Oviposition Behavior of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Panama Under Experimental L4-Larval Co-Occurrence Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Sampling and Colony Establishment
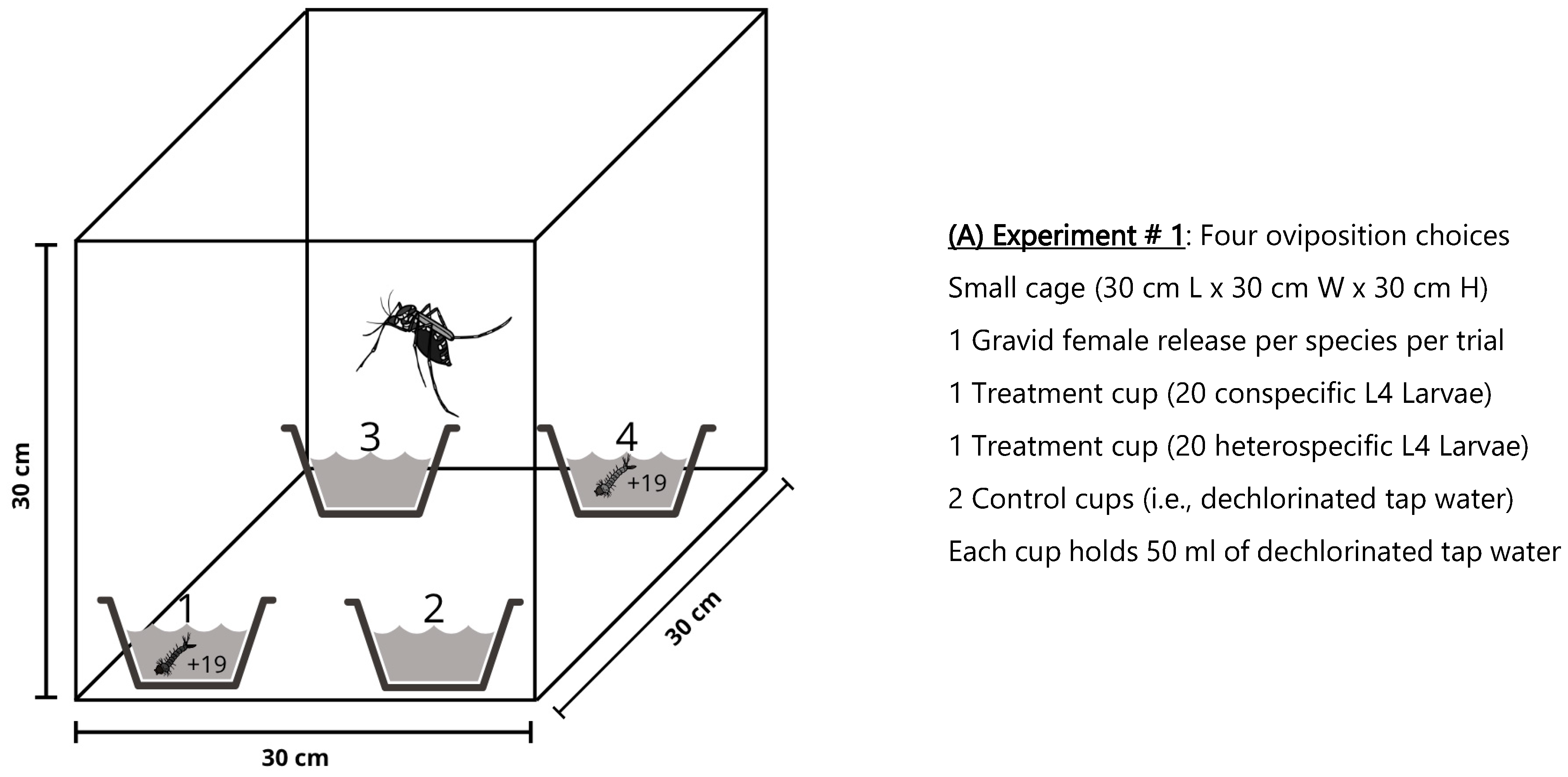
2.1.1. Experiment # 1: Oviposition Choices of Aedes Species in Small Cages
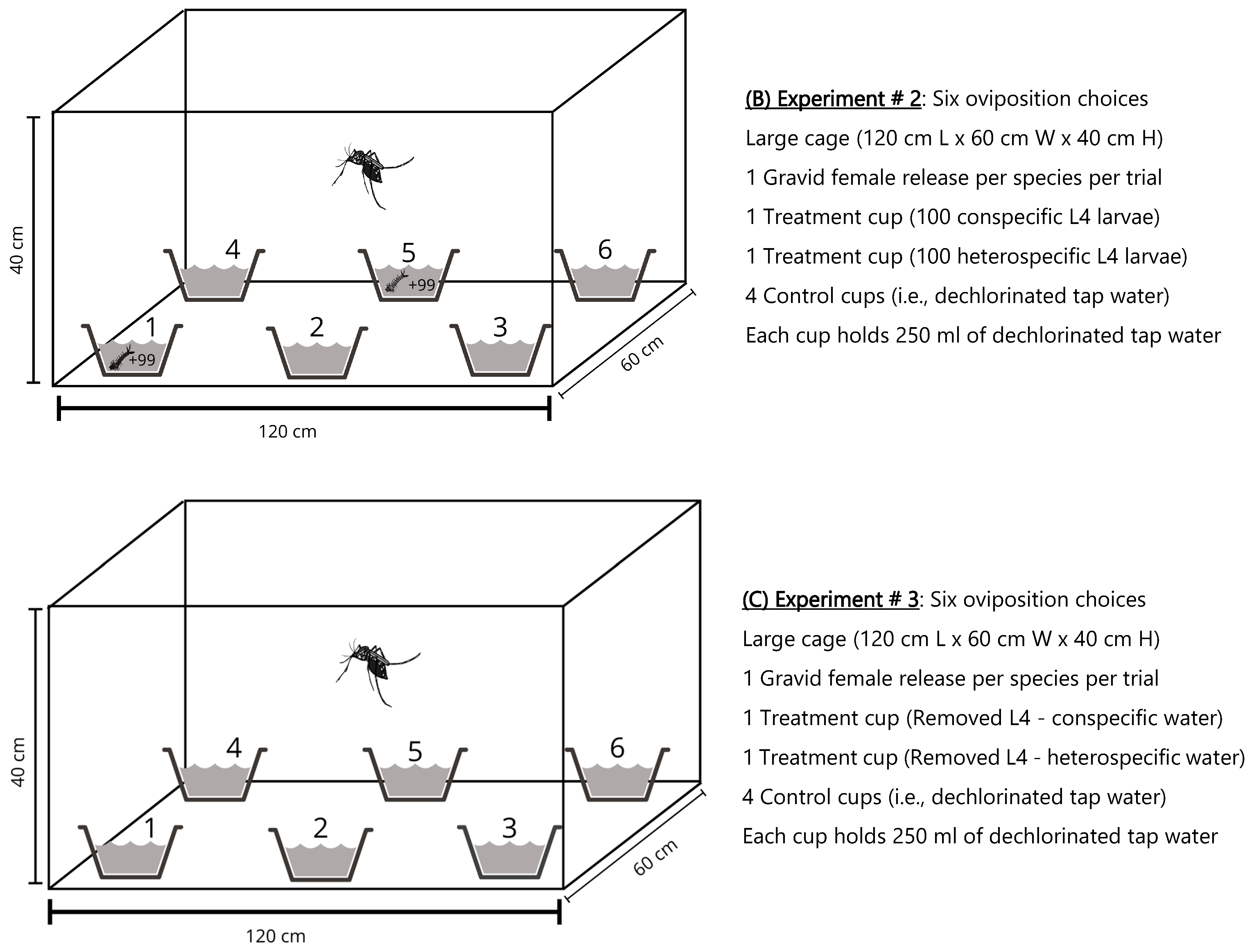
2.1.2. Experiment # 2: Oviposition Choices of Aedes Species in Large Cages
2.1.3. Experiment # 3: Oviposition Choices of Aedes Species in the Absence of L4-Larvae
2.2. Metabolic Rates
2.3. Statistical Analysis
3. Results
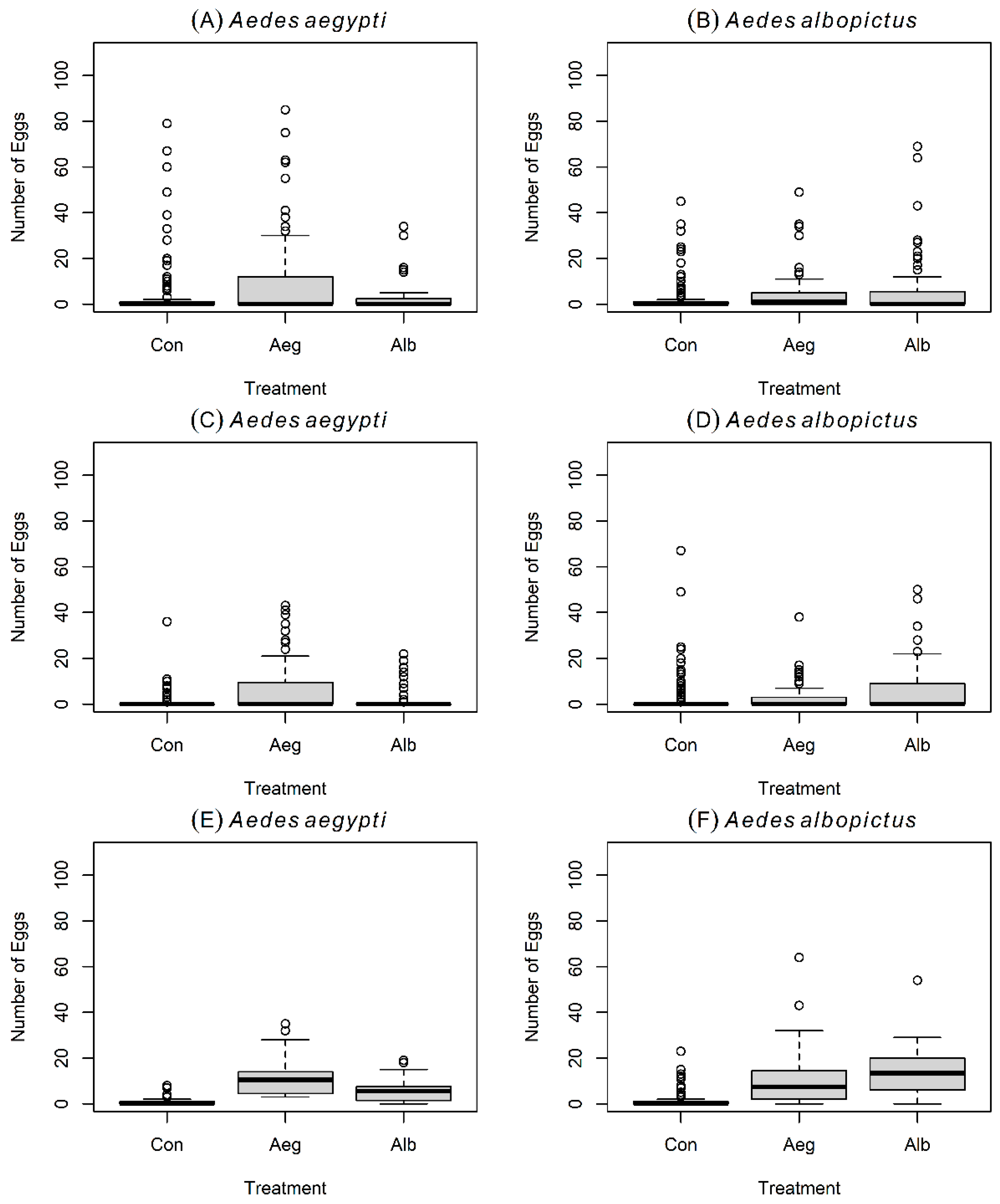
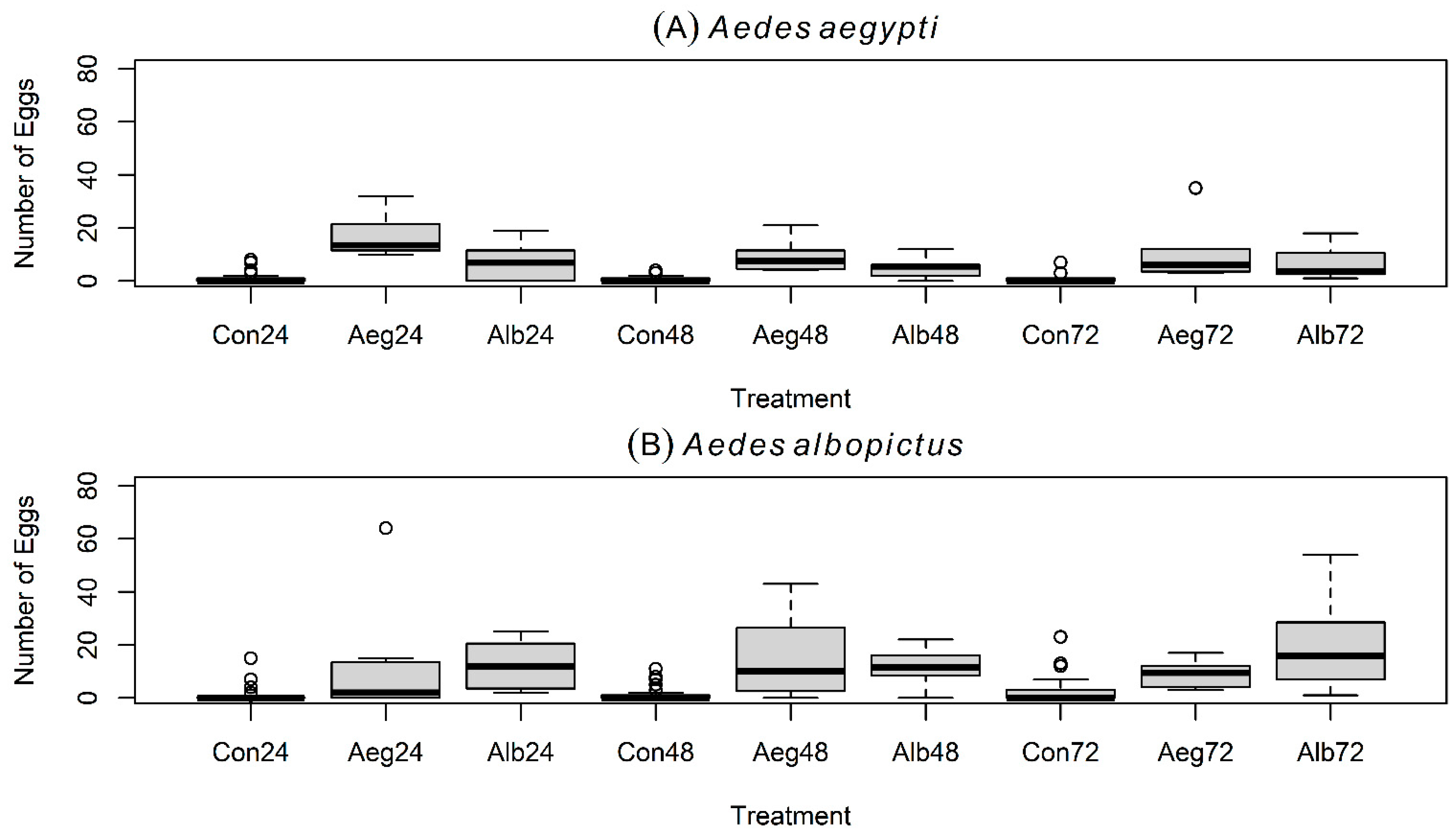
3.1. Oviposition Experiment # 1 with Gravid Aedes aegypti
3.2. Oviposition Experiment # 1 with Gravid Aedes albopictus
3.3. Oviposition Experiment # 2 with Gravid Aedes aegypti
3.4. Oviposition Experiment # 2 with Gravid Aedes albopictus
3.5. Oviposition Experiment # 3 with Gravid Aedes aegypti
3.6. Oviposition Experiment # 3 with Gravid Aedes albopictus
4. Discussion
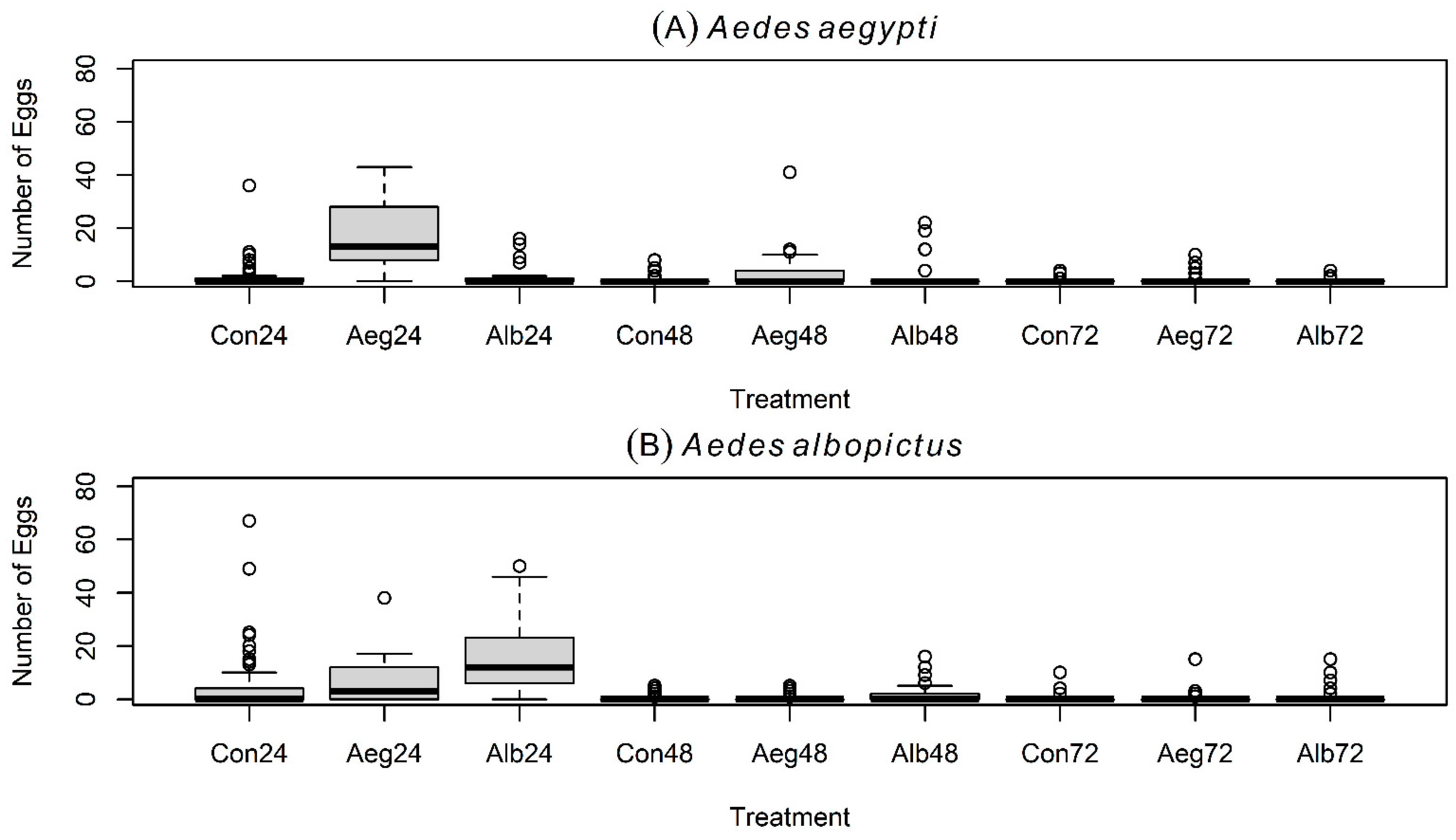
4.1. Spatial–Temporal Oviposition Performances of Aedes aegypti and Aedes albopictus
4.2. Favorite Cup Versus Skip Oviposition
4.3. Implications for the Control of Aedes aegypti in Panama
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bti | Bacillus thuringiensis israelensis |
| AP | Azuero Peninsula |
| GLMM | Generalized Linear Mixed Effects Models |
| AIC | Akaike Information Criterion |
| ANOVA | Analysis of Variance |
| HSD | Post Hoc Tukey’s |
| Aeg | Aedes aegypti |
| Alb | Aedes albopictus |
| Con | Control |
| INDICASAT | Instituto de Investigaciones Científicas y Servicios de Alta Tecnología |
| SENACYT | Secretariat for Science, Technology, and Innovation |
| STRI | Smithsonian Tropical Research Institute |
| UP | University of Panama |
| SNI | National System of Investigation |
References
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.B.; Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef]
- Medlock, J.M.; Hansford, K.M.; Versteirt, V.; Cull, B.; Kampen, H.; Fontenille, D.; Hendrickx, G.; Zeller, H.; Van Bortel, W.; Schaffner, F. An entomological review of invasive mosquitoes in Europe. Bull. Entomol. Res. 2015, 105, 637–663. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.B. