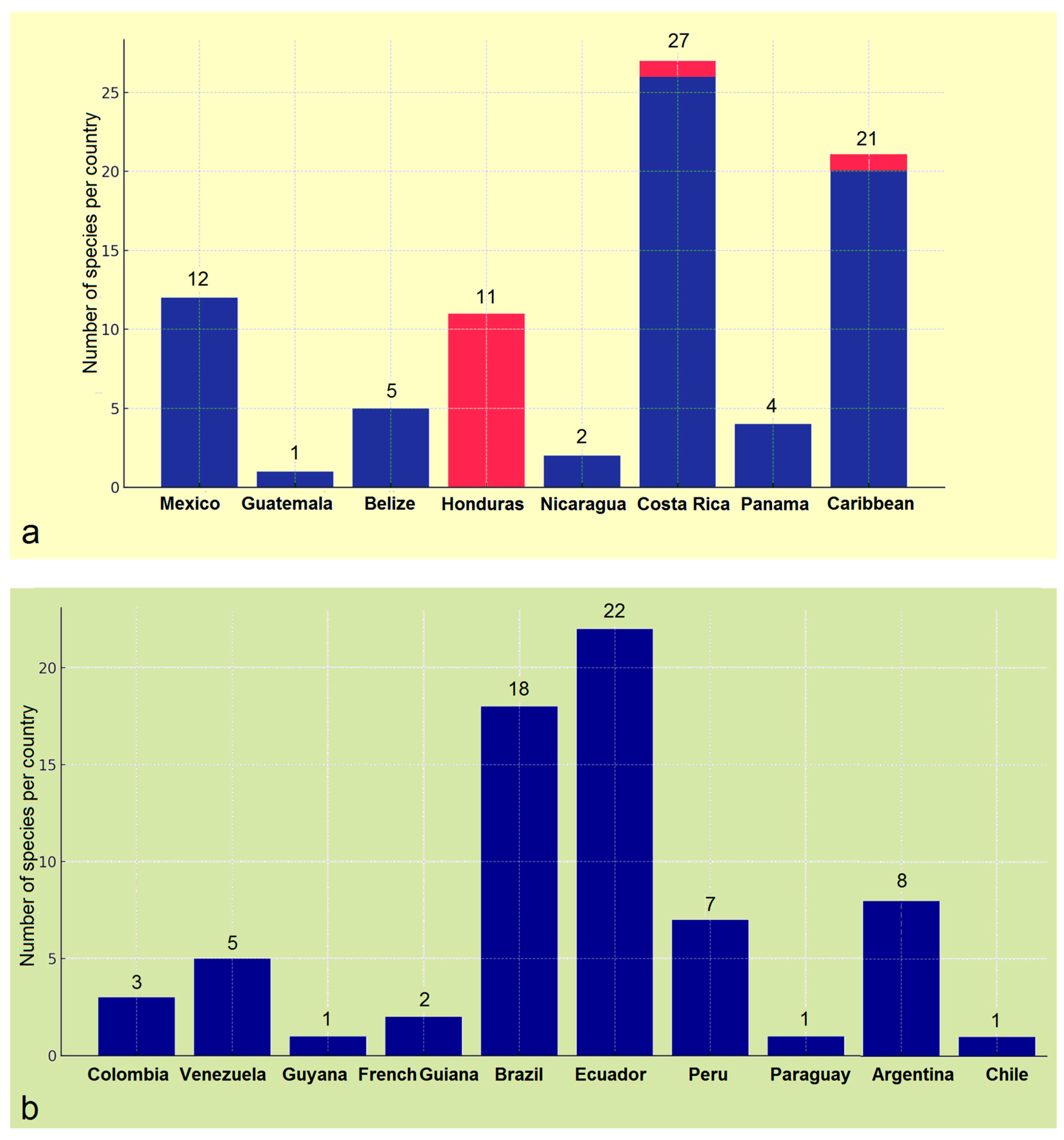
3.4. Updating the Checklist of Central America and the Caribbean Opostegidae with an Expanded Neotropical Coverage
| Genus Notiopostega Davis, 1989 Notiopostega atrata Davis, 1989 Distribution. The new species is known from Chile (Valdivia) [10]. Genus Neopostega Davis & Stonis, 2007 Neopostega longispina Davis & Stonis, 2007 Distribution. The species is known from the lowland Amazonian rainforest of southern Venezuela. Neopostega falcata Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia). Neopostega asymmetra Davis & Stonis, 2007 Distribution. The species is known from the Atlantic coastal forest of southern Brazil. Neopostega petila Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia). Neopostega distola Davis & Stonis, 2007 Distribution. The species is known from northern Costa Rica (Alad) and southwestern Brazil (Mato Grosso). Neopostega nigrita Heppner & Davis, 2009 Distribution. The species is known from Guatemala [11]. Neopostega dondavisi Stonis & Remeikis, 2020 Distribution. The species is known from a single locality: La Merced, Junín Region, central Peru, at an elevation of about 900 m (selva central/selva alta) [19]. Genus Pseudopostega Kozlov, 1985 The rotunda group Pseudopostega rotunda Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia and Guanacaste), and the Amazonian rainforest of Ecuador (Napo Province). Pseudopostega ovatula Davis & Stonis, 2007 Distribution. The species is known from the Amazonian lowland and premontane rainforest of Ecuador (Napo Province). Pseudopostega attenuata Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia, Cartago, and Guanacaste), northwestern Brazil (Ceara), and southwestern Ecuador (Bucay). Pseudopostega latisaccula Davis & Stonis, 2007 Distribution. The species is known from Dominica (NW Pont Casse) and Puerto Rico (Carite). The latifurcata group Pseudopostega latifurcata Davis & Stonis, 2007 Distribution. The species is known from US Virgin Islands (St. Thomas), Puerto Rico (Cayey, Carite), British Virgin Islands (Tortola Island), and Dominica (Cabrit Swamp). Pseudopostega apoclina Davis & Stonis, 2007 (=P. latifurcata apoclina Davis & Stonis, 2007). Distribution. The species is known from Costa Rica (Guanacaste, Cartago). Pseudopostega bidorsalis Davis & Stonis, 2007 Distribution. The species is known from northern Costa Rica (Heredia, Cartago). Pseudopostega bestiola Stonis & Remeikis, sp. nov. (described herein). Distribution. The species is known from a single locality in Honduras: Cantarranas, Francisco Morazán Department, at elevations of about 660–690 m. The lateriplicata group Pseudopostega lateriplicata Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia). Pseudopostega floridensis Davis & Stonis, 2007 Distribution. The species is known from southern Florida, USA. Pseodopostega robusta Remeikis & Stonis, 2009 Distribution. The species is known from the Pacific coast of Costa Rica [21]. Pseudopostega abrupta (Walsingham, 1897) Distribution. The species is known from the US Virgin Islands (St. Thomas) and British Virgin Islands (Guana Island). Pseudopostega ferruginea Davis & Stonis, 2007 Distribution. The species is known from the US Virgin Islands (St. Thomas) and Puerto Rico. Pseudopostega uncinata Davis & Stonis, 2007 