A New Genus of Four-Legged Mites from Palms in Vietnam: The Morphology and Phylogeny of Calventer arengii n. g. & sp. (Eriophyoidea, Phytoptidae) †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
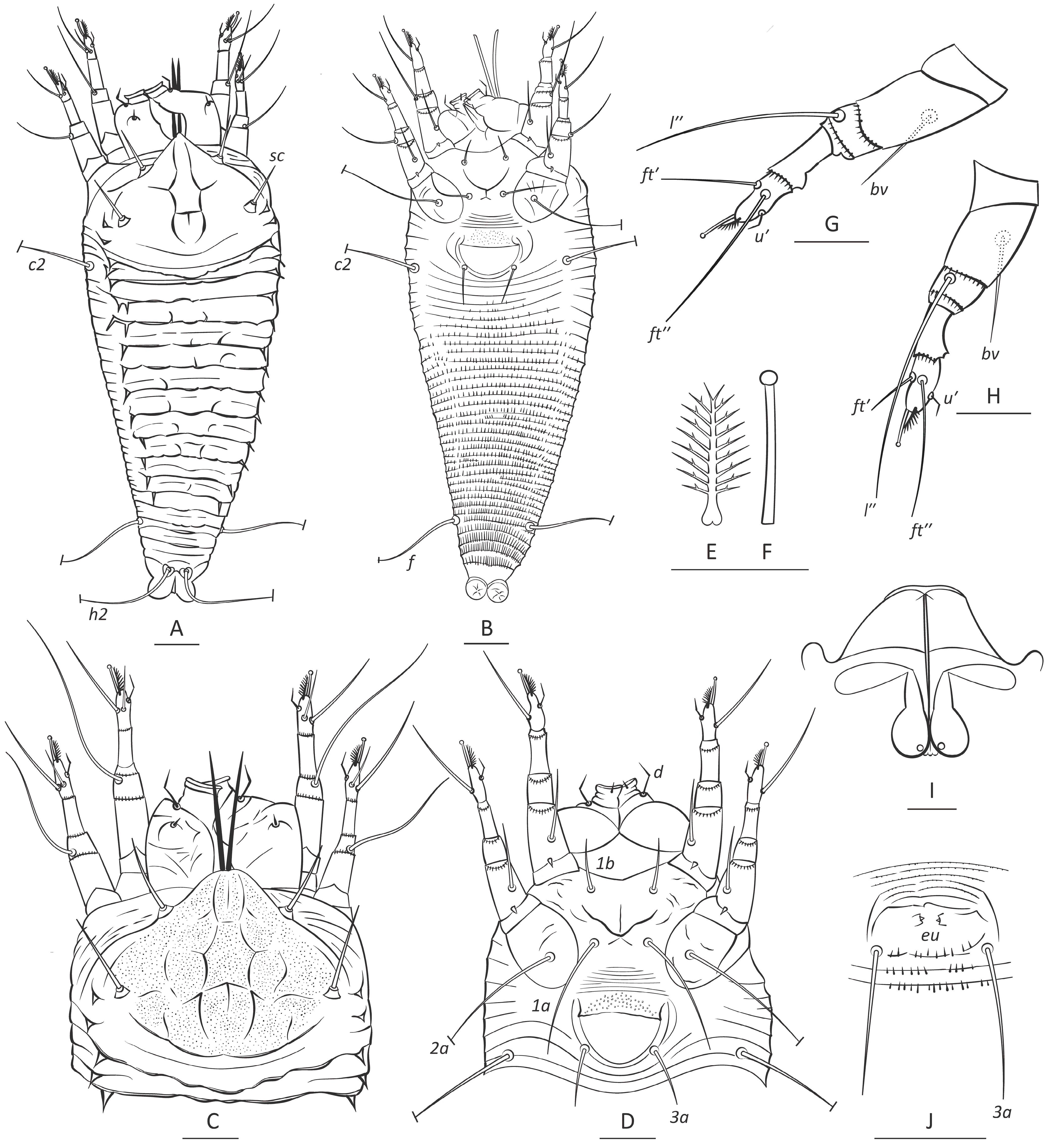
3. Results
3.1. Systematics
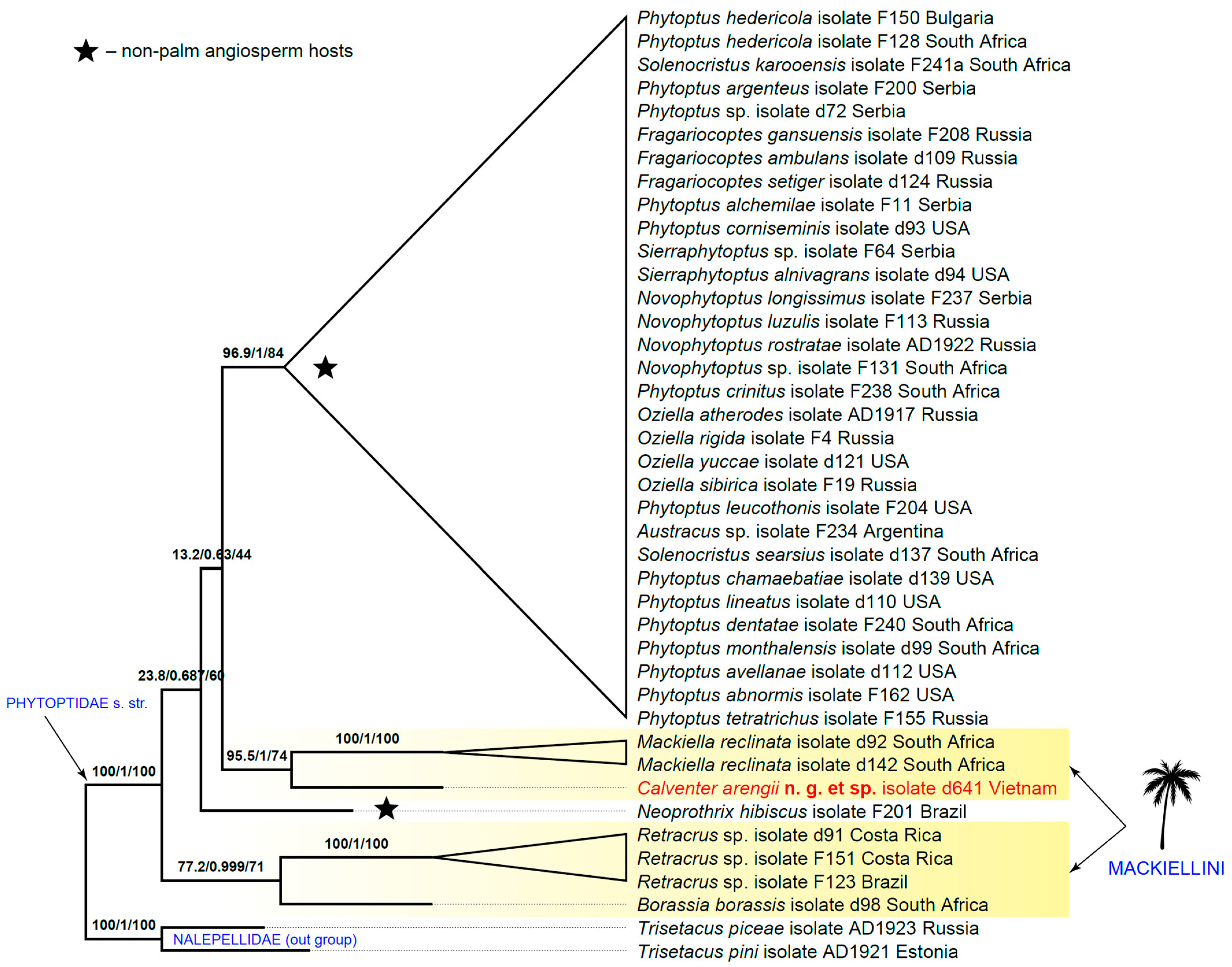
3.2. Molecular Phylogenetics
4. Discussion
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindquist, E.E. External anatomy and notation of structures. In Eriophyoid Mites Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests 6; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 3–31. [Google Scholar]
- Bolton, S.J.; Chetverikov, P.E.; Klompen, H. Morphological support for a clade comprising two vermiform mite lineages: Eriophyoidea (Acariformes) and Nematalycidae (Acariformes). Syst. Appl. Acarol. 2017, 22, 1096–1131. [Google Scholar] [CrossRef]
- Bolton, S.J.; Chetverikov, P.E.; Ochoa, R.; Klimov, P.B. Where Eriophyoidea (Acariformes) Belong in the Tree of Life. Insects 2023, 14, 527. [Google Scholar] [CrossRef]
- Klimov, P.B.; OConnor, B.M.; Chetverikov, P.E.; Bolton, S.J.; Pepato, A.R.; Mortazavi, A.L.; Tolstikov, A.V.; Bauchan, G.R.; Ochoa, R. Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Mol. Phylogenet. Evol. 2018, 119, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Klimov, P.B.; Chetverikov, P.E.; Dodueva, I.E.; Vishnyakov, A.E.; Bolton, S.J.; Paponova, S.S.; Lutova, L.A.; Tolstikov, A.V. Symbiotic bacteria of the gall-inducing mite Fragariocoptes setiger (Eriophyoidea) and phylogenomic resolution of the eriophyoid position among Acari. Sci. Rep. 2022, 12, 3811. [Google Scholar] [CrossRef]
- Klimov, P.B.; Kolesnikov, V.B.; Vorontsov, D.D.; Ball, A.D.; Bolton, S.J.; Mellish, C.; Edgecombe, G.D.; Pepato, A.R.; Chetverikov, P.E.; He, Q.; et al. The evolutionary history and timeline of mites in ancient soils. Sci. Rep. 2025, 15, 13555. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, P.E.; Craemer, C.; Gankevich, V.D.; Zhuk, A.S. Integrative Taxonomy of the Gall Mite Nothopoda todeica n. sp. (Eriophyidae) from the Disjunct Afro-Australasian Fern Todea barbara: Morphology, Phylogeny, and Mitogenomics. Insects 2023, 14, 507. [Google Scholar] [CrossRef]
