Vector Potential of Nosema-Infected Drones in Honey Bees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Experimental Approaches
2.2. Group Inoculation
2.3. Drifting and Space Use
2.4. Intruder Assay
2.5. Social Interactions
2.6. Energetic Stress
3. Results
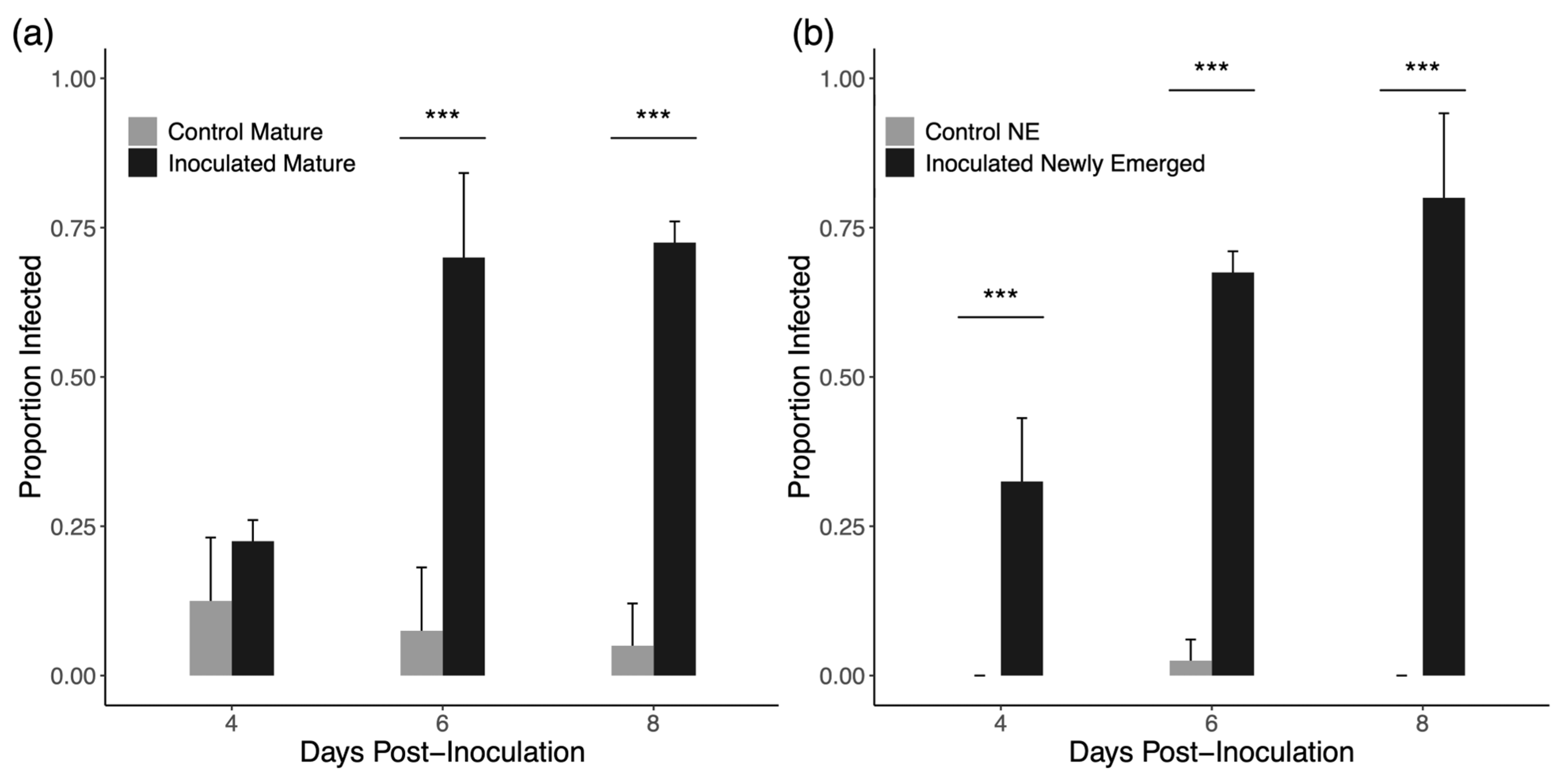
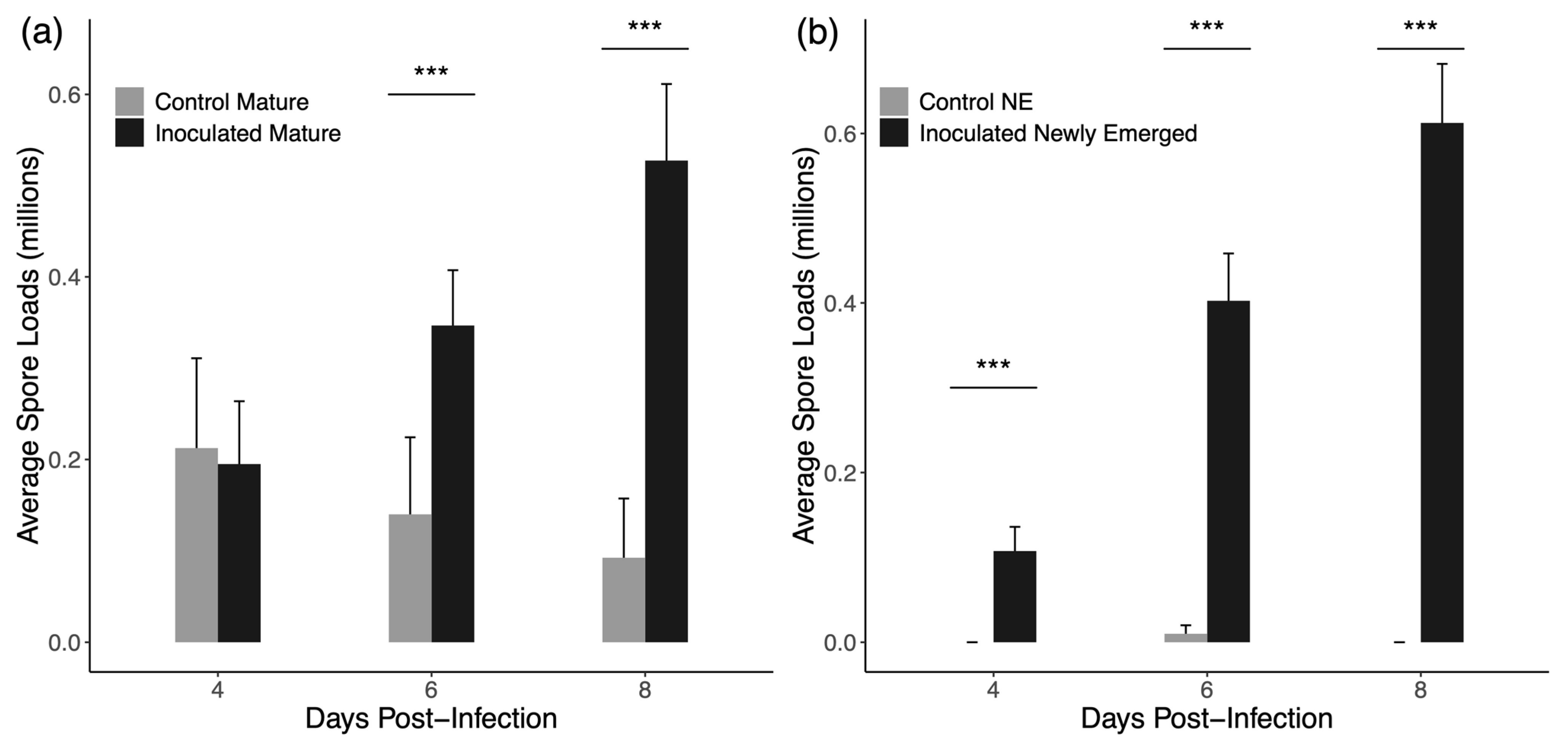
3.1. Group Inoculation
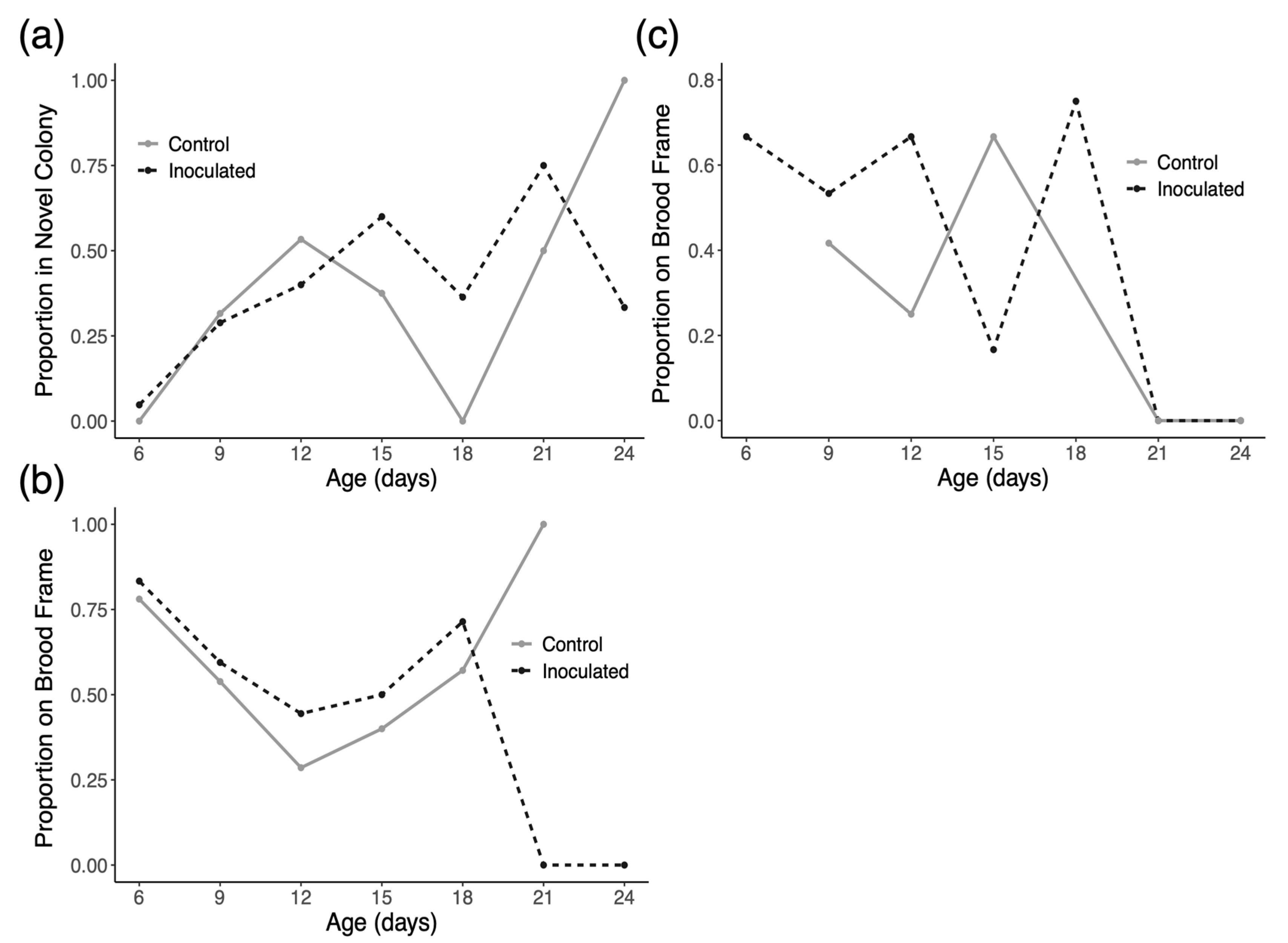
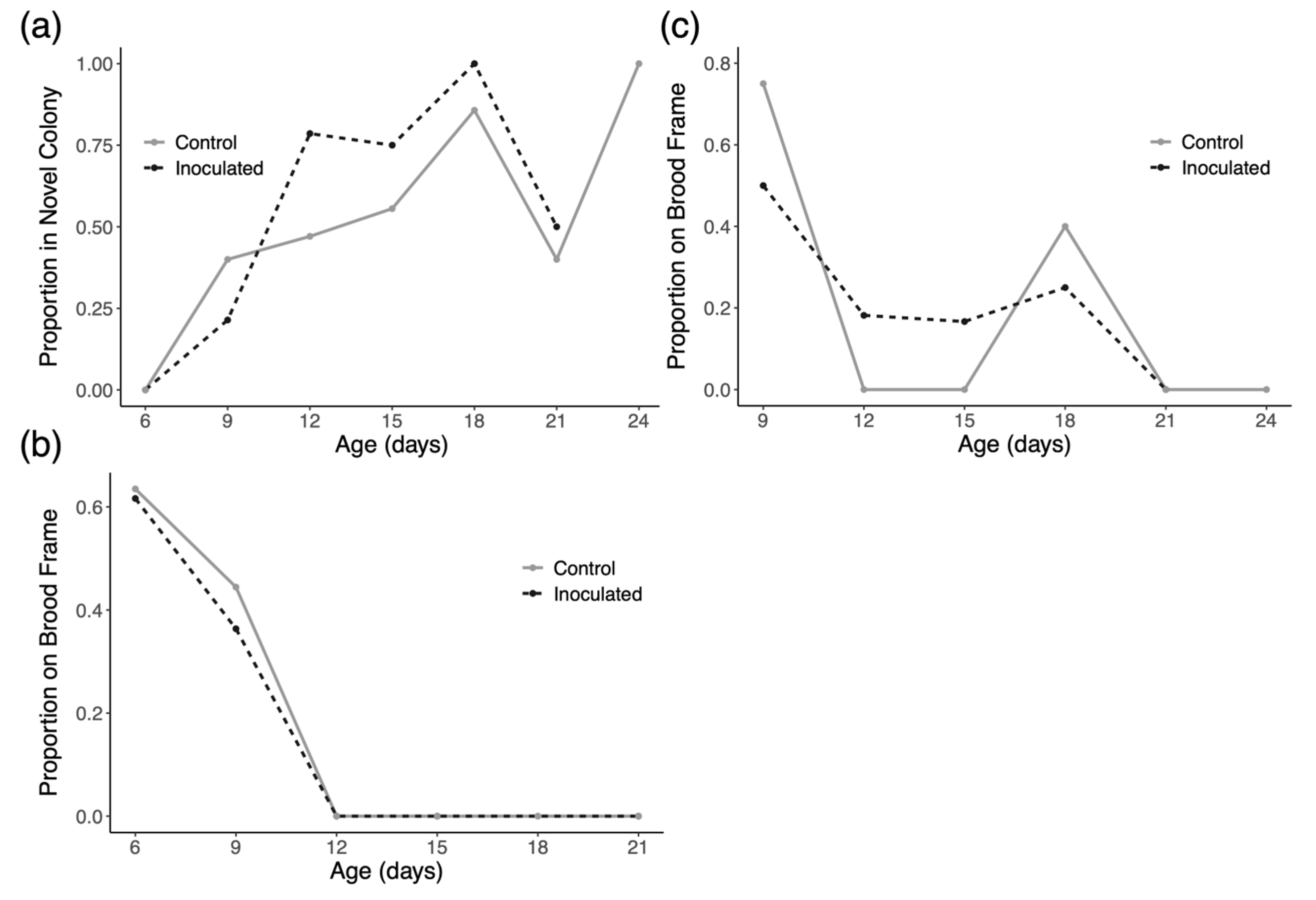
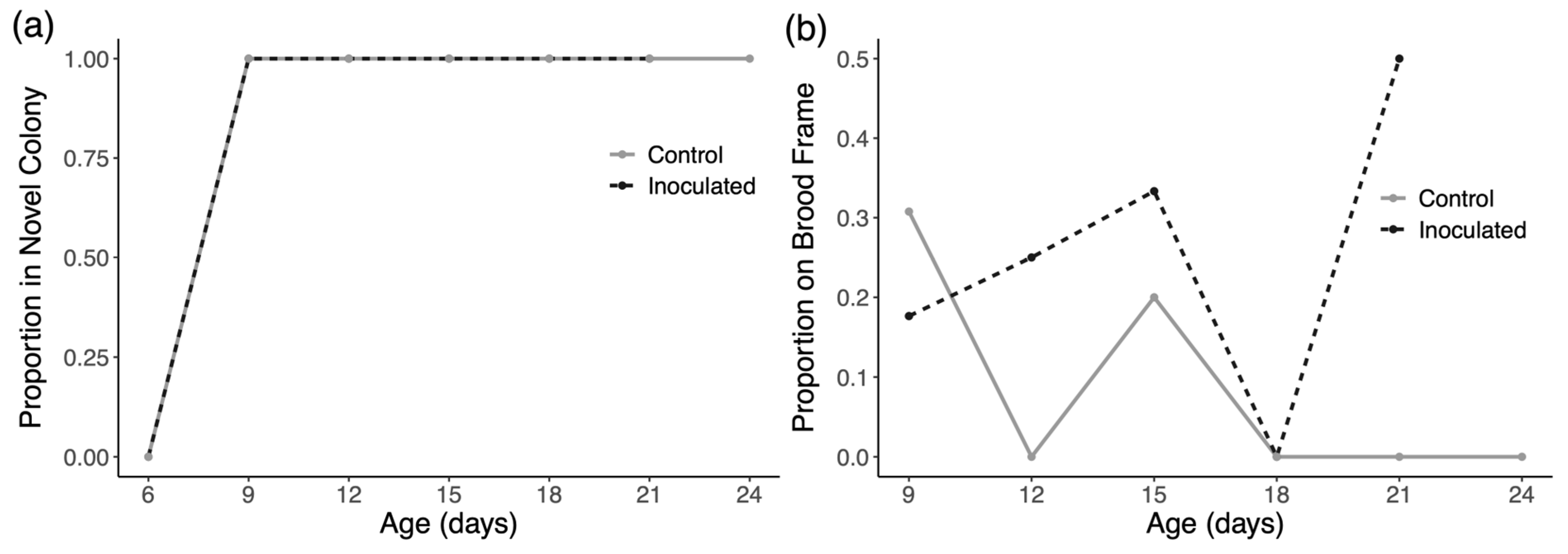
3.2. Drifting and Space Use
3.3. Intruder Assay
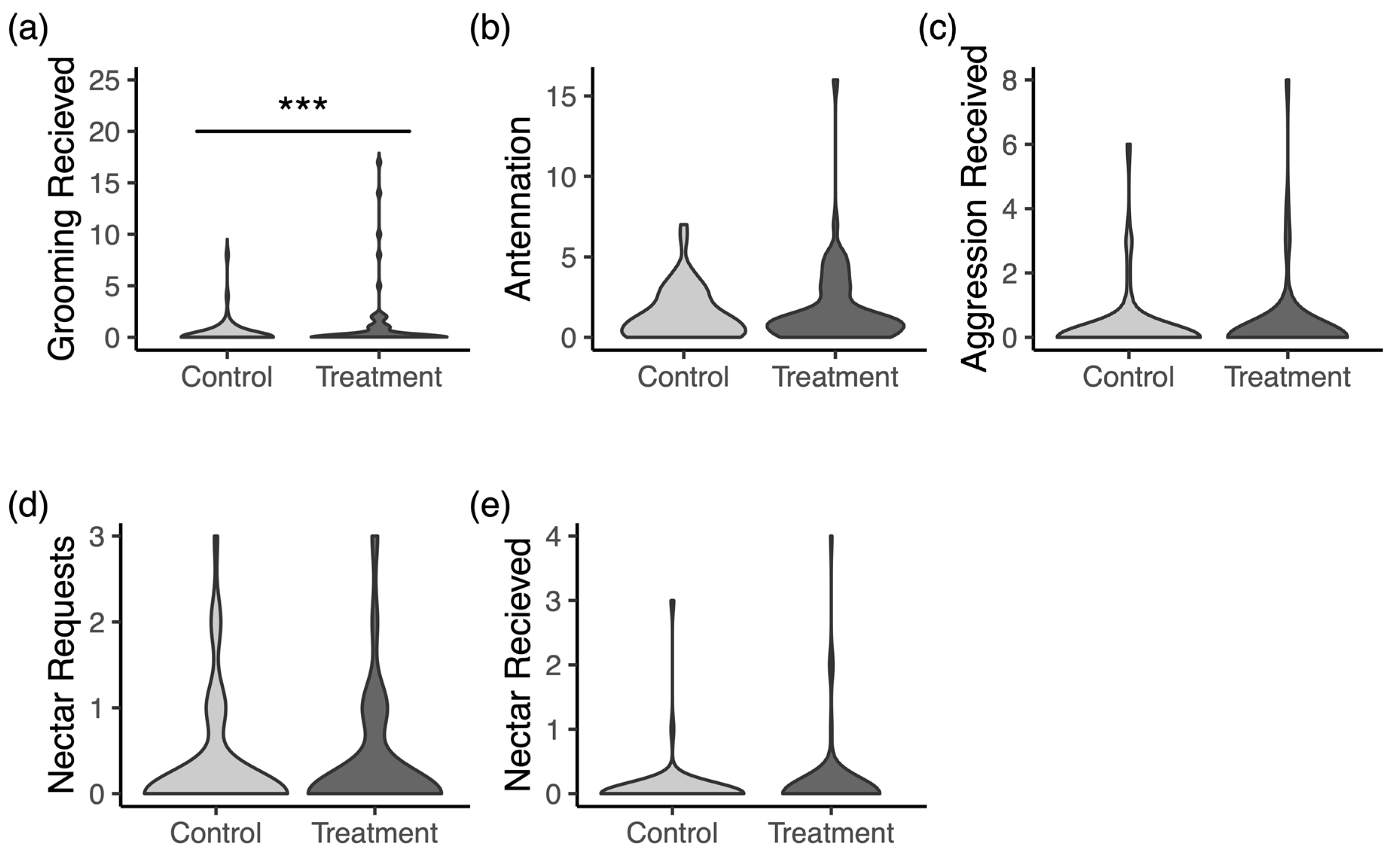
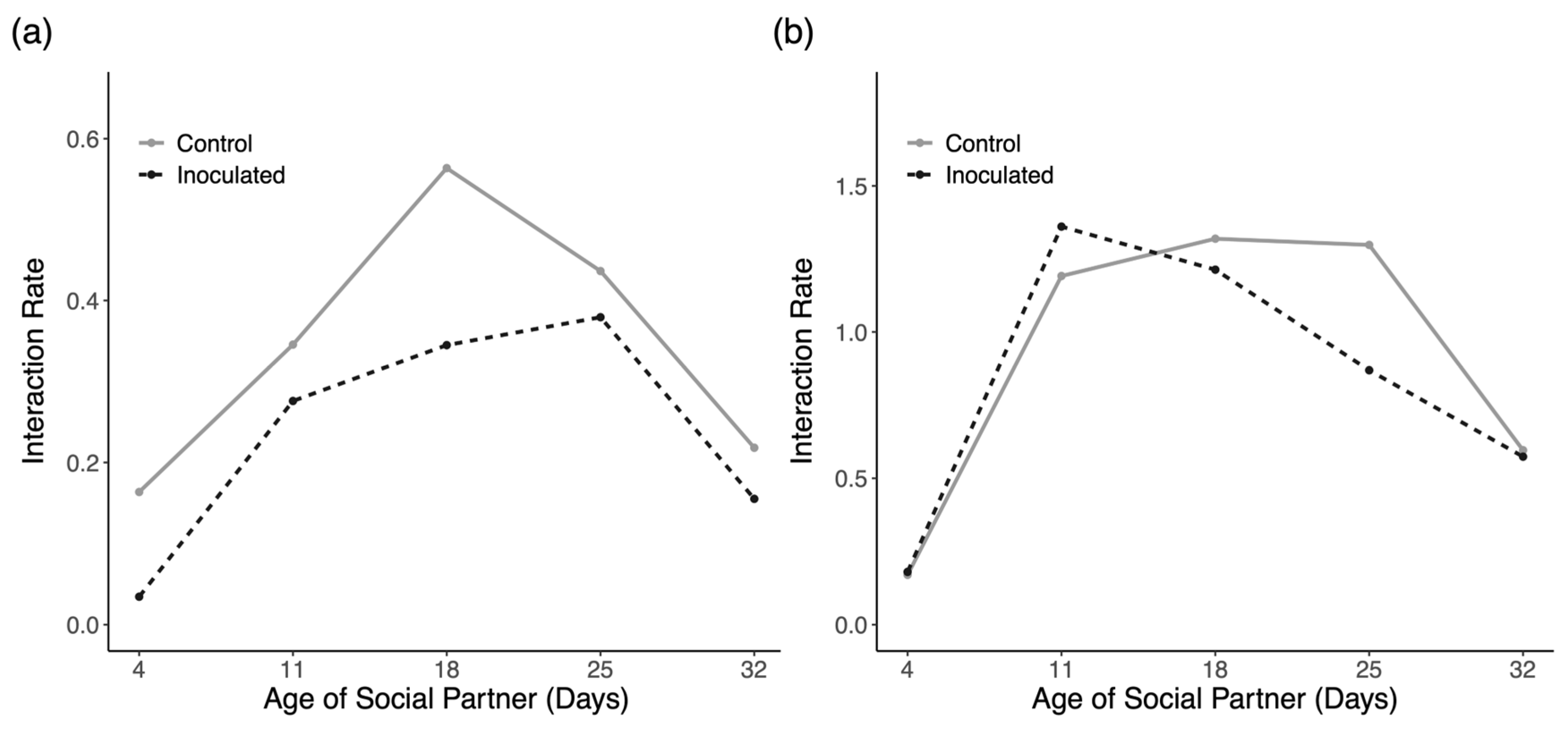
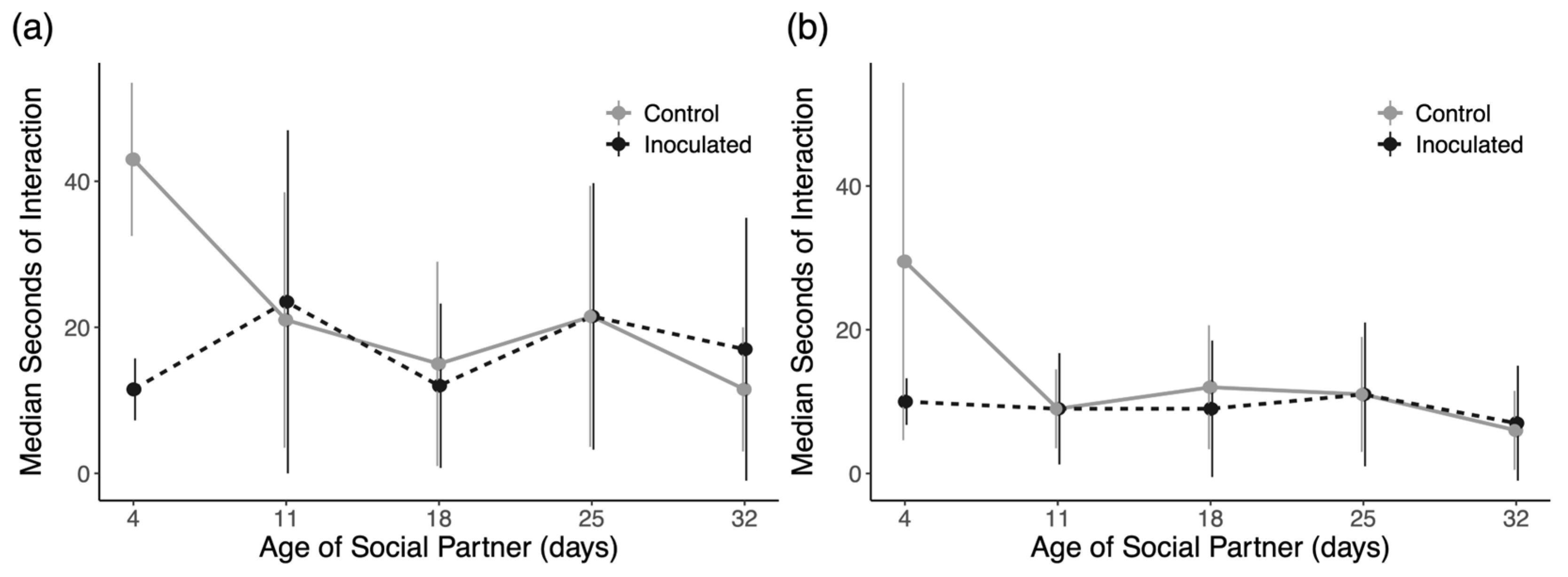
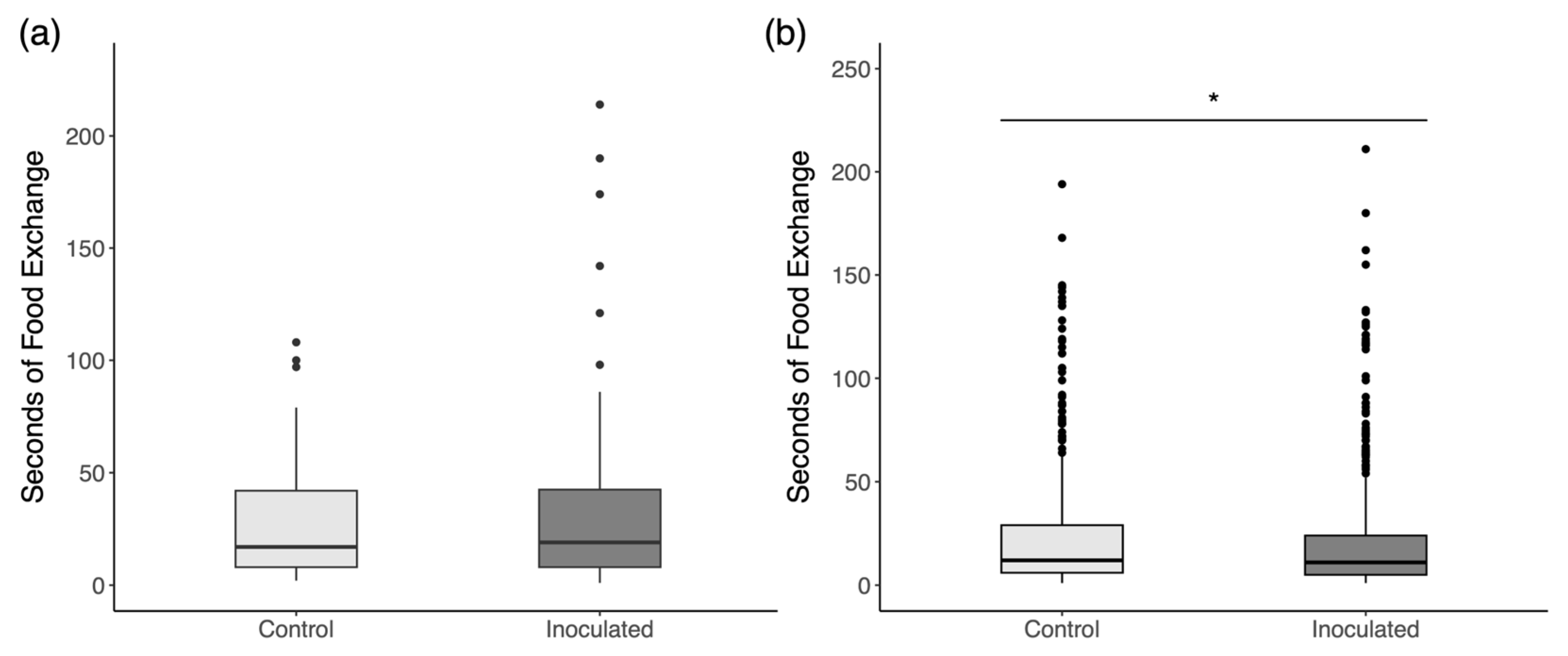
3.4. Social Interactions
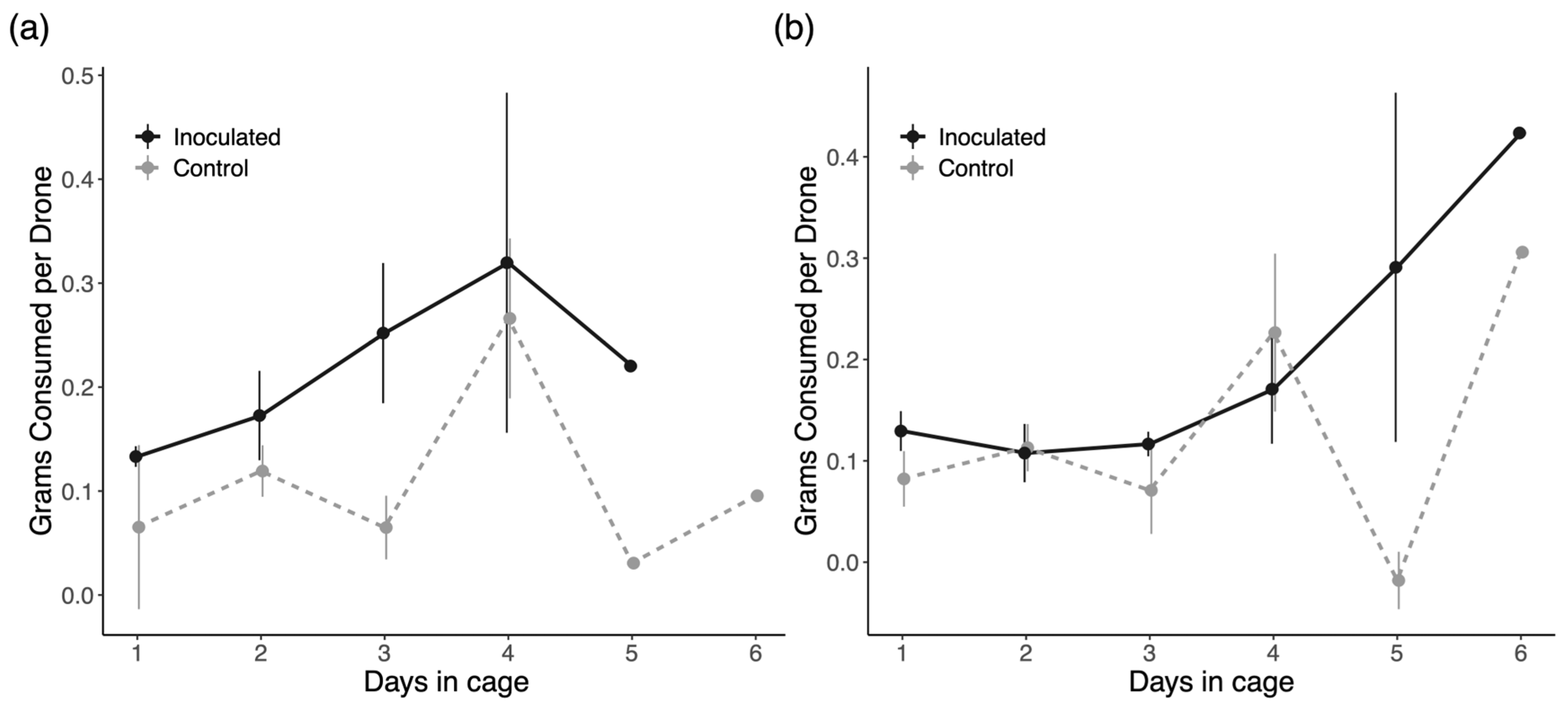
3.5. Energetic Stress
4. Discussion
4.1. Group Inoculation
4.2. Drifting and Space Use
4.3. Intruder Assay
4.4. Social Interactions
4.5. Energetic Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderone, N.W. Insect pollinated crops, insect pollinators and US agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE 2012, 7, e37235. [Google Scholar] [CrossRef]
- Hipólito, J.; Sousa, B.D.S.B.; Borges, R.C.; de Brito, R.M.; Jaffé, R.; Dias, S.; Fonseca, V.L.I.; Giannini, T.C. Valuing nature’s contribution to people: The pollination services provided by two protected areas in Brazil. Glob. Ecol. Conserv. 2019, 20, e00782. [Google Scholar] [CrossRef]
