Implication of Two Small Heat Shock Proteins in the Thermotolerance of Bradysia odoriphaga (Diptera: Sciaridae) Yang et Zhang
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. RNA Extraction and cDNA Synthesis
2.3. Cloning and Confirmation of BoHsp21.9 and BoHsp22.3
2.4. Bioinformatic Analysis
2.5. Sampling of BoHsp21.9 and BoHsp22.3 in Different Developmental Stages and Body Segments
2.6. Sampling of BoHsp21.9 and BoHsp22.3 in Response to Heat Stress
2.7. Real-Time Quantitative PCR
2.8. Double-Stranded (dsRNA) Synthesis
2.9. dsRNA Feeding
2.10. Heat Stress After RNA Interference
2.11. Statistical Analysis
3. Results
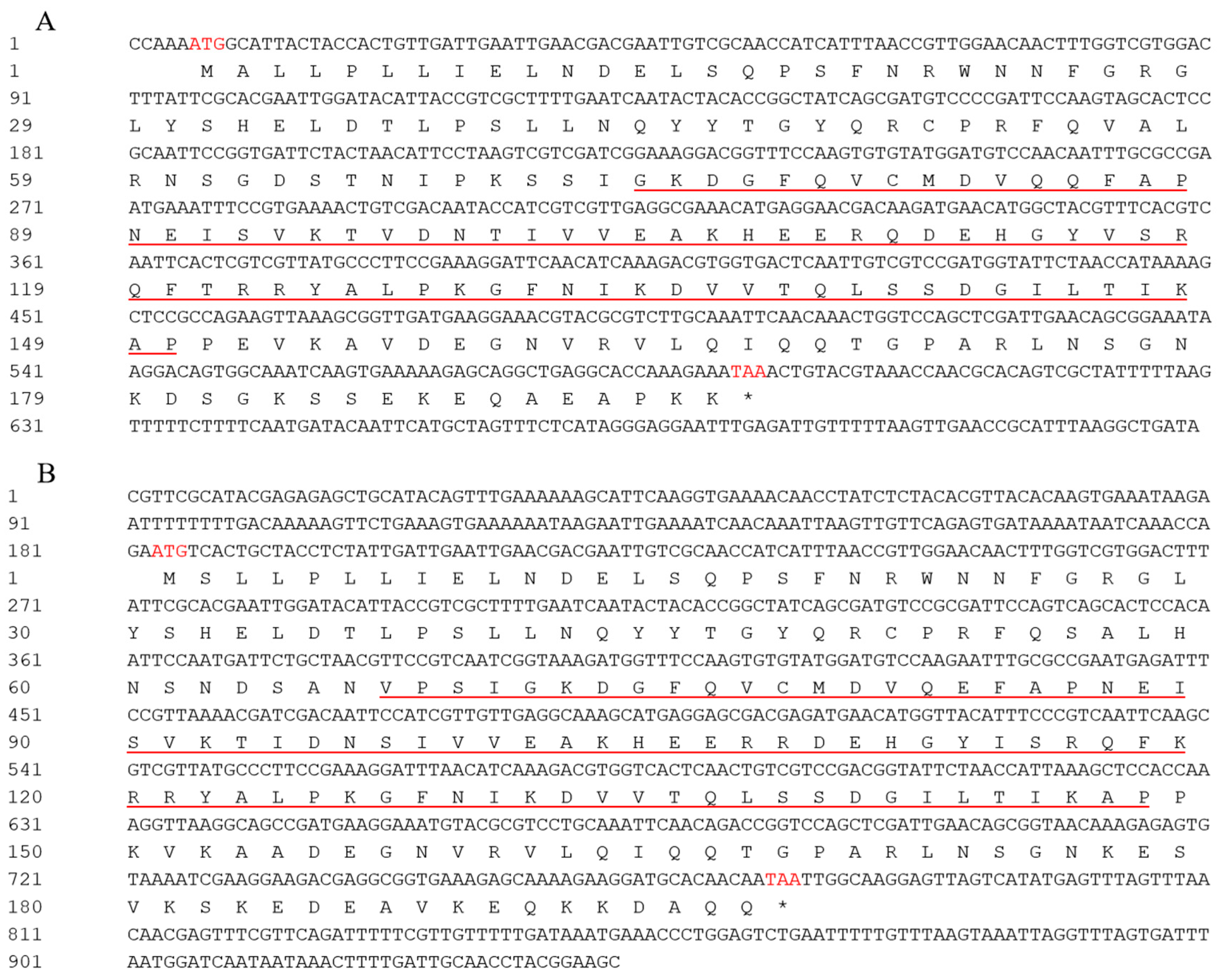
3.1. Cloning and Sequence Analysis of BoHsp21.9 and BoHsp22.3
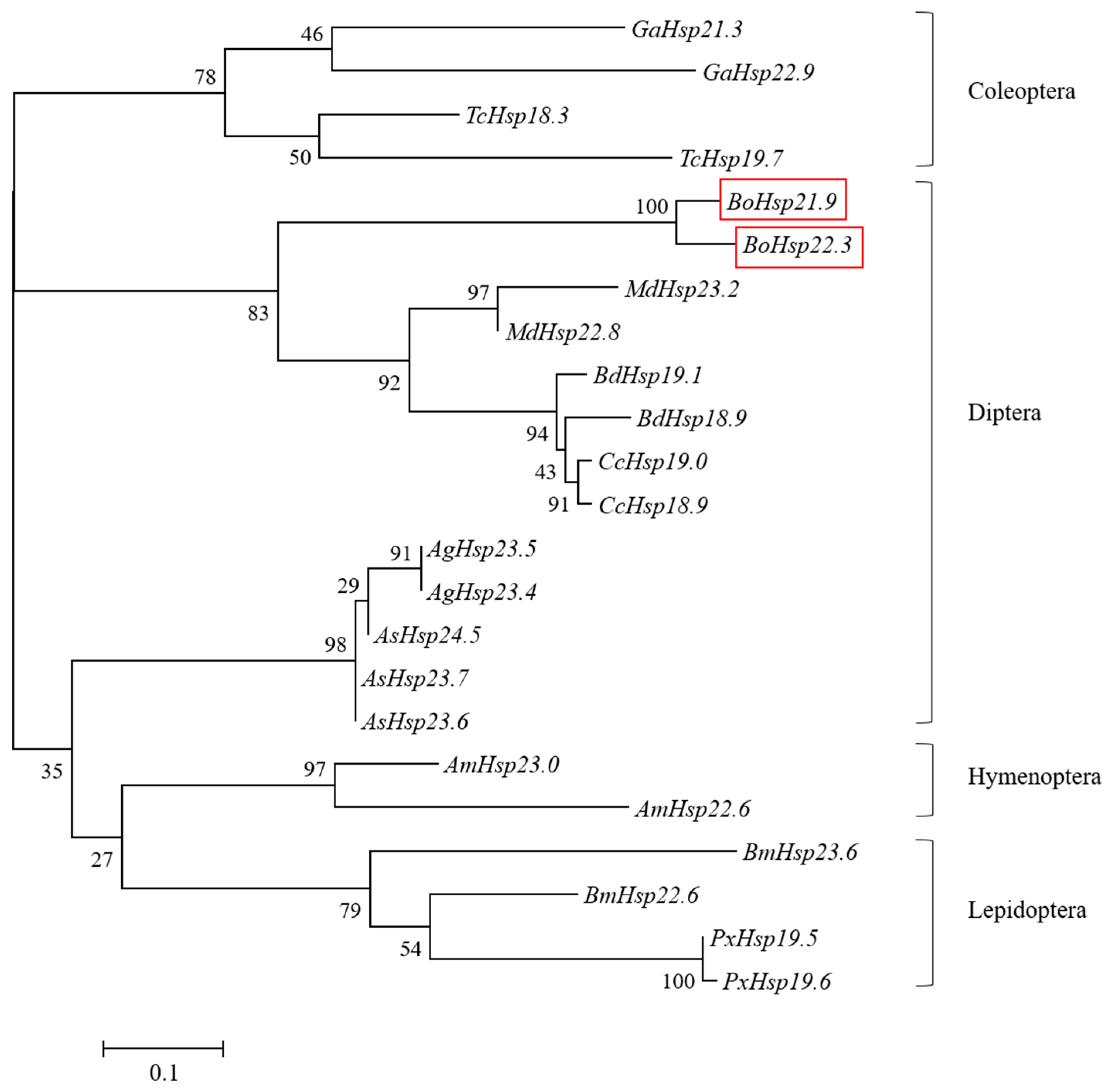
3.2. Phylogenetic Analysis
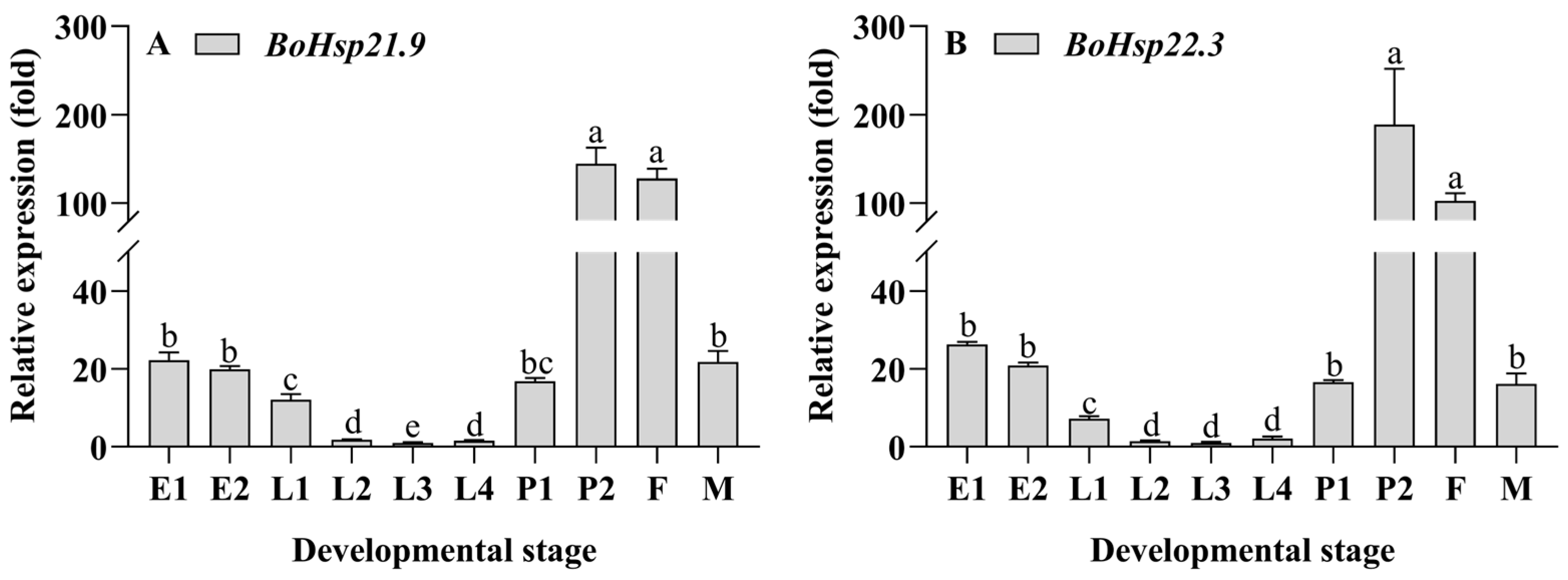
3.3. Developmental Stage Expression Patterns of BoHsp21.9 and BoHsp22.3
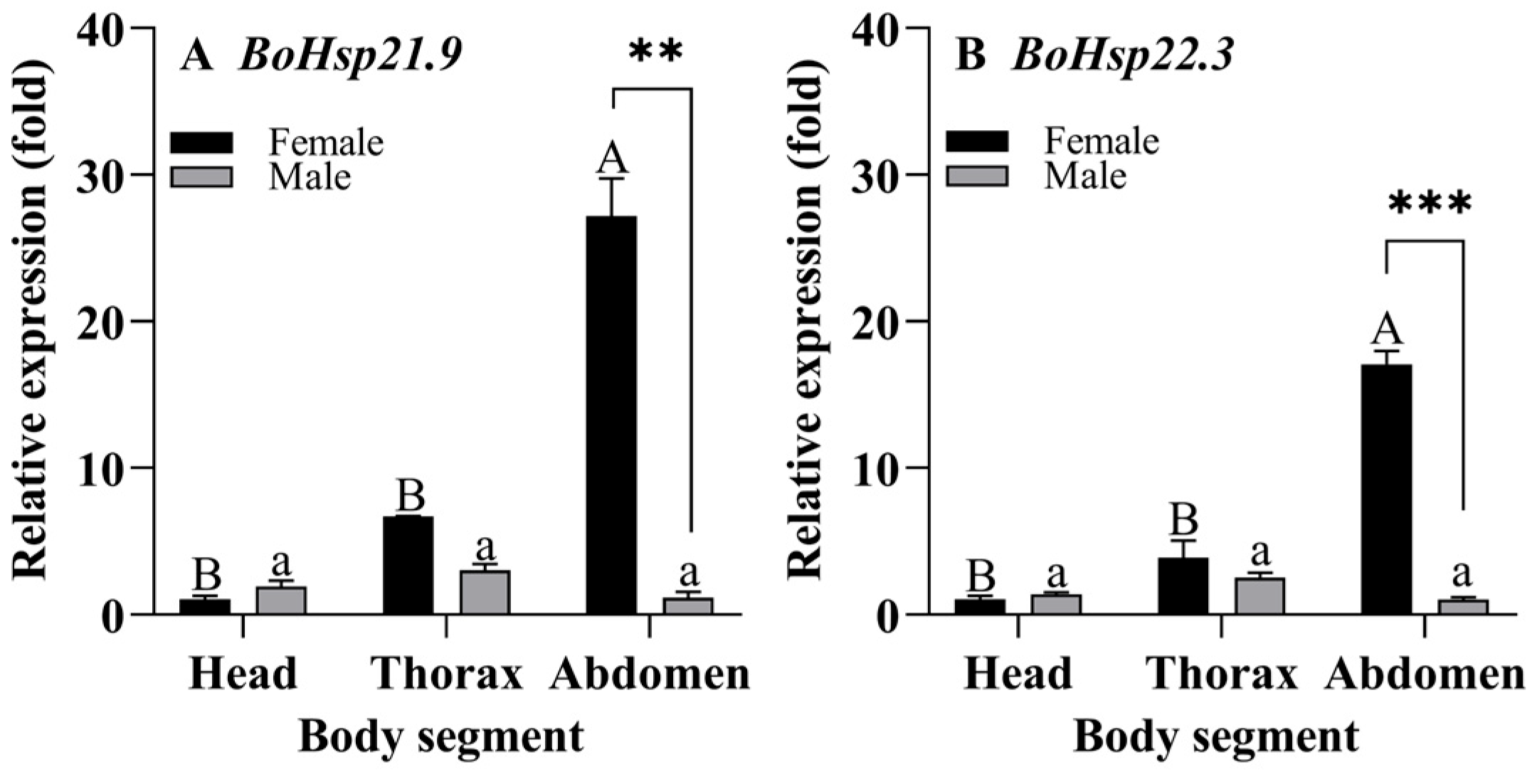
3.4. Body Segment Expression Patterns of BoHsp21.9 and BoHsp22.3
3.5. Expression Patterns of BoHsp21.9 and BoHsp22.3 After Heat Stress
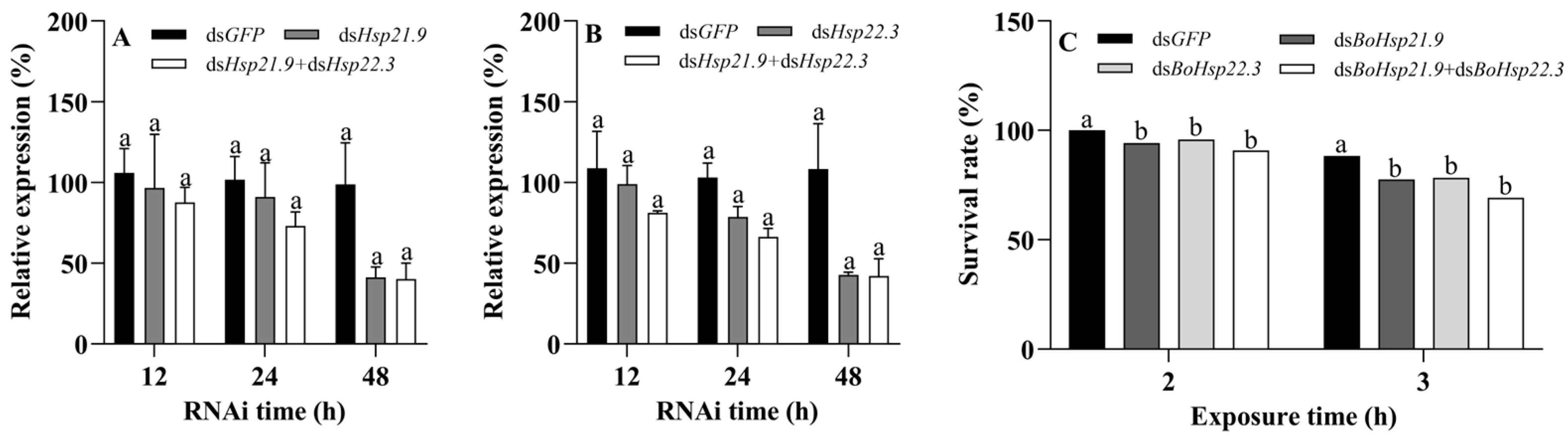
3.6. Functional Analysis of BoHsp21.9 and BoHsp22.3 by RNAi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.K.; Zhang, X.M. Notes on the fragrant onion gnats with descriptions of two new species of Bradysia (Sciaridae: Diptera). Acta Agric. Univ. Pekin. 1985, 11, 153–156. [Google Scholar]
- Xue, M.; Pang, Y.H.; Wang, C.X.; Li, Q. Biological effect of liliaceous host plants on Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae). Acta Entomol. Sin. 2005, 48, 914–921. [Google Scholar]
- Zhu, G.D.; Xue, M.; Luo, Y.; Ji, G.X.; Liu, F.; Zhao, H.P.; Sun, X. Effects of short-term heat shock and physiological responses to heat stress in two Bradysia adults, Bradysia odoriphaga and Bradysia difformis. Sci. Rep. 2017, 7, 13381. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, F.; Mu, W.; Wang, Q.H.; Li, H. Comparison of Bradysia odoriphaga Yang and Zhang reared on artificial diet and different host plants based on an age-stage, two-sex life table. Phytoparasitica 2015, 43, 107–120. [Google Scholar] [CrossRef]