- Amrine, J.W., Jr.; Stasny, T.A.H.; Flechtmann, C.H.W. Revised Keys to the World Genera of the Eriophyoidea (Acari: Prostigmata); Indira Publishing House: West Bloomfield, MI, USA, 2003; pp. 1–244. [Google Scholar]
- Chetverikov, P.E.; Cvrković, T.; Makunin, A.; Sukhareva, S.; Vidović, B.; Petanović, R. Basal divergence of Eriophyoidea (Acariformes, Eupodina) inferred from combined partial COI and 28S gene sequences and CLSM genital anatomy. Exp. Appl. Acarol. 2015, 67, 219–245. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Cvrković, T.; Eimov, P.G.; Klimov, P.B.; Petanović, R.U.; Romanovich, A.E.; Schubert, M.A.; Sukhareva, S.I.; Zukof, S.N.; Amrine, J. Molecular phylogenetic analyses reveal a deep dichotomy in the conifer-inhabiting genus Trisetacus (Eriophyoidea: Nalepellidae), with the two lineages differing in their female genital morphology and host associations. Exp. Appl. Acarol. 2020, 81, 287–316. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Craemer, C.; Cvrković, T.; Klimov, P.B.; Petanović, R.U.; Romanovich, A.E.; Sukhareva, S.I.; Zukoff, S.N.; Bolton, S.; Amrine, J. Molecular phylogeny of the phytoparasitic mite family Phytoptidae (Acariformes: Eriophyoidea) identified the female genitalic anatomy as a major macroevolutionary factor and revealed multiple origins of gall induction. Exp. Appl. Acarol. 2021, 83, 31–68. [Google Scholar] [CrossRef]
- Oldfield, G.N. Diversity and host plant specificity. In Eriophyoid Mites Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests 6; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 199–216. [Google Scholar]
- Skoracka, A. Host specificity of eriophyoid mites: Specialists or generalists. Biol. Lett. 2006, 43, 289–298. [Google Scholar]
- Skoracka, A.; Smith, L.; Oldfield, G.; Cristofaro, M.; Amrine, J.W. Host-plant specificity and specialization in eriophyoid mites and their importance for the use of eriophyoid mites as biocontrol agents of weeds. In Eriophyoid Mites: Progress and Prognoses; Euckermann, E.A., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 93–113. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Craemer, C. Two new genera of eriophyoid mites (Eriophyoidea) from Hyphaene coriacea linking eriophyoid faunas of South American, Indian and African palms: An insight from paleobiography of Arecaceae. Syst. Appl. Acarol. 2017, 22, 925–947. [Google Scholar] [CrossRef]