Distribution. The species is known from north-central Venezuela (Guarico). Pseudopostega serrata Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia, Cartago, Limón, and Puntarenas), southern Panama, and the Amazonian rainforest of Ecuador (Napo Province). Pseudopostega monstruosa Davis & Stonis, 2007 Distribution. The species is known from the Amazonian premontane rainforest of Ecuador (Napo Province). The triangularis group Pseudopostega triangularis Davis & Stonis, 2007 Distribution. The species is known from northern Argentina (Salta). Pseudopostega conicula Davis & Stonis, 2007 Distribution. The species is known from northwestern Costa Rica (Guanacaste). Pseudopostega sacculata (Meyrick, 1915) Distribution. The species is known from Ecuador (Chimborazo: Huigra; Guayas: Bucay) [53]; recently, it was discovered in the Central Amazonian forest of Peru (La Merced) [19]. Pseudopostega fumida Davis & Stonis, 2007 Distribution. The species is known from the tropical humid forest of Belize (Cayo District). Pseudopostega breviapicula Davis & Stonis, 2007 Distribution. The species is known from Panama (La Chorrera), Brazil (Pará, Minas Gerais), and northern Argentina (Jujuy). Pseudopostega mignonae Davis & Stonis, 2007 Distribution. The species is known from Jamaica (St Catherine Parish) and Cuba (Pinar del Rio). Pseudopostega acuminata Davis & Stonis, 2007 Distribution. The species is known from northern Venezuela (Mérida) and northern Argentina (Tucumán). Pseudopostega trinidadensis (Busck, 1910) Distribution. The species is known from Trinidad and Tobago (Trinidad). Pseudopostega plicatella Davis & Stonis, 2007 Distribution. The species is known from northeastern Brazil (Pará) and the Amazonian rainforest of Ecuador (Napo Province). Pseudopostega subtila Davis & Stonis, 2007 Distribution. The species is known from southeastern Brazil (Minas Gerais). Pseudopostega kempella (Eyer, 1967) Distribution. The species is known from Monroe County of southern Florida, USA [55]. Pseudopostega paraplicatella Davis & Stonis, 2007 Distribution. The species is known from the Amazonian rainforest of Ecuador (Napo Province). Pseudopostega gracilis Davis & Stonis, 2007 Distribution. The species is know from the rainforest of French Guiana. Pseudopostega tanygnatha Davis & Stonis, 2007 Distribution. The species is known from northwestern Costa Rica (Guanacaste). The spatulata group Pseudopostega spatulata Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia). Pseudopostega truncata Davis & Stonis, 2007 Distribution. The species is known from central Brazil (Distrito Federal: Maranhao). Pseudopostega mexicana Remeikis & Stonis, 2009 Distribution. The species is known from the Pacific coast of Mexico (Oaxaca region) [21]. In the present article, we provide new distributional data from the Pacific coast of Honduras. Pseudopostega apotoma Davis & Stonis, 2007 Distribution. The species is known from Brazil (Minas Gerais and Pará); recently it has also been recorded from the Amazonian rainforest of Peru (Satipo) [19]. Pseudopostega pexa (Meyrick, 1920) Distribution. The species is known from northeastern Brazil (Pará) [54]. Pseudopostega diskusi Davis & Stonis, 2007 Distribution. The species is known from the tropical humid forest of Belize (Cayo District). Pseudopostega