- Li, W.X.; Yang, Y.T.; Xie, W.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Zhu, X.; Wang, S.J.; Zhang, Y.J. Effects of Temperature on the Age-Stage, Two-Sex Life Table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2015, 108, 126–134. [Google Scholar] [CrossRef]
- Cheng, J.X.; Su, Q.; Jiao, X.G.; Shi, C.H.; Yang, Y.T.; Han, H.L.; Xie, W.; Guo, Z.J.; Wu, Q.J.; Xu, B.Y.; et al. Effects of Heat Shock on the Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2017, 110, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.X. Studies on Biological Characteristics and Cold Hardness of Bradysia odoriphaga. Master’s Thesis, Northwest Sci-Tech University of Agriculture and Forestry, Yangling, China, 2003. [Google Scholar]
- Shi, C.H.; Yang, Y.T.; Han, H.L.; Cheng, J.X.; Wu, Q.J.; Xu, B.Y.; Zhang, Y.J. Population dynamics and summer and winter habitats of Bradysia odoriphaga in the Beijing area. Chin. J. Appl. Entomol. 2016, 53, 1174–1183. [Google Scholar]
- Shi, C.H.; Hu, J.R.; Wei, Q.W.; Yang, Y.T.; Cheng, J.X.; Han, H.L.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Su, Q.; et al. Control of Bradysia odoriphaga (Diptera: Sciaridae) by soil solarization. Crop Prot. 2018, 114, 76–82. [Google Scholar] [CrossRef]
- Cheng, J.X.; Su, Q.; Xia, J.X.; Yang, Z.Z.; Shi, C.H.; Wang, S.L.; Wu, Q.J.; Li, C.R.; Zhang, Y.J. Comparative transcriptome analysis of differentially expressed genes in Bradysia odoriphaga Yang et Zhang (Diptera: Sciaridae) at different acute stress temperatures. Genomics 2020, 112, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Kim, R.; Kim, S.H. Crystal structure of a small heat-shock protein. Nature 1998, 394, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.R.; Rioflorido, I. Evolutionary analysis of the small heat shock proteins in five complete algal genomes. J. Mol. Evol. 2007, 65, 162–174. [Google Scholar] [CrossRef]