- Henderson, A. Palms of Southern Asia; Princeton University Press: Princeton, NJ, USA, 2009; pp. 1–199. [Google Scholar]
- Amrine, J.W., Jr.; Manson, D.C.M. Preparation, mounting and descriptive study of eriophyoid mites. In Eriophyoid Mites Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests 6; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 383–396. [Google Scholar] [CrossRef]
- Chetverikov, P.E. Video projector: A digital replacement for camera lucida for drawing mites and other microscopic objects. Syst. Appl. Acarol. 2016, 21, 1278–1280. [Google Scholar]
- Chetverikov, P.E.; Bertone, M. First rhyncaphytoptine mite (Eriophyoidea, Diptilomiopidae) parasitizing american hazelnut (Corylus americana): Molecular identification, confocal microscopy, and phylogenetic position. Exp. Appl. Acarol. 2022, 88, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2017, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Petanović, R. Towards an integrative approach to taxonomy of Eriophyoidea (Acari, Prostigmata)-an overview. Ecol. Montenegrina 2016, 7, 580–599. [Google Scholar] [CrossRef]
- Mohanasundaram, M. New genera and species of Eriophyoidea (Acarina) from South India. Indian J. Acarol. 1983, 7, 53–58. [Google Scholar]
- Sukhareva, S.I. Family Phytoptidae Murray 1877 (Acari: Tetrapodili), its consisting, structure and suggested ways of evolution. Acarina 1994, 2, 47–72. [Google Scholar]
- Chetverikov, P.E.; Craemer, C.; Vishnyakov, A.E.; Sukhareva, S.I. CLSM anatomy of internal genitalia of Mackiella reclinata n. sp. and systematic remarks on eriophyoid mites from the tribe Mackiellini Keifer, 1946 (Eriophyoidea, Phytoptidae). Zootaxa 2014, 3860, 261–279. [Google Scholar] [CrossRef]