microacris Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia and Cartago). Pseudopostega tucumanae Davis & Stonis, 2007 Distribution. The species is known from northern Argentina (Tucumán). The saltatrix group Pseudopostega colognatha Davis & Stonis, 2007 Distribution. The species is known from Puerto Rico (Patillas and Carite). Pseudopostega obtusa Davis & Stonis, 2007 Distribution. The species is known from Ecuador (Carchí). Pseudopostega pontifex (Meyrick, 1915) Distribution. The species is known from western Colombia (Cali) [54]. Pseudopostega cucullata Stonis & Vargas, 2020 Distribution. The species is known from a single locality in Colombia: Valle del Cauca, on the border of the National Park de los Farallones de Cali, at an elevation of about 1700 m (Desarrollo Biodiverso) [19]. Pseudopostega bogotensis Vargas, 2020 Distribution. The species is known from green urban areas of Bogotá, Colombia, at an elevation of about 2600 m [19]. Pseudopostega galapagosae Davis & Stonis, 2007 Distribution. The species is known from the Galápagos Islands, Ecuador. Pseudopostega saltatrix (Walsingham, 1897) Distribution. The species is known from the US Virgin Islands (St. Thomas) [40]. All other previously reported records from the Caribbean and Central and South America [10] are most likely incorrect. Pseudopostega dorsalis Davis & Stonis, 2007 (=P. dorsalis dorsalis Davis & Stonis, 2007) Distribution. The species is known from Costa Rica (San José and Cartago). Pseudopostega fasciata Davis & Stonis, 2007 (=P. dorsalis fasciata Davis & Stonis, 2007) Distribution. The species is known from Costa Rica (Alajuela, Cartago, Heredia, and Guanacaste). Pseudopostega parakempella Davis & Stonis, 2007 Distribution. The species is known from Florida, USA [10], and the Pacific coast of Mexico (Oaxaca region: Puerto Angel) [21]. Pseudopostega latiplana Remeikis & Stonis, 2009 Distribution. The species is known from the Pacific coast of Mexico (Oaxaca region: Puerto Angel) [21]. Pseudopostega jamaicensis Stonis & Remeikis, sp. nov. (described herein) Distribution. The species is known from Jamaica. Pseudopostega geometra Stonis & Remeikis, sp. nov. (described herein) Distribution. The species is known from various localities of Honduras; the published DNA sequences also indicated that this species is in Costa Rica. Pseudopostega cristagalli Stonis & Remeikis, sp. nov. (described herein) Distribution. The species is known from the tropical humid forest of northern Honduras (Tela). The longipedicella group Pseudopostega longipedicella Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Puntarenas) and Panama (Canal Zone). Pseudopostega adusta (Walsingham, 1897) Distribution. The species is known from the US Virgin Islands (St. Thomas) [40], Cuba (Central Baragua), Dominica (Cabrit Swamp), Belize (Cayo District) [10]. It has also been recorded from the Pacific coast of Costa Rica [21] and from Ecuador (Napo Province) [21]. In the present article, we provide new distributional data from Honduras. The lobata group Pseudopostega lobata Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia and Guanacaste), Belize (Cayo District), Nicaragua (Estelí) [10], Ecuador (Napo Province) [21], and Argentina (Jujuy and Salta) [10]. In the present article, we provide new