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Neglected Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- Hahn, M.B.; Eisen, L.; McAllister, J.; Savage, H.M.; Mutebi, J.P.; Eisen, R.J. Updated Reported Distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the United States, 1995–2016. J. Med. Entomol. 2017, 54, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Murrell, E.G.; Juliano, S.A. Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2008, 45, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Murrell, E.G.; Noden, B.H.; Juliano, S.A. Contributions of temporal segregation, oviposition choice, and non-additive effects of competitors to invasion success of Aedes japonicus (Diptera: Culicidae) in North America. Biol. Invasions 2015, 17, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Bargielowski, I.E.; Lounibos, L.P. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci. 2016, 23, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, M.U.G.; Reiner, R.C., Jr.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Benzon, G.L.; Apperson, C.S. Reexamination of chemically mediated oviposition behavior in Aedes aegypti (L.) (Diptera: Culicidae). J. Med. Entomol. 1988, 25, 158–164. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Greene, K.L.; Lounibos, L.P. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecol. Entomol. 2009, 34, 447–456. [Google Scholar] [CrossRef]
- Davis, T.J.; Kaufman, P.E.; Hogsette, J.A.; Kline, D.L. The Effects of Larval Habitat Quality on Aedes albopictus Skip Oviposition. J. Am. Mosq. Control Assoc. 2015, 31, 321–328. [Google Scholar] [CrossRef]
- Davis, T.J.; Kline, D.L.; Kaufman, P.E. Aedes albopictus (Diptera: Culicidae) Oviposition Preference as Influenced by Container Size and Buddleja davidii Plants. J. Med. Entomol. 2016, 53, 273–278. [Google Scholar] [CrossRef]
- Suman, D.S.; Shrivastava, A.R.; Pant, S.C.; Parashar, B.D. Differentiation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) with egg surface morphology and morphometrics using scanning electron microscopy. Arthropod Struct. Dev. 2011, 40, 479–483. [Google Scholar] [CrossRef]
- Beilhe, L.B.; Arnoux, S.; Delatte, H.; Lajoie, G.; Fontenille, D. Spread of invasive Aedes albopictus and decline of resident Aedes aegypti in urban areas of Mayotte 2007–2010. Biol. Invasions 2012, 14, 1623–1633. [Google Scholar] [CrossRef]