- Bellot, S.J.; Condamine, F.L.; Matsunaga, K.K.; Morley, R.J.; Cano, Á.; Couvreur, T.L.P.; Cowan, R.; Eiserhardt, W.L.; Kuhnhäuser, B.G.; Maurin, O.; et al. Early Cretaceous origin and evolutionary history of palms (Arecaceae) inferred from 1033 nuclear genes and a new synthesis of fossil evidence. bioRxiv 2024. [Google Scholar] [CrossRef]
- Reis, A.C.; Gondim Jr, M.G.C.; Flechtmann, C.H.W.; Navia, D. New eriophyoid mites (Acari: Prostigmata: Eriophyoidea) from cultivated plants from northeastern Brazil, including the second taxon in the Prothricinae. J. Nat. Hist. 2014, 48, 1135–1152. [Google Scholar]
- Chetverikov, P.E.; Desnitskiy, A.G.; Navia, D. Confocal microscopy refines generic concept of a problematic taxon: Rediagnosis of the genus Neoprothrix and remarks on female anatomy of eriophyoids (Acari: Eriophyoidea). Zootaxa 2015, 3919, 179–191. [Google Scholar] [CrossRef]
- Navia, D.; Ferreira, C.B.; Reis, A.C.; Gondim, M.G. Traditional and geometric morphometrics supporting the differentiation of two new Retracrus (Phytoptidae) species associated with heliconias. Exp. Appl. Acarol. 2015, 67, 87–121. [Google Scholar] [CrossRef] [PubMed]
- Navia, D.; Duarte, M.E.; Flechtmann, C.H.W. Eriophyoid mites (Acari: Prostigmata) from Brazil: An annotated checklist. Zootaxa 2021, 4997, 1–152. [Google Scholar] [CrossRef]
- Navia, D.; Gondim, M.G.C.; de Moraes, G.J. Eriophyoid mites (Acari: Eriophyoidea) associated with palm trees. Zootaxa 2007, 1389, 1–30. [Google Scholar] [CrossRef][Green Version]
- de Lillo, E.; Skoracka, A. What’s “cool” on eriophyoid mites? Exp. Appl. Acarol. 2010, 51, 3–30. [Google Scholar] [CrossRef]
- Ozman-Sullivan, S.K.; Sullivan, G.T. Global patterns of the species richness and distribution of eriophyoid mites: A response to Li et al. 2023. J. Biogeogr. 2024, 51, 57–60. [Google Scholar] [CrossRef]
- Ozman-Sullivan, S.K.; Sullivan, G.T. More Insights into the Species Richness and Distribution of Eriophyoid Mites: A Reply to Li and Xue (2024). J. Biogeogr. 2025, 52, e15118. [Google Scholar] [CrossRef]
- Li, N.; Sun, J.T.; Yin, Y.; Hong, X.Y.; Xue, X.F. Global patterns and drivers of herbivorous eriophyoid mite species diversity. J. Biogeogr. 2023, 50, 330–340. [Google Scholar] [CrossRef]