- Ferrier, P.M.; Rucker, R.R.; Thurman, W.N.; Burgett, M. Economic Effects and Responses to Changes in Honey Bee Health; United States Department of Agriculture, Economic Research Service: Washington, DC, USA, 2018. [Google Scholar]
- Khalifa, S.A.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; El-Seedi, H.R. Overview of bee pollination and its economic value for crop production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Schwarz, R.S. Bees brought to their knees: Microbes affecting honey bee health. Trends Microbiol. 2011, 19, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Cremer, S.; Armitage, S.A.; Schmid-Hempel, P. Social immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef]
- Durrer, S. Schmid-Hempel PShared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 258, 299–302. [Google Scholar]
- Naug, D. Structure of the social network and its influence on transmission dynamics in a honeybee colony. Behav. Ecol. Sociobiol. 2008, 62, 1719–1725. [Google Scholar] [CrossRef]
- McArt, S.H.; Koch, H.; Irwin, R.E.; Adler, L.S. Arranging the bouquet of disease: Floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 2014, 17, 624–636. [Google Scholar] [CrossRef]
- Graystock, P.; Goulson, D.; Hughes, W.O. Parasites in bloom: Flowers aid dispersal and transmission of pollinator parasites within and between bee species. Proc. R. Soc. B 2015, 282, 20151371. [Google Scholar] [CrossRef]
- Free, J.B. The drifting of honey-bees. J. Agric. Sci. 1958, 51, 294–306. [Google Scholar] [CrossRef]
- Bailey, L. Nosema Apis in Drone Honeybees. J. Apic. Res. 1972, 11, 171–174. [Google Scholar] [CrossRef]
- Currie, R.W. The biology and behaviour of drones. Bee World 1987, 68, 129–143. [Google Scholar] [CrossRef]
- Fievet, J.; Tentcheva, D.; Gauthier, L.; De Miranda, J.; Cousserans, F.; Colin, M.E.; Bergoin, M. Localization of deformed wing virus infection in queen and drone Apis mellifera L. Virol. J. 2006, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Schröder, M.; Bienefeld, K.; Genersch, E. Detection of viral sequences in semen of honeybees (Apis mellifera): Evidence for vertical transmission of viruses through drones. J. Invertebr. Pathol. 2006, 92, 105–108. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Fries, I. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 2008, 98, 184–189. [Google Scholar] [CrossRef]