- Bennett, K.L.; McMillan, W.O.; Enríquez, V.; Barraza, E.; Díaz, M.; Baca, B.; Whiteman, A.; Cerro Medina, J.; Ducasa, M.; Gómez Martínez, C.; et al. The role of heterogenous environmental conditions in shaping the spatiotemporal distribution of competing Aedes mosquitoes in Panama: Implications for the landscape of arboviral disease transmission. Biol. Invasions 2021, 23, 1933–1948. [Google Scholar] [CrossRef]
- Bennett, K.L.; McMillan, W.O.; Loaiza, J.R. The genomic signal of local environmental adaptation in Aedes aegypti mosquitoes. Evol. Appl. 2021, 14, 1301–1313. [Google Scholar] [CrossRef]
- Thongsripong, P.; Carter, B.H.; Ward, M.J.; Jameson, S.B.; Michaels, S.R.; Yukich, J.O.; Wesson, D.M. Aedes aegypti and Aedes albopictus (Diptera: Culicidae) Oviposition Activity and the Associated Socio-environmental Factors in the New Orleans Area. J. Med. Entomol. 2023, 60, 392–400. [Google Scholar] [CrossRef]
- Rey, J.R.; O’Connell, S.M. Oviposition by Aedes aegypti and Aedes albopictus: Influence of congeners and of oviposition site characteristics. J. Vector Ecol. J. Soc. Vector Ecol. 2014, 39, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.V.; González Audino, P.A.; Masuh, H.M. Oviposition Behavior in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Response to the Presence of Heterospecific and Conspecific Larvae. J. Med. Entomol. 2016, 53, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, N.; Ranathunge, T.; Udayanga, L.; Wijegunawardena, A.; Abeyewickreme, W. Oviposition preferences of dengue vectors; Aedes aegypti and Aedes albopictus in Sri Lanka under laboratory settings. Bull. Entomol. Res. 2018, 108, 442–450. [Google Scholar] [CrossRef]
- Reiter, P. Oviposition, dispersal, and survival in Aedes aegypti: Implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 2007, 7, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Day, J.F. Mosquito Oviposition Behavior and Vector Control. Insects 2016, 7, 65. [Google Scholar] [CrossRef]
- Allan, S.A.; Kline, D.L. Larval rearing water and preexisting eggs influence oviposition by Aedes aegypti and Ae. albopictus (Diptera: Culicidae). J. Med. Entomol. 1998, 35, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Zahiri, N.; Rau, M.E. Oviposition attraction and repellency of Aedes aegypti (Diptera: Culicidae) to waters from conspecific larvae subjected to crowding, confinement, starvation, or infection. J. Med. Entomol. 1998, 35, 782–787. [Google Scholar] [CrossRef]