- Waters, E.R.; Aevermann, B.D.; Sanders-Reed, Z. Comparative analysis of the small heat shock proteins in three angiosperm genomes identifies new subfamilies and reveals diverse evolutionary patterns. Cell Stress Chaperon. 2008, 13, 127–142. [Google Scholar]
- Aevermann, B.D.; Waters, E.R. A comparative genomic analysis of the small heat shock proteins in Caenorhabditis elegans and briggsae. Genetica 2008, 133, 307–319. [Google Scholar]
- Singh, M.K.; Sharma, B.; Tiwari, P.K. The small heat shock protein Hsp27: Present understanding and future prospects. J. Therm. Biol. 2017, 69, 149–154. [Google Scholar] [CrossRef]
- de Jong, W.W.; Caspers, G.J.; Leunissen, J.A.M. Genealogy of the α-crystallin-small heat-shock protein superfamily. Int. J. Biol. Macromol. 1998, 22, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Franck, E.; Madsen, O.; Rheede, T.V.; Ricard, G.; Huynen, M.A.; de Jong, W.W. Evolutionary Diversity of Vertebrate Small Heat Shock Proteins. J. Mol. Evol. 2004, 59, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Caspers, G.J.; Leunissen, J.A.M.; de Jong, W.W. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain”. J. Mol. Evol. 1995, 40, 238–248. [Google Scholar] [PubMed]
- Fu, X.M.; Jiao, W.W.; Chang, Z.Y. Phylogenetic and Biochemical Studies Reveal a Potential Evolutionary Origin of Small Heat Shock Proteins of Animals from Bacterial Class A. J. Mol. Evol. 2006, 62, 257–266. [Google Scholar] [CrossRef]
- Stromer, T.; Fischer, E.; Richter, K.; Haslbeck, M.; Buchner, J. Analysis of the Regulation of the Molecular Chaperone Hsp26 by Temperature-induced Dissociation: The N-terminal domain is important for oligomer assembly and the binding of unfolding proteins. J. Biol. Chem. 2004, 279, 11222–11228. [Google Scholar]
- Pérez-Morales, D.; Ostoa-Saloma, P.; Espinoza, B. Trypanosoma cruzi SHSP16: Characterization of an a-crystallin small heat shock protein. Exp. Parasitol. 2009, 123, 182–189. [Google Scholar]
- Li, Z.W.; Li, X.; Yu, Q.Y.; Xiang, Z.H.; Kishino, H.; Zhang, Z. The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol. Biol. 2009, 9, 215. [Google Scholar] [CrossRef]