distributional data from Honduras. Pseudopostega clavata Davis & Stonis, 2007 Distribution. The species is known from southeastern Puerto Rico (Cayey). Pseudopostega sublobata Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia), Ecuador (Bucay) [10], and Peru (Satipo: selva central) [19]. In the current article, we provide new distribution data from Honduras. Pseudopostega merendoni Stonis & Remeikis, sp. nov. (described herein). Distribution. The species is known from northwestern Honduras (El Merendon). The duplicata group Pseudopostega duplicata Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia, Cartago, Guanacaste, and Limón) and the British Virgin Islands (Tortola Island). In the present article, we provide new distributional data from Honduras. Pseudopostega didyma Davis & Stonis, 2007 Distribution. The species is known from the western slopes of the Andes (Bucay) in Ecuador [10]; recently, it was also discovered in the Amazonian rainforest of Ecuador (Napo Province) [21]. Pseudopostega acidata (Meyrick, 1915) Distribution. The species is known from southern Ecuador (Chimborazo: Huigra) [53], as well as the USA (Texas) [10] but the latter record needs to be re-examined. Pseudopostega tenuifurcata Davis & Stonis, 2007 Distribution. The species is known from northeastern Costa Rica (Heredia). Pseudopostega sectila Davis & Stonis, 2007 Distribution. The species is known from the British Virgin Islands (Tortola Island) and Puerto Rico (Maricao). The microlepta group Pseudopostega microlepta (Meyrick, 1915) Distribution. The species is known from Guyana (Mazaruni-Potaro) and Ecuador (Duran) [53]. The divaricata group Pseudopostega curtarama Davis & Stonis, 2007 Distribution. The species is known from southern Brazil (Minas Gerais and Goias). Pseudopostega crassifurcata Davis & Stonis, 2007 Distribution. The species is known from southeastern Cuba (Sierra Maestra). Pseudopostega turquinoensis Davis & Stonis, 2007 Distribution. The species is known from southeastern Cuba (Santiago). Pseudopostega concava Davis & Stonis, 2007 Distribution. The species is known from northeastern Mexico (Tamaulipas). Pseudopostega acrodicra Davis & Stonis, 2007 Distribution. The species is known from southern Brazil (Minas Gerais). Pseudopostega caulifurcata Davis & Stonis, 2007 Distribution. The species is known from southwestern Brazil (Mato Grosso). Pseudopostega contigua Davis & Stonis, 2007 Distribution. The species is known from Venezuela (Teritorio Federal de Amazonas). Pseudopostega divaricata Davis & Stonis, 2007 Distribution. The species is known from northern Argentina (Jujuy). Pseudopostega denticulata Davis & Stonis, 2007 Distribution. The species is known from the Amazonian rainforest of Ecuador (Napo Province). Pseudopostega brevifurcata Davis & Stonis, 2007 Distribution. The species is known from northern Costa Rica (Heredia and Guanacaste). Pseudopostega brevivalva Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia). Pseudopostega resimafurcata Davis & Stonis, 2007 Distribution. The species is known from southeastern Brazil (Minas Gerais). Pseudopostega ocellata Stonis & Remeikis, sp. nov. (described herein) Distribution. The species is known from the Caribbean coast of northwestern Honduras. The brachybasis group Pseudopostega