- Yañez, O.; Jaffé, R.; Jarosch, A.; Fries, I.; Moritz, R.F.; Paxton, R.J.; de Miranda, J.R. Deformed wing virus and drone mating flights in the honey bee (Apis mellifera): Implications for sexual transmission of a major honey bee virus. Apidologie 2012, 43, 17–30. [Google Scholar] [CrossRef]
- Amiri, E.; Kryger, P.; Meixner, M.D.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Quantitative patterns of vertical transmission of deformed wing virus in honey bees. PLoS ONE 2018, 13, e0195283. [Google Scholar] [CrossRef]
- Le Conte, Y.; Arnold, G.; Trouiller, J.; Masson, C.; Chappe, B.; Ourisson, G. Attraction of the parasitic mite Varroa to the drone larvae of honey bees by simple aliphatic esters. Science 1989, 245, 638–639. [Google Scholar] [CrossRef]
- Fuchs, S. Choice in Varroa jacobsoni Oud. between honey bee drone or workerbrood cells for reproduction. Behav. Ecol. Sociobiol. 1992, 31, 429–435. [Google Scholar] [CrossRef]
- Traver, B.E.; Fell, R.D. Nosema ceranae in drone honey bees (Apis mellifera). J. Invertebr. Pathol. 2011, 107, 234–236. [Google Scholar] [CrossRef]
- Laughton, A.M.; Boots, M.; Siva-Jothy, M.T. The ontogeny of immunity in the honey bee Apis mellifera L. following an immune challenge. J. Insect Physiol. 2011, 57, 1023–1032. [Google Scholar] [CrossRef]