- Wong, J.; Stoddard, S.T.; Astete, H.; Morrison, A.C.; Scott, T.W. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Neglected Trop. Dis. 2011, 5, e1015. [Google Scholar] [CrossRef]
- Yap, H.H.; Lee, C.Y.; Chong, N.L.; Foo, A.E.; Lim, M.P. Oviposition site preference of Aedes albopictus in the laboratory. J. Am. Mosq. Control Assoc. 1995, 11, 128–132. [Google Scholar]
- Edman, J.D.; Scott, T.W.; Costero, A.; Morrison, A.C.; Harrington, L.C.; Clark, G.G. Aedes aegypti (Diptera: Culicidae) movement influenced by availability of oviposition sites. J. Med. Entomol. 1998, 35, 578–583. [Google Scholar] [CrossRef]
- Harrington, L.C.; Ponlawat, A.; Edman, J.D.; Scott, T.W.; Vermeylen, F. Influence of container size, location, and time of day on oviposition patterns of the dengue vector, Aedes aegypti, in Thailand. Vector Borne Zoonotic Dis. 2008, 8, 415–423. [Google Scholar] [CrossRef]
- David, M.R.; Maciel-de-Freitas, R.; Petersen, M.T.; Bray, D.; Hawkes, F.M.; Fernández-Grandon, G.M.; Young, S.; Gibson, G.; Hopkins, R.J. Aedes aegypti oviposition-sites choice under semi-field conditions. Med. Vet. Entomol. 2023, 37, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, L.; Xu, N.; Böröczky, K.; Wesson, D.M.; Abu Ayyash, L.; Schal, C.; Apperson, C.S. Oviposition responses of the mosquitoes Aedes aegypti and Aedes albopictus to experimental plant infusions in laboratory bioassays. J. Chem. Ecol. 2010, 36, 709–719. [Google Scholar] [CrossRef]
- Faierstein, G.B.; Lu, W.; Sena, A.K.L.S.; Barbosa, R.M.R.; Leal, W.S. Conspecific and allospecific larval extracts entice mosquitoes to lay eggs and may be used in attract-and-kill control strategy. Sci. Rep. 2019, 9, 13747. [Google Scholar] [CrossRef]
- Boullis, A.; Mulatier, M.; Delannay, C.; Héry, L.; Verheggen, F.; Vega-Rúa, A. Behavioural and antennal responses of Aedes aegypti (L.) (Diptera: Culicidae) gravid females to chemical cues from conspecific larvae. PLoS ONE 2021, 16, e0247657. [Google Scholar] [CrossRef]
- Mulatier, M.; Boullis, A.; Vega-Rúa, A. Semiochemical oviposition cues to control Aedes aegypti gravid females: State of the art and proposed framework for their validation. Parasites Vectors 2022, 15, 228. [Google Scholar] [CrossRef]
- Khan, Z.; Bohman, B.; Ignell, R.; Hill, S.R. Odour-mediated oviposition site selection in Aedes aegypti depends on aquatic stage and density. Parasites Vectors 2023, 16, 264. [Google Scholar] [CrossRef]