ecuadoriana Davis & Stonis, 2007 Distribution. The species is known from the Amazonian rainforest of Ecuador (Napo Province). Pseudopostega beckeri Davis & Stonis, 2007 Distribution. The species is known from southern and central Brazil (Goias, Minas Gerais, and Paraná). Pseudopostega protomochla (Meyrick, 1935) Distribution. The species is known from northern Argentina (Córdoba, Catamarca, Salta, and Tucumán) and southern Brazil (Minas Gerais) [10,56]. Pseudopostega venticola (Walsingham, 1897) Distribution. The species is known from Puerto Rico, Dominica, Grenada (Balthazar), Mexico (Campeche), Costa Rica (Guanacaste), Panama (Cabima), Venezuela (Guarico), Brazil (Mato Grosso and Pará), and Ecuador (Napo Province) [10,40], as well as from Florida and Texas, USA [10]. Recently, the species was also recorded from the central Amazonian rainforest of Peru (Satipo) [19]. Pseudopostega longifurcata Davis & Stonis, 2007 Distribution. The species is known from Jamaica (St. Catherine Parish). Pseudopostega spinosa Stonis and Diškus, 2020 Distribution. The species is known from two areas on opposite sides of the Ecuadorian Andes: the western Andean foothills at an elevation of about 700 m, and the premontane Amazonian rainforest at an elevation of about 500 m [19]. Pseudopostega monosperma (Meyrick, 1931) Distribution. The species is known from eastern Brazil (Bahia) [57]. Pseudopostega suffuscula Davis & Stonis, 2007 Distribution. The species is known from northern Argentina (Salta and Tucumán). Pseudopostega bicornuta Davis & Stonis, 2007 Distribution. The species is known from southern Mexico (Chiapas). Pseudopostega brachybasis Davis & Stonis, 2007 Distribution. The species is known from northeastern Mexico (Tamaulipas). Pseudopostega constricta Davis & Stonis, 2007 Distribution. The species is known from southern Mexico (Chiapas). Pseudopostega bifida Stonis & Remeikis, 2020 Distribution. The species is known from the central selva of Peru (Departamento de Junín: Satipo), at an elevation of about 750 m [19]. Pseudopostega latiapicula Davis & Stonis, 2007 Distribution. The species is known from Costa Rica (Heredia) and Brazil (Paraná). Pseudopostega pumila (Walsingham, 1914) Distribution. The species is known from Mexico (Tabasco) [41]. In the present article, we provide new distributional data from Honduras. Ungrouped species Pseudopostega congruens (Walsingham, 1914) Distribution. The species is known from southwestern Mexico (Guerrero) [41]. Pseudopostega elachista (Walsingham, 1914) Distribution. The species is known from southwestern Mexico (Guerrero) [41]. Pseudopostega paromias (Meyrick, 1915) Distribution. The species is known from western Peru (Lima) [53]. Pseudopostega perdigna (Walsingham, 1914) Distribution. The species is known from southwestern Mexico (Guerrero) [41]. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Regier, J.C.; Mitter, C.; Kristensen, N.P.; Davis, D.R.; van Nieukerken, E.J.; Rota, J.; Simonsen, T.J.; Mitter, K.T.; Kawahara, A.Y.; Yen, S.-H.; et al. A molecular phylogeny for the oldest (nonditrysian) lineages of extant Lepidoptera, with implications for classification, comparative morphology and life-history evolution. Syst. Entomol. 2015, 40, 671–704. [Google Scholar] [CrossRef]