- Currie, R.W.; Jay, S.C. Drifting behaviour of drone honey bees (Apis mellifera L.) in commercial apiaries. J. Apic. Res. 1991, 30, 61–68. [Google Scholar] [CrossRef]
- Smith, M.L. The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS ONE 2012, 7, e43319. [Google Scholar] [CrossRef]
- Goblirsch, M. Nosema ceranae disease of the honey bee (Apis mellifera). Apidologie 2018, 49, 131–150. [Google Scholar] [CrossRef]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Botías, C.; Anderson, D.L.; Meana, A.; Garrido-Bailón, E.; Martín-Hernández, R.; Higes, M. Further evidence of an oriental origin for Nosema ceranae (Microsporidia: Nosematidae). J. Invertebr. Pathol. 2012, 110, 108–113. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- VanEngelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 8, e6481. [Google Scholar] [CrossRef]
- Forsgren, E.; Fries, I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Moritz, R.F.A.; De Miranda, J.; Fries, I.; Le Conte, Y.; Neumann, P.; Paxton, R. Research strategies to improve honeybee health in Europe. Apidologie 2010, 41, 227–242. [Google Scholar] [CrossRef]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors associated with honey bee colony losses: A mini-review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef]
- Ostap-Chec, M.; Cait, J.; Scott, R.W.; Arct, A.; Moroń, D.; Rapacz, M.; Miler, K. Nosemosis negatively affects honeybee survival: Experimental and meta-analytic evidence. Parasitology 2024, 151, 1530–1542. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Naug, D.; Gibbs, A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 2009, 40, 595–599. [Google Scholar] [CrossRef]
- Campbell, J.; Kessler, B.; Mayack, C.; Naug, D. Behavioural fever in infected honeybees: Parasitic manipulation or coincidental benefit? Parasitology 2010, 137, 1487–1491. [Google Scholar] [CrossRef]
- Holt, H.L.; Aronstein, K.A.; Grozinger, C.M. Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genom. 2013, 14, 799. [Google Scholar] [CrossRef]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef]
- Poulin, R. “Adaptive” changes in the behaviour of parasitized animals: A critical review. Int. J. Parasitol. 1995, 25, 1371–1383. [Google Scholar] [CrossRef]
- Heil, M. Host manipulation by parasites: Cases, patterns, and remaining doubts. Front. Ecol. Evol. 2016, 4, 80. [Google Scholar] [CrossRef]
- Richard, F.J.; Aubert, A.; Grozinger, C.M. Modulation of social interactions by immune stimulation in honey bee, Apis mellifera, workers. BMC Biol. 2008, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.E.; Hughes, W. Horizontal transmission of a parasite is influenced by infected host phenotype and density. Parasitology 2015, 142, 395–405. [Google Scholar] [CrossRef]
- Chen, Y.P.; Huang, Z.Y. Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie 2010, 41, 364–374. [Google Scholar] [CrossRef]
- Urbieta-Magro, A.; Higes, M.; Meana, A.; Barrios, L.; Martín-Hernández, R. Age and method of inoculation influence the infection of worker honey bees (Apis mellifera) by Nosema ceranae. Insects 2019, 10, 417. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P. Widespread dispersal of the microsporidian Nosema ceranae an emergent pathogen of the western honey bee Apis mellifera. J. Invert. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.D.; Zhou, L.; Boncristiani, H.; Kimura, K.; Xiao, T.; Pettis, J.S. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invertebr. Pathol. 2009, 101, 204–209. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; Higes, M. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef]
- Grupe, A.C.; Quandt, C.A. A growing pandemic: A review of Nosema parasites in globally distributed domesticated and native bees. PLoS Pathog. 2020, 16, e1008580. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard methods for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 1 January 2023).