- Kawada, H.; Takemura, S.Y.; Arikawa, K.; Takagi, M. Comparative study on nocturnal behavior of Aedes aegypti and Aedes albopictus. J. Med. Entomol. 2005, 42, 312–318. [Google Scholar] [CrossRef]
- Barredo, E.; Raji, J.I.; Ramon, M.; DeGennaro, M.; Theobald, J. Carbon dioxide and blood-feeding shift visual cue tracking during navigation in Aedes aegypti mosquitoes. Biol. Lett. 2022, 18, 20220270. [Google Scholar] [CrossRef] [PubMed]
- Guerenstein, P.G.; Hildebrand, J.G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2008, 53, 161–178. [Google Scholar] [CrossRef]
- Corbet, P.S.; Chadee, D.D. An improved method for detecting substrate preferences shown by mosquitoes that exhibit ‘skip oviposition’. Physiol. Entomol. 1993, 18, 114–118. [Google Scholar] [CrossRef]
- Chadee, D.D. The diel oviposition periodicity of Aedes aegypti (L.) (Diptera: Culicidae) in Trinidad, West Indies: Effects of forced egg retention. Bull. Entomol. Res. 2010, 100, 599–603. [Google Scholar] [CrossRef]
- Swan, T.; Lounibos, L.P.; Nishimura, N. Comparative Oviposition Site Selection in Containers by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Florida. J. Med. Entomol. 2018, 55, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Reinbold-Wasson, D.D.; Reiskind, M.H. Comparative Skip-Oviposition Behavior Among Container Breeding Aedes spp. Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2021, 58, 2091–2100. [Google Scholar] [CrossRef]
- Harrington, L.C.; Edman, J.D. Indirect evidence against delayed “skip-oviposition” behavior by Aedes aegypti (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2001, 38, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.V.; Morais, M.M.; Ribeiro, S.P.; Eiras, Á.E. Influence of breeding site availability on the oviposition behaviour of Aedes aegypti. Memórias Inst. Oswaldo 2015, 110, 669–676. [Google Scholar] [CrossRef]
- Shragai, T.; Harrington, L.; Alfonso-Parra, C.; Avila, F. Oviposition site attraction of Aedes albopictus to sites with conspecific and heterospecific larvae during an ongoing invasion in Medellín, Colombia. Parasites Vectors 2019, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Braks, M.A.H.; Honório, N.A.; Lounibos, L.P.; Lourenço-de-Oliveira, R.; Juliano, S.A. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 2004, 97, 130–139. [Google Scholar] [CrossRef]