- Johansson, R.; Nielsen, E.S.; van Nieukerken, E.J.; Gustafsson, B. The Nepticulidae and Opostegidae (Lepidoptera) of North West Europe, Fauna Entomologica Scandinavica; Brill: Leiden, The Netherlands, 1990; pp. 1–739. ISBN 978-900-408-698-2. [Google Scholar]
- Minet, J. Contribution a l’analyse phylogénétique des Néolepidopteres (Lepidoptera, Glossata). Nouv. Rev. Entomol. 1984, 1, 139–149. [Google Scholar]
- Kristensen, N.P.; Nielsen, E.S. The ventral diaphragm of primitive (non-ditrysian) Lepidoptera: A morphological and phylogenetic study. J. Zool. Syst. Evol. Res. 1980, 18, 123–146. [Google Scholar] [CrossRef]
- Kristensen, N.P.; Skalski, A.W. Phylogeny and Palaentology. In Lepidoptera, Moths and Butterflies. 1. Evolution, Systematics and Biogeography; Kristensen, N.P., Ed.; De Gruyter: Berlin, Germany, 1999; pp. 7–25. ISBN 978-311-015-704-8. [Google Scholar]
- Davis, D.R. The monotrysian Heteroneura. In Lepidoptera, Moths and Butterflies. 1. Evolution, Systematics and Biogeography; Kristensen, N.P., Ed.; De Gruyter: Berlin, Germany, 1999; pp. 65–90. ISBN 978-311-015-704-8. [Google Scholar]
- Doorenweerd, C.; van Nieukerken, E.J.; Hoare, R.J.B. Phylogeny, classification and divergence times of pygmy leaf-mining moths (Lepidoptera: Nepticulidae): The earliest lepidopteran radiation on Angiosperms? Syst. Entomol. 2016, 42, 267–287. [Google Scholar] [CrossRef]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Baryshnikova, S.; Solis, M.A. What are the smallest moths (Lepidoptera) in the world? Zootaxa 2021, 4942, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Puplesis, R.; Robinson, G.S. Revision of the Oriental Opostegidae (Lepidoptera) with general comments on phylogeny within the family. Bull. Nat. Hist. Mus. Lond. (Ent.) 1999, 68, 1–92. [Google Scholar]
- Davis, D.R.; Stonis, J.R. A Revision of the New World Plant-Mining Moths of the Family Opostegidae (Lepidoptera: Nepticuloidea); Smithsonian Institution Scholarly Press: Washington, DC, USA, 2007; 212p. [Google Scholar]
- Heppner, B.J.; Davis, D.R. Guatemala moth notes, 2. A new Neopostega from Guatemala (Lepidoptera: Opostegidae). Lepid. Novae 2009, 2, 31–34. [Google Scholar]
- Stonis, J.R.; Remeikis, A.; Solis, M.A.; Karsholt, O. Diagnostics and updated checklist of Oriental Pseudopostega (Opostegidae), including the matrona species group with a new, extralimital species discovered in the Mediterranean. Zootaxa 2021, 4933, 341–360. [Google Scholar] [CrossRef]
- Davis, D.R. Generic Revision of the Opostegidae, with a Synoptic Catalog of the World’s Species (Lepidoptera: Nepticuloidea); Smithsonian Institution Press: Washington, DC, USA, 1989; 97p. [Google Scholar]
- Kumata, T. Cambium miners making pith flecks in broad-leaved trees. Hoppo Ringyo 1984, 36, 6–15. [Google Scholar]
- Grossenbacher, J.G. Medullary spots: A contribution to the life history of some cambium miners. N. Y. Agric. Exp. Sta. Techn. Bull. 1910, 15, 49–65. [Google Scholar]
- Swezey, O.H. Opostega in the Hawaiian Islands (Lep.). Proc. Hawaii. Entomol. Soc. 1921, 4, 531–536. [Google Scholar]
- Puplesis, R.; Diškus, A. The Nepticuloidea & Tischerioidea (Lepidoptera)—A Global Review, with Strategic Regional Revisions; Lututė Publishers: Kaunas, Lithuania, 2003; 512p, ISBN 995-557-509-3. [Google Scholar]