- Reineke, A. Identification and expression of a small heat shock protein in two lines of the endoparasitic wasp Venturia canescens. Comp. Biochem. Phys. A 2005, 141, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Xi, D.M.; Kang, M.J.; Guo, X.Q.; Xu, B.H. Molecular cloning and characterization of Hsp27.6: The first reported small heat shock protein from Apis cerana cerana. Cell Stress Chaperon. 2012, 17, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.; Pauli, D.; Tissières, A. Transcriptional regulation in Drosophila during heat shock: A nuclear run-on analysis. Chromosoma 1993, 102, 233–248. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Liu, Y.L.; Guo, X.L.; Li, Y.L.; Gao, H.R.; Guo, X.Q.; Xu, B.H. sHsp22.6, an intronless small heat shock protein gene, is involved in stress defence and development in Apis cerana cerana. Insect Biochem. Mol. Biol. 2014, 53, 1–12. [Google Scholar] [CrossRef]
- Zhao, L.M.; Becnel, J.J.; Clark, G.G.; Linthicum, K.J. Expression of AeaHsp26 and AeaHsp83 in Aedes aegypti (Diptera: Culicidae) larvae and Pupae in Response to Heat Shock Stress. J. Med. Entomol. 2010, 47, 367–375. [Google Scholar]
- Sakano, D.; Lin, B.; Xia, Q.Y.; Yamamoto, K.; Aso, Y. Genes encoding small heat shock proteins of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2006, 70, 2443–2450. [Google Scholar] [CrossRef]
- Sheng, Q.; Xia, J.Y.; Nie, Z.M.; Zhang, Y.Z. Cloning, Expression, and Cell Localization of a Novel Small Heat Shock Protein Gene: BmHSP25.4. Appl. Biochem. Biotechnol. 2010, 162, 1297–1305. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Y.; Bao, Z.Z.; Jin, X.; Zhu, W.J.; Wang, L.P.; Liu, T.; Ji, H.P.; Wang, H.Y.; Xu, S.Q.; et al. Identification of a Bombyx mori gene encoding small heat shock protein BmHsp27.4 expressed in response to high-temperature stress. Gene 2014, 538, 56–62. [Google Scholar]