- Miller, M.J.; Loaiza, J.R. Geographic expansion of the invasive mosquito Aedes albopictus across Panama--implications for control of dengue and Chikungunya viruses. PLoS Neglected Trop. Dis. 2015, 9, e0003383. [Google Scholar] [CrossRef]
- Eskildsen, G.A.; Rovira, J.R.; Smith, O.; Miller, M.J.; Bennett, K.L.; McMillan, W.O.; Loaiza, J. Maternal invasion history of Aedes aegypti and Aedes albopictus into the Isthmus of Panama: Implications for the control of emergent viral disease agents. PLoS ONE 2018, 13, e0194874. [Google Scholar] [CrossRef]
- Whiteman, A.; Gomez, C.; Rovira, J.; Chen, G.; McMillan, W.O.; Loaiza, J. Aedes Mosquito Infestation in Socioeconomically Contrasting Neighborhoods of Panama City. EcoHealth 2019, 16, 210–221. [Google Scholar] [CrossRef]
- Whiteman, A.; Desjardins, M.R.; Eskildsen, G.A.; Loaiza, J.R. Detecting space-time clusters of dengue fever in Panama after adjusting for vector surveillance data. PLoS Neglected Trop. Dis. 2019, 13, e0007266. [Google Scholar] [CrossRef]
- Bennett, K.L.; Gómez Martínez, C.; Almanza, A.; Rovira, J.R.; McMillan, W.O.; Enriquez, V.; Barraza, E.; Diaz, M.; Sanchez-Galan, J.E.; Whiteman, A.; et al. High infestation of invasive Aedes mosquitoes in used tires along the local transport network of Panama. Parasites Vectors 2019, 12, 264. [Google Scholar] [CrossRef]
- Bennett, K.L.; Gómez-Martínez, C.; Chin, Y.; Saltonstall, K.; McMillan, W.O.; Rovira, J.R.; Loaiza, J.R. Dynamics and diversity of bacteria associated with the disease vectors Aedes aegypti and Aedes albopictus. Sci. Rep. 2019, 9, 12160. [Google Scholar] [CrossRef]
- Rueda, L.M. Zootaxa. Pictorial Keys for the Identification of Mosquitoes (Diptera: Culicidae) Associated with Dengue Virus Transmission; Magnolia Press: Auckland, New Zealand, 2004; Volume 589. [Google Scholar] [CrossRef]
- Casas-Martínez, M.; Tamayo-Domínguez, R.; Bond-Compeán, J.G.; Rojas, J.C.; Weber, M.; Ulloa-García, A. Desarrollo oogénico y ciclo gonotrófico de Aedes aegypti y Aedes albopictus en laboratorio. Salud Publica Mex. 2020, 62, 372–378. [Google Scholar] [PubMed]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chaves, L.F. An entomologist guide to demystify pseudoreplication: Data analysis of field studies with design constraints. J. Med. Entomol. 2010, 47, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Faraway, J.J. Linear Models with R; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 16 May 2021).