- van Nieukerken, E.J.; Doorenweerd, C.; Hoare, R.; Davis, D.R. Revised classification and catalogue of global Nepticulidae and Opostegidae (Lepidoptera, Nepticuloidea). ZooKeys 2016, 628, 65–246. [Google Scholar] [CrossRef]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Vargas, S.A.; Solis, M.A. Opostegidae (Lepidoptera) of the Americas: Updated catalog, diagnostics, and new species descriptions. Proc. Entomol. Soc. Wash. 2020, 122, 929–972. [Google Scholar] [CrossRef]
- Stonis, J.R.; Remeikis, A.; Sruoga, V. An annotated list of the Opostegidae of the Himalaya, with a description of Pseudopostega brevicaudata sp. nov. (Lepidoptera: Nepticuloidea). Zootaxa 2013, 3609, 182–194. [Google Scholar] [CrossRef]
- Remeikis, A.; Stonis, J.R.; Diškus, A.; Davis, D.R. Contribution to the Opostegidae fauna of Central America, with an updated checklist and description of new species from Costa Rica and Mexico (Insecta: Lepidoptera). Acta Zoologica Lituanica 2009, 19, 278–286. [Google Scholar] [CrossRef]
- Stonis, J.R.; Diškus, A.; Remeikis, A.; Orlovskytė, S. Dvidulopsis gen. nov., a rare Neotropical genus of pygmy moths (Nepticulidae) endemic to lowland humid forests, a biome of conservation priority. Zootaxa 2025, 5609, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Stonis, J.R.; Diškus, A.; Remeikis, A.; Orlovskytė, S.; Katinas, L. First documentation of Nepticulidae feeding on Gondwanan relict Nothofagus from Andean Patagonia and the unexpected discovery of morphologically similar pygmy moth species from distant Central America. Biologija 2025, 71, 1–31. [Google Scholar] [CrossRef]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Dobrynina, V.; Orlovskytė, S. A phenomenon: What are the minuscule grey moths abundant in the dry season in the tropical dry forests of the Pacific coast of Honduras? Insects 2024, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- Faber-Langendoen, D. F020 Tropical Lowland Humid Forest Formation. Available online: https://www1.usgs.gov/csas/nvcs/unitDetails/860256 (accessed on 28 August 2025).
- Faber-Langendoen, D.; Keeler-Wolf, T.; Meidinger, D.; Josse, C.; Weakley, A.; Tart, D.; Navarro, G.; Hoagland, B.; Ponomarenko, S.; Fults, G.; et al. Classification and Description of World Formation Types; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016; 222p. [Google Scholar]
- Brehm, G. A new LED lamp for the collection of nocturnal Lepidoptera and a spectral comparison of lighttrapping lamps. Nota Lepidopterol. 2017, 40, 87–108. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. PM 7/129 (1) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull. 2016, 46, 501–537. [Google Scholar] [CrossRef]
- European and Mediterranean Plant Protection Organization. PM 7/129 (2) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull. 2021, 51, 100–143. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2025. Nucleic Acids Res. 2025, 53, D30–D44. [Google Scholar] [CrossRef]
- Stonis, J.R.; Diškus, A.; Orlovskytė, S. Discovery of the genus Dishkeya (Lepidoptera: Tischeriidae) in Honduras: Unveiling the unique traits of D. gouaniae. Biologija 2024, 70, 116–129. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data system. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, version 1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 28 August 2025).