- Shi, C.H.; Yang, F.S.; Zhu, X.; Du, E.; Yang, Y.T.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Evalution of Housekeeing Genes for Quantitative Real-Time PCR Analysis of Bradysia odoriphaga (Diptera: Sciaridae). Int. J. Mol. Sci. 2016, 17, 1034. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shan, T.S.; Liu, Y.; Shi, X.Y.; Gao, X.W. Identification of a novel cytochrome P450 CYP3356A1 linked with insecticide detoxification in Bradysia odoriphaga. Pest Manag. Sci. 2019, 75, 1006–1013. [Google Scholar] [CrossRef]
- Michaud, S.; Morrow, G.; Marchand, J.; Tanguay, R.M. Drosophila small heat shock proteins: Cell and organelle-specific chaperones? Prog. Mol. Subcell. Biol. 2002, 28, 79–101. [Google Scholar]
- Sonoda, S.; Ashfaq, M.; Tsumuki, H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch. Insect Biochem. Physiol. 2006, 62, 80–90. [Google Scholar] [CrossRef]
- Sonoda, S.; Ashfaq, M.; Tsumuki, H. A comparison of heat shock protein genes from cultured cells of the Cabbage armyworm, Mamestra brassicae, in response to heavy metals. Arch. Insect Biochem. Physiol. 2007, 65, 210–222. [Google Scholar] [CrossRef]
- Kokolakis, G.; Tatari, M.; Zacharopoulou, A.; Mintzas, A.C. The hsp27 gene of the Mediterranean fruit fly, Ceratitis capitata: Structural characterization, regulation and developmental expression. Insect Mol. Biol. 2008, 17, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Gkouvitsas, T.; Kontogiannatos, D.; Kourti, A. Differential expression of two small Hsps during diapause in the corn stalk borer Sesamia nonagrioides (Lef.). J. Insect Physiol. 2008, 54, 1503–1510. [Google Scholar] [CrossRef]
- Huang, L.H.; Wang, C.Z.; Kang, L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J. Insect Physiol. 2009, 55, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.J.; Xiao, J.H.; Liu, L.; Li, T.; Huang, D.W. Molecular cloning and characterization of four heat shock protein genes from Macrocentrus cingulum (Hymenoptera: Braconidae). Mol. Biol. Rep. 2010, 37, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Tiwari, P.K. Cloning & sequence identification of Hsp27 gene and expression analysis of the protein on thermal stress in Lucilia cuprina. Insect Sci. 2016, 23, 555–568. [Google Scholar]