- Juliano, S.A. Coexistence, Exclusion, or Neutrality? A metanalysis of competition between Aedes albopictus and resident mosquitoes. Isr. J. Ecol. Evol. 2010, 56, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Morrison, A.C.; Stoddard, S.T.; Astete, H.; Chu, Y.Y.; Baseer, I.; Scott, T.W. Linking oviposition site choice to offspring fitness in Aedes aegypti: Consequences for targeted larval control of dengue vectors. PLoS Neglected Trop. Dis. 2012, 6, e1632. [Google Scholar] [CrossRef]
- Tuñon, A.; García, J.; Carrera, L.C.; Chaves, L.F.; Lenhart, A.E.; Loaiza, J.R. Chemical control of medically important arthropods in Panama: A systematic literature review of historical efforts. Acta Trop. 2024, 255, 107217. [Google Scholar] [CrossRef]

| Experiment | Aedes Stegomyia aegypti | Aedes Stegomyia albopictus | ||||
|---|---|---|---|---|---|---|
| Control | Conspecific | Heterospecific | Control | Conspecific | Heterospecific | |
| # 1 (n = 120, 60, 60) | 531 (4.43 ± 12.93) | 635 (10.58 ± 20.63) | 161 (2.68 ± 6.47) | 320 (2.67 ± 7.30) | 394 (6.57 ± 14.27) | 340 (5.67 ± 11.17) |
| # 2 (n = 348, 87, 87) | 189 (0.54 ± 2.38) | 636 (7.31 ± 11.41) | 130 (1.49 ± 4.13) | 499 (1.43 ± 5.52) | 549 (6.31 ± 10.70) | 231 (2.66 ± 5.78) |
| # 3 (n = 96, 24, 24) | 72 (0.75 ± 1.56) | 288 (12.00 ± 8.89) | 147 (6.13 ± 5.68) | 146 (1.52 ± 3.72) | 351 (14.63 ± 11.93) | 287 (11.96 ± 15.34) |
| Experiment # 1 | ||||
| Fixed effects | Estimate | Std. Error | z value | Pr (>|z|) |
| Intercept | 1.7169 | 0.112 | 15.291 | <2 × 10−16 *** |
| Larvae (Conspecific) | 1.008 | 0.084 | 11.987 | <2 × 10−16 *** |
| Larvae (Heterospecific) | −0.311 | 0.123 | −2.520 | 0.01173 * |
| Time at 48 h. | −0.261 | 0.096 | −2.701 | 0.00691 ** |
| Time at 72 h. | −0.877 | 0.118 | −7.439 | 1.02 × 10−13 *** |
| Larvae (Conspecific) × 48 h. | −0.257 | 0.131 | −1.955 | 0.05059 |
| Larvae (Heterospecific) × 48 h. | −0.615 | 0.217 | −2.827 | 0.00469 ** |
| Larvae (Conspecific) × 72 h. | −0.285 | 0.162 | −1.757 | 0.07897 |
| Larvae (Heterospecific) × 72 h. | −0.055 | 0.231 | −0.239 | 0.81093 |
| Random effects | Variance | Std. Dev. | AIC | |
| Assay | 0.1676 | 0.4094 | 4198.0 | |
| Experiment # 2 | ||||
| Fixed effects | Estimate | Std. Error | z value | Pr (>|z|) |
| Intercept | 0.064 | 0.128 | 0.504 | 0.6143 |
| Larvae (Conspecific) | 2.666 | 0.094 | 28.277 | <2 × 10−16 *** |
| Larvae (Heterospecific) | 0.419 | 0.158 | 2.640 | 0.0083 ** |
| Time at 48 h. | −1.318 | 0.181 | −7.260 | 3.88 × 10−13 *** |
| Time at 72 h. | −2.758 | 0.341 | −8.072 | 6.90 × 10−16 *** |
| Larvae (Conspecific) × 48 h. | −0.323 | 0.211 | −1.525 | 0.1273 |
| Larvae (Heterospecific) × 48 h. | 1.503 | 0.257 | 5.831 | 5.50 × 10−9 *** |
| Larvae (Conspecific) × 72 h. | −0.219 | 0.395 | −0.554 | 0.5793 |
| Larvae (Heterospecific) × 72 h. | 1.167 | 0.474 | 2.462 | 0.0138 * |
| Random effects | Variance | Std. Dev. | AIC | |
| Assay | 0.266 | 0.516 | 2081.3 | |
| Experiment # 3 | ||||
| Fixed effects | Estimate | Std. Error | z value | Pr (>|z|) |
| Intercept | −0.094 | 0.207 | −0.457 | 0.6473 |
| Water (Conspecific) | 2.772 | 0.130 | 21.197 | <2 × 10−16 *** |
| Water (Heterospecific) | 2.101 | 0.142 | 14.707 | <2 × 10−16 *** |
| Time at 48 h. | −0.529 | 0.255 | −2.070 | 0.0385 * |
| Time at 72 h. | −0.461 | 0.255 | −1.807 | 0.0707 |
| Random effects | Variance | Std. Dev. | AIC | |
| Assay | 0.208 | 0.456 | 621.5 | |
| Experiment # 1 | ||||
| Fixed effects | Estimate | Std. Error | z value | Pr (>|z|) |