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.J.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K.A. TCS: Estimating gene genealogies. In Proceedings of the 16th International Parallel and Distributed Processing Symposium, Fort Lauderdale, FL, USA, 15–19 April 2002; p. 184. [Google Scholar]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Walsingham, T.G. Revision of the West-Indian Micro-Lepidoptera, with descriptions of new species. Proc. Gen. Meet. Sci. Bus. Zool. Soc. Lond. 1897, 1, 54–183. [Google Scholar]
- Walsingham, T.G. Insecta. Lepidoptera—Heterocera. In Biologia Centrali-Americana; Godman, F.D., Salvin, O., Eds.; Taylor & Francis: London, UK, 1914; pp. 225–393. [Google Scholar]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Janzen, D.H.; Burns, J.M.; Hallwachs, W.; Hebert, P.D.N. DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. USA 2006, 103, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, A.; Godfray, H.C.J.; Huemer, P.; Mutanen, M.; Rougerie, R.; van Nieukerken, E.J.; Ratnasingham, S.; Hebert, P.D.N. Genetic patterns in European geometrid moths revealed by the Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e84518. [Google Scholar] [CrossRef]
- Nneji, L.M.; Adeola, A.C.; Ayoola, A.O.; Oladipo, S.O.; Wang, Y.-Y.; Malann, Y.D.; Anyaele, O.; Nneji, I.C.; Rahman, M.M.; Olory, C.S. DNA barcoding and species delimitation of butterflies (Lepidoptera) from Nigeria. Mol. Biol. Rep. 2020, 47, 9441–9457. [Google Scholar] [CrossRef]
- Huemer, P.; Wieser, C. DNA barcode library of megadiverse Lepidoptera in an alpine nature park (Italy) reveals unexpected species. Diversity 2023, 15, 214. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographical regionalisation of the Neotropical region. Zootaxa 2014, 3782, 1–110. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Biogeographical regionalisation of the Andean region. Zootaxa 2015, 3936, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Gerulaitis, V. The Ando-Patagonian Stigmella magnispinella group (Lepidoptera, Nepticulidae) with description of new species from Ecuador, Peru and Argentina. Zootaxa 2016, 4200, 561–579. [Google Scholar] [CrossRef]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Megoran, N. New species of leaf-mining Nepticulidae (Lepidoptera) from the Neotropical and Ando-Patagonian regions, with new data on host-plants. Zootaxa 2017, 4272, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, E. Descriptions of South American Micro-Lepidoptera. Trans. R. Entomol. Soc. Lond. 1915, 63, 201–256. [Google Scholar] [CrossRef]
- Meyrick, E. Exotic Microlepidoptera; Taylor and Francis: London, UK, 1920; Volume 2, pp. 353–384. [Google Scholar]
- Eyer, J.R. A new species of Opostegidae from Florida. Fla. Entomol. 1967, 50, 39–42. [Google Scholar] [CrossRef]
- Meyrick, E. Exotic Microlepidoptera; Taylor and Francis: London, UK, 1935; Volume 4, pp. 545–576. [Google Scholar]
- Meyrick, E. Exotic Microlepidoptera; Taylor and Francis: London, UK, 1931; Volume 4, pp. 161–192. [Google Scholar]
- Sun, H.; Wang, S. First report of the genus Opostega Zeller, 1839 (Lepidoptera, Opsotegidae) from China, with descriptions of two new species. Zootaxa 2025, 5660, 267–272. [Google Scholar] [CrossRef] [PubMed]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stonis, J.R.; Remeikis, A.; Orlovskytė, S. New Discoveries Supporting the Exceptional Species Diversity of Opostegidae in Central America and the Caribbean, Alerting on Misidentified Barcodes. Insects 2025, 16, 1170. https://doi.org/10.3390/insects16111170
Stonis JR, Remeikis A, Orlovskytė S. New Discoveries Supporting the Exceptional Species Diversity of Opostegidae in Central America and the Caribbean, Alerting on Misidentified Barcodes. Insects. 2025; 16(11):1170. https://doi.org/10.3390/insects16111170
Chicago/Turabian StyleStonis, Jonas R., Andrius Remeikis, and Svetlana Orlovskytė. 2025. "New Discoveries Supporting the Exceptional Species Diversity of Opostegidae in Central America and the Caribbean, Alerting on Misidentified Barcodes" Insects 16, no. 11: 1170. https://doi.org/10.3390/insects16111170
APA StyleStonis, J. R., Remeikis, A., & Orlovskytė, S. (2025). New Discoveries Supporting the Exceptional Species Diversity of Opostegidae in Central America and the Caribbean, Alerting on Misidentified Barcodes. Insects, 16(11), 1170. https://doi.org/10.3390/insects16111170