- Michaud, S.; Marin, R.; Tanguay, R.M. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol. Life Sci. 1997, 53, 104–113. [Google Scholar] [CrossRef]
- Gu, J.; Huang, L.X.; Shen, Y.; Huang, L.H.; Feng, Q.L. Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol. Bio. 2012, 5, 535–543. [Google Scholar] [CrossRef]
- Wang, H.S.; Wang, X.H.; Zhou, C.S.; Huang, L.H.; Zhang, S.F.; Guo, W.; Kang, L. cDNA cloning of heat shock proteins and their expression in the two phases of the migratory locust. Insect Mol. Biol. 2007, 16, 207–219. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, J.; Huang, L.H.; Zheng, S.C.; Liu, L.; Xu, W.H.; Feng, Q.L.; Kang, L. Cloning and expression analysis of six small heat shock protein genes in the common cutworm, Spodoptera litura. J. Insect Physiol. 2011, 57, 908–914. [Google Scholar] [CrossRef]
- Collett, M.; Despland, E.; Simpson, S.J.; Krakauer, D. Spatial scales of desert locust gregarization. Proc. Natl. Acad. Sci. USA 1998, 95, 13052–13055. [Google Scholar]
- Pener, M.P. Locust Phase Polymorphism and its Endocrine Relations. Adv. Insect Physiol. 1991, 23, 1–79. [Google Scholar]
- Xie, J.; Hu, X.X.; Zhai, M.F.; Yu, X.J.; Song, X.W.; Gao, S.S.; Wu, W.; Li, B. Characterization and functional analysis of hsp18.3 gene in the red flour beetle, Tribolium castaneum. Insect Sci. 2019, 26, 263–273. [Google Scholar] [CrossRef]
- Zhang, C.F.; Dai, L.S.; Wang, L.; Qian, C.; Wei, G.Q.; Li, J.; Zhu, B.J.; Liu, C.L. Eicosanoids mediate sHSP 20.8 gene response to biotic stress in larvae of the Chinese oak silkworm Antheraea pernyi. Gene 2015, 562, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.C.; Wan, F.H. Using double-stranded RNA to explore the role of heat shock protein genes in heat tolerance in Bemisia tabaci (Gennadius). J. Exp. Biol. 2011, 214, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, S. The Heat-Shock Response. Ann. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef]