- Delaplane, K.S.; Van Der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Geffre, A.C.; Gernat, T.; Harwood, G.P.; Jones, B.M.; Morselli Gysi, D.; Hamilton, A.R.; Dolezal, A.G. Honey bee virus causes context-dependent changes in host social behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 10406–10413. [Google Scholar] [CrossRef]
- Pettis, J.S.; Vanengelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef]
- Tanner, G.; Williams, G.R.; Mehmann, M.; Neumann, P. Comparison of mass versus individual inoculation of worker honey bees with Nosema ceranae. In Proceedings of the 8th COLOSS Conference/MC Meeting FA0803, Halle-Saale, Germany, 1–3 September 2012. [Google Scholar]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Gisder, S.; Möckel, N.; Linde, A.; Genersch, E. A cell culture model for Nosema ceranae and Nosema apis allows new insights into the life cycle of these important honey bee-pathogenic microsporidia. Environ. Microbiol. 2011, 13, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.D.; Sheppard, W.S. Nosema ceranae in age cohorts of the western honey bee (Apis mellifera). J. Invertebr. Pathol. 2012, 109, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Retschnig, G.; Williams, G.R.; Mehmann, M.M.; Yanez, O.; De Miranda, J.R.; Neumann, P. Sex-specific differences in pathogen susceptibility in honey bees (Apis mellifera). PLoS ONE 2014, 9, e85261. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Lin, Z.G.; Huang, S.K.; Sohr, A.; Wu, L.; Chen, Y.P. Spore loads may not be used alone as a direct indicator of the severity of Nosema ceranae infection in honey bees Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 2014, 107, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.; Fuchs, S. Nosema sp. influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 2009, 4, 21–28. [Google Scholar]
- Wolf, S.; McMahon, D.P.; Lim, K.S.; Pull, C.D.; Clark, S.J.; Paxton, R.J.; Osborne, J.L. So Near and Yet So Far: Harmonic Radar Reveals Reduced Homing Ability of Nosema Infected Honeybees. PLoS ONE 2014, 9, e103989. [Google Scholar] [CrossRef] [PubMed]
- Forfert, N.; Natsopoulou, M.E.; Frey, E.; Rosenkranz, P.; Paxton, R.J.; Moritz, R.F. Parasites and pathogens of the honeybee (Apis mellifera) and their influence on inter-colonial transmission. PLoS ONE 2015, 10, e0140337. [Google Scholar] [CrossRef]
- Bordier, C.; Pioz, M.; Crauser, D.; Le Conte, Y.; Alaux, C. Should I stay or should I go: Honeybee drifting behaviour as a function of parasitism. Apidologie 2016, 48, 286–297. [Google Scholar] [CrossRef]
- Pfeiffer, K.J.; Crailsheim, K.J.I.S. Drifting of honeybees. Insectes Sociaux 1998, 45, 151–167. [Google Scholar] [CrossRef]
- Southwick, E.E.; Buchmann, S.L. Effects of horizon landmarks on homing success in honey bees. Am. Nat. 1995, 146, 748–764. [Google Scholar] [CrossRef]
- Smart, M.D.; Pettis, J.S.; Euliss, N.; Spivak, M.S. Land use in the Northern Great Plains region of the US influences the survival and productivity of honey bee colonies. Agric. Ecosyst. Environ. 2016, 230, 139–149. [Google Scholar] [CrossRef]
- Ohtani, T.; Fukuda, H. Factors governing the spatial distribution of adult drone honeybees in the hive. J. Apic. Res. 1977, 16, 14–26. [Google Scholar] [CrossRef]
- Free, J.B. The food of adult drone honeybees (Apis mellifera). Br. J. Anim. Behav. 1957, 5, 7–11. [Google Scholar] [CrossRef]
- Johnson, B.R. Honey Bee Biology; Princeton University Press: Princeton, NJ, USA, 2023. [Google Scholar]
- Konrad, M.; Vyleta, M.L.; Theis, F.J.; Stock, M.; Tragust, S.; Klatt, M.; Drescher, V.; Marr, C.; Ugelvig Cremer, S. Social. transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 2012, 10, e1001300. [Google Scholar] [CrossRef]
- Baracchi, D.; Fadda, A.; Turillazzi, S. Evidence for antiseptic behaviour towards sick adult bees in honey bee colonies. J. Insect Physiol. 2012, 58, 1589–1596. [Google Scholar] [CrossRef]
- Leclerc, J.B.; Detrain, C. Ants detect but do not discriminate diseased workers within their nest. Naturwissenschaften 2016, 103, 70. [Google Scholar] [CrossRef]
- Perez, A.; Johnson, B.R. Centrality of Hygienic Honey Bee Workers in Colony Social Networks. Insects 2025, 16, 58. [Google Scholar] [CrossRef]
- Baracchi, D.; Cini, A. A socio-spatial combined approach confirms a highly compartmentalised structure in honeybees. Ethology 2014, 120, 1167–1176. [Google Scholar] [CrossRef]
- Crailsheim, K. Interadult feeding of jelly in honeybee (Apis mellifera L.) colonies. J. Comp. Physiol. B 1991, 161, 55–60. [Google Scholar] [CrossRef]
- Crailsheim, K. The flow of jelly within a honeybee colony. J. Comp. Physiol. B 1992, 162, 681–689. [Google Scholar] [CrossRef]
- Crailsheim, K. Trophallactic interactions in the adult honeybee (Apis mellifera L.). Apidologie 1998, 29, 97–112. [Google Scholar] [CrossRef]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with ml. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, A.; Johnson, B.R. Vector Potential of Nosema-Infected Drones in Honey Bees. Insects 2025, 16, 1142. https://doi.org/10.3390/insects16111142
Perez A, Johnson BR. Vector Potential of Nosema-Infected Drones in Honey Bees. Insects. 2025; 16(11):1142. https://doi.org/10.3390/insects16111142
Chicago/Turabian StylePerez, Adrian, and Brian R. Johnson. 2025. "Vector Potential of Nosema-Infected Drones in Honey Bees" Insects 16, no. 11: 1142. https://doi.org/10.3390/insects16111142
APA StylePerez, A., & Johnson, B. R. (2025). Vector Potential of Nosema-Infected Drones in Honey Bees. Insects, 16(11), 1142. https://doi.org/10.3390/insects16111142






