Environmental and Dispersal-Related Drivers of Color Morph Distribution in Triatoma infestans (Klug, 1834) (Hemiptera, Reduviidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