- Feder, J.H.; Rossi, J.M.; Solomon, J.M.; Solomon, N.M.; Lindquist, S. The consequences of expressing hsp70 in Drosophila cells at normal temperatures. Gene Dev. 1992, 6, 1402–1413. [Google Scholar] [CrossRef]
- Krebs, R.A. A comparison of Hsp70 expression and thermotolerance in adults and larvae of three Drosophila species. Cell Stress Chaperon. 1999, 4, 243–249. [Google Scholar] [CrossRef]
- Mahadav, A.; Kontsedalov, S.; Czosnek, H.; Ghanim, M. Thermotolerance and gene expression following heat stress in the whitefly Bemisia tabaci B and Q biotypes. Insect Biochem. Mol. Biol. 2009, 39, 668–676. [Google Scholar] [CrossRef]
- Liang, P.; MacRae, T.H. Molecular chaperones and the cytoskeleton. J. Cell Sci. 1997, 110, 1431–1440. [Google Scholar] [CrossRef]
- Wang, K.Y.; Spector, A. α-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur. J. Biochem. 1996, 242, 56–66. [Google Scholar]
- Somero, G.N. Proteins and Temperature. Annu. Rev. Physiol. 1995, 57, 43–68. [Google Scholar] [CrossRef]
- Colinet, H.; Lee, S.F.; Hoffmann, A.A. Knocking down expression of Hsp22 and Hsp23 by RNA interference affects recovery from chill coma in Drosophila melanogaster. J. Exp. Biol. 2010, 213, 4146–4150. [Google Scholar] [CrossRef]
- Chapman, T.; Bangham, J.; Vinti, G.; Seifried, B.; Lung, O.; Wolfner, M.F.; Smith, H.K.; Partridge, L. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc. Natl. Acad. Sci. USA 2003, 100, 9923–9928. [Google Scholar] [PubMed]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Olson, K.E.; Blair, C.D. Arbovirus-mosquito interactions: RNAi pathway. Curr. Opin. Virol. 2015, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]



| Name | Primer Used | Primer Sequence (5′-3′) | Efficiency (%) | R2 |
|---|---|---|---|---|
| GSP1-Hsp21.9 | 5′-RACE | CCTTCATCAACCGCTTTTACTTCTGGCG | ||
| GSP2-Hsp21.9 | 5′-RACE | TTTGGAAGGGCATAACGGCGAGTGA | ||
| 10 × Universal Primer A Mix (UPM) | RACE | TAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | ||
| 10 × Universal Primer short (UPS) | RACE | CTAATACGACTCACTATAGGGC | ||
| Hsp21.9-F | PCR | CCAAAATGGCATTACTACCAC | ||
| Hsp21.9-R | PCR | TATCAGCCTTAAATGCGGTTC | ||