- Li, N.; Xue, X.F. Global patterns of herbivorous eriophyoid mite species diversity: A reply to Ozman-Sullivan and Sullivan (2024). J. Biogeogr. 2024, 51, 675–676. [Google Scholar] [CrossRef]

| Genus | vi | ve | sc | ppd | c1 | c2 | d | e | f | h1 | 1a | 1b | 2a | bv I/II | l″ I/II | l′ I | φ I | ft′ I/II | ft″ I/II | u′ I/II |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acathrix Keifer 1962 | − | + | + | + | + | + | + | + | + | + | + | + | + | +/+ | +/+ | + | + | +/+ | +/+ | +/+ |
| Borassia Chetverikov, Craemer 2017 | − | + | + | + | − | + | + | + | + | + | + | + | + | +/+ | +/+ | + | − | +/+ | +/+ | +/+ |
| Calventer n. g. | − | + | + | + | − | + | − | − | + | − | + | + | + | +/+ | +/+ | − | − | +/+ | +/+ | +/+ |
| Mackiella Keifer 1939 | − | + | + | + | − | + | + | + | + | + | + | + | + | +/+ | +/+ | + | + | +/+ | +/+ | +/+ |
| Palmiphytoptus Navia & Flechtmann 2002 | − | + | − | + | − | + | + | + | + | + | + | + | + | +/+ | +/+ | − | − | +/+ | +/+ | +/+ |
| Propilus Keifer 1975 | − | + | − | + | − | + | + | + | + | + | + | + | + | +/+ | +/+ | − | − | +/+ | +/+ | +/+ |
| Retracrus Keifer 1965 | − | + | + | + | − | + | + | + | + | − | + | + | + | +/+ | +/+ | + | + | +/+ | +/+ | +/+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chetverikov, P.E. A New Genus of Four-Legged Mites from Palms in Vietnam: The Morphology and Phylogeny of Calventer arengii n. g. & sp. (Eriophyoidea, Phytoptidae). Insects 2025, 16, 1113. https://doi.org/10.3390/insects16111113
Chetverikov PE. A New Genus of Four-Legged Mites from Palms in Vietnam: The Morphology and Phylogeny of Calventer arengii n. g. & sp. (Eriophyoidea, Phytoptidae). Insects. 2025; 16(11):1113. https://doi.org/10.3390/insects16111113
Chicago/Turabian StyleChetverikov, Philipp E. 2025. "A New Genus of Four-Legged Mites from Palms in Vietnam: The Morphology and Phylogeny of Calventer arengii n. g. & sp. (Eriophyoidea, Phytoptidae)" Insects 16, no. 11: 1113. https://doi.org/10.3390/insects16111113
APA StyleChetverikov, P. E. (2025). A New Genus of Four-Legged Mites from Palms in Vietnam: The Morphology and Phylogeny of Calventer arengii n. g. & sp. (Eriophyoidea, Phytoptidae). Insects, 16(11), 1113. https://doi.org/10.3390/insects16111113