| Intercept | 1.401 | 0.101 | 13.869 | <2 × 10−16 *** |
| Larvae (Heterospecific) | 0.681 | 0.108 | 6.263 | 3.78 × 10−10 *** |
| Larvae (Conspecific) | 1.215 | 0.096 | 12.561 | <2 × 10−16 *** |
| Time at 48 h. | −0.524 | 0.125 | −4.172 | 3.02 × 10−5 *** |
| Time at 72 h. | −1.198 | 0.159 | −7.522 | 5.38 × 10−14 *** |
| Larvae (Heterospecific) × 48 h. | 0.266 | 0.171 | 1.553 | 0.12051 |
| Larvae (Conspecific) × 48 h. | −0.502 | 0.170 | −2.950 | 0.00318 ** |
| Larvae (Heterospecific) × 72 h. | −0.135 | 0.232 | −0.585 | 0.55881 |
| Larvae (Conspecific) × 72 h. | −2.508 | 0.413 | −6.069 | 1.29 × 10−9 *** |
| Random effects | Variance | Std. Dev. | AIC | |
| Assay | 0.084 | 0.2911 | 2950.9 | |
| Experiment # 2 | ||||
| Fixed effects | Estimate | Std. Error | z value | Pr (>|z|) |
| Intercept | 1.286 | 0.088 | 14.547 | <2 × 10−16 *** |
| Larvae (Heterospecific) | 0.533 | 0.085 | 6.252 | 4.05 × 10−10 *** |
| Larvae (Conspecific) | 1.384 | 0.066 | 20.953 | <2 × 10−16 *** |
| Time at 48 h. | −2.862 | 0.200 | −14.254 | <2 × 10−16 *** |
| Time at 72 h. | −3.229 | 0.239 | −13.496 | <2 × 10−16 *** |
| Larvae (Heterospecific) × 48 h. | 0.366 | 0.327 | 1.120 | 0.26288 |
| Larvae (Conspecific) × 48 h. | 0.787 | 0.244 | 3.216 | 0.00130 ** |
| Larvae (Heterospecific) × 72 h. | 1.006 | 0.331 | 3.041 | 0.00236 ** |
| Larvae (Conspecific) × 72 h. | 0.749 | 0.292 | 2.562 | 0.01040 * |
| Random effects | Variance | Std. Dev. | AIC | |
| Assay | 0.159 | 0.399 | 2975.1 | |
| Experiment # 3 | ||||
| Fixed effects | Estimate | Std. Error | z value | Pr (>|z|) |
| Intercept | −0.128 | 0.224 | −0.571 | 0.5687 |
| Water (Heterospecific) | 2.506 | 0.205 | 12.182 | <2 × 10−16 *** |
| Larvae (Conspecific) | 2.547 | 0.204 | 12.445 | <2 × 10−16 *** |
| Time at 48 h. | 0.331 | 0.304 | 1.088 | 0.27679 |
| Time at 72 h. | 0.887 | 0.286 | 3.098 | 0.00195 ** |
| Water (Heterospecific) × 48 h. | −0.037 | 0.273 | −0.138 | 0.89025 |
| Water (Conspecific) × 48 h. | −0.331 | −0.331 | −1.198 | 0.23111 |
| Water (Heterospecific) × 72 h. | −1.161 | 0.264 | −4.401 | 1.08 × 10−5 *** |
| Water (Conspecific) × 72 h. | −0.402 | 0.2481 | −1.623 | 0.10467 |
| Random effects | Variance | Std. Dev. | AIC | |
| Assay | 0.143 | 0.378 | 1227.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuñón, R.; Chong, M.; Rojas, A.L.; Castillo, A.; Kingwell, C.; Chaves, L.F.; Loaiza, J.R. Oviposition Behavior of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Panama Under Experimental L4-Larval Co-Occurrence Scenarios. Insects 2025, 16, 1110. https://doi.org/10.3390/insects16111110
Tuñón R, Chong M, Rojas AL, Castillo A, Kingwell C, Chaves LF, Loaiza JR. Oviposition Behavior of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Panama Under Experimental L4-Larval Co-Occurrence Scenarios. Insects. 2025; 16(11):1110. https://doi.org/10.3390/insects16111110
Chicago/Turabian StyleTuñón, Reyna, Mabelle Chong, Ambar L. Rojas, Armando Castillo, Callum Kingwell, Luis F. Chaves, and Jose R. Loaiza. 2025. "Oviposition Behavior of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Panama Under Experimental L4-Larval Co-Occurrence Scenarios" Insects 16, no. 11: 1110. https://doi.org/10.3390/insects16111110
APA StyleTuñón, R., Chong, M., Rojas, A. L., Castillo, A., Kingwell, C., Chaves, L. F., & Loaiza, J. R. (2025). Oviposition Behavior of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Panama Under Experimental L4-Larval Co-Occurrence Scenarios. Insects, 16(11), 1110. https://doi.org/10.3390/insects16111110