| Hsp22.3-F | PCR | CGTTCGCATACGAGAGAGC | ||
| Hsp22.3-R | PCR | GCTTCCGTAGGTTGCAATC | ||
| Hsp21.9-FP | qRT-PCR | TCGTCCGATGGCATTCTAACC | 93.60 | 0.995 |
| Hsp21.9-RP | qRT-PCR | TTCCGCTGTTCAATCGAGCT | ||
| Hsp22.3-FP | qRT-PCR | GTCGATCGGAAAGGACGGTT | 105.13 | 0.990 |
| Hsp22.3-RP | qRT-PCR | TCTTGGCGTTCCTCATGCTT | ||
| RPS15-FP | qRT-PCR | ATCGTGGCGTCGATTTGGAT | 101.03 * | 0.997 * |
| RPS15-RP | qRT-PCR | CTCATTTGGTGGGGCTTCCT | ||
| RPL28-FP | qRT-PCR | CGTGCCCGACATTTTCATCA | 105.18 * | 1.000 * |
| RPL28-RP | qRT-PCR | GACCAAGCCACTGTAACGGA | ||
| Hsp21.9-RNAi FP | RNAi | TAATACGACTCACTATAGGGTTGGAACAACTTTGGTCGTG | ||
| Hsp21.9-RNAi RP | RNAi | TAATACGACTCACTATAGGGGAAGGGCATAACGACGAGTG | ||
| Hsp22.3-RNAi FP | RNAi | TAATACGACTCACTATAGGGAATTCAAGCGTCGTTATGCC | ||
| Hsp22.3-RNAi RP | RNAi | TAATACGACTCACTATAGGGTTCTTTTGCTCTTTCACCGC | ||
| dsGFP-FP | RNAi | TAATACGACTCACTATAGGCAGTGCTTCAGCCGCTAC | ||
| dsGFP-RP | RNAi | TAATACGACTCACTATAGGGTTCACCTTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Zheng, H.; Feng, S.; Cao, W.; Wu, Q.; Song, J. Implication of Two Small Heat Shock Proteins in the Thermotolerance of Bradysia odoriphaga (Diptera: Sciaridae) Yang et Zhang. Insects 2025, 16, 1107. https://doi.org/10.3390/insects16111107
Cheng J, Zheng H, Feng S, Cao W, Wu Q, Song J. Implication of Two Small Heat Shock Proteins in the Thermotolerance of Bradysia odoriphaga (Diptera: Sciaridae) Yang et Zhang. Insects. 2025; 16(11):1107. https://doi.org/10.3390/insects16111107
Chicago/Turabian StyleCheng, Jiaxu, Huixin Zheng, Shuo Feng, Weiping Cao, Qingjun Wu, and Jian Song. 2025. "Implication of Two Small Heat Shock Proteins in the Thermotolerance of Bradysia odoriphaga (Diptera: Sciaridae) Yang et Zhang" Insects 16, no. 11: 1107. https://doi.org/10.3390/insects16111107
APA StyleCheng, J., Zheng, H., Feng, S., Cao, W., Wu, Q., & Song, J. (2025). Implication of Two Small Heat Shock Proteins in the Thermotolerance of Bradysia odoriphaga (Diptera: Sciaridae) Yang et Zhang. Insects, 16(11), 1107. https://doi.org/10.3390/insects16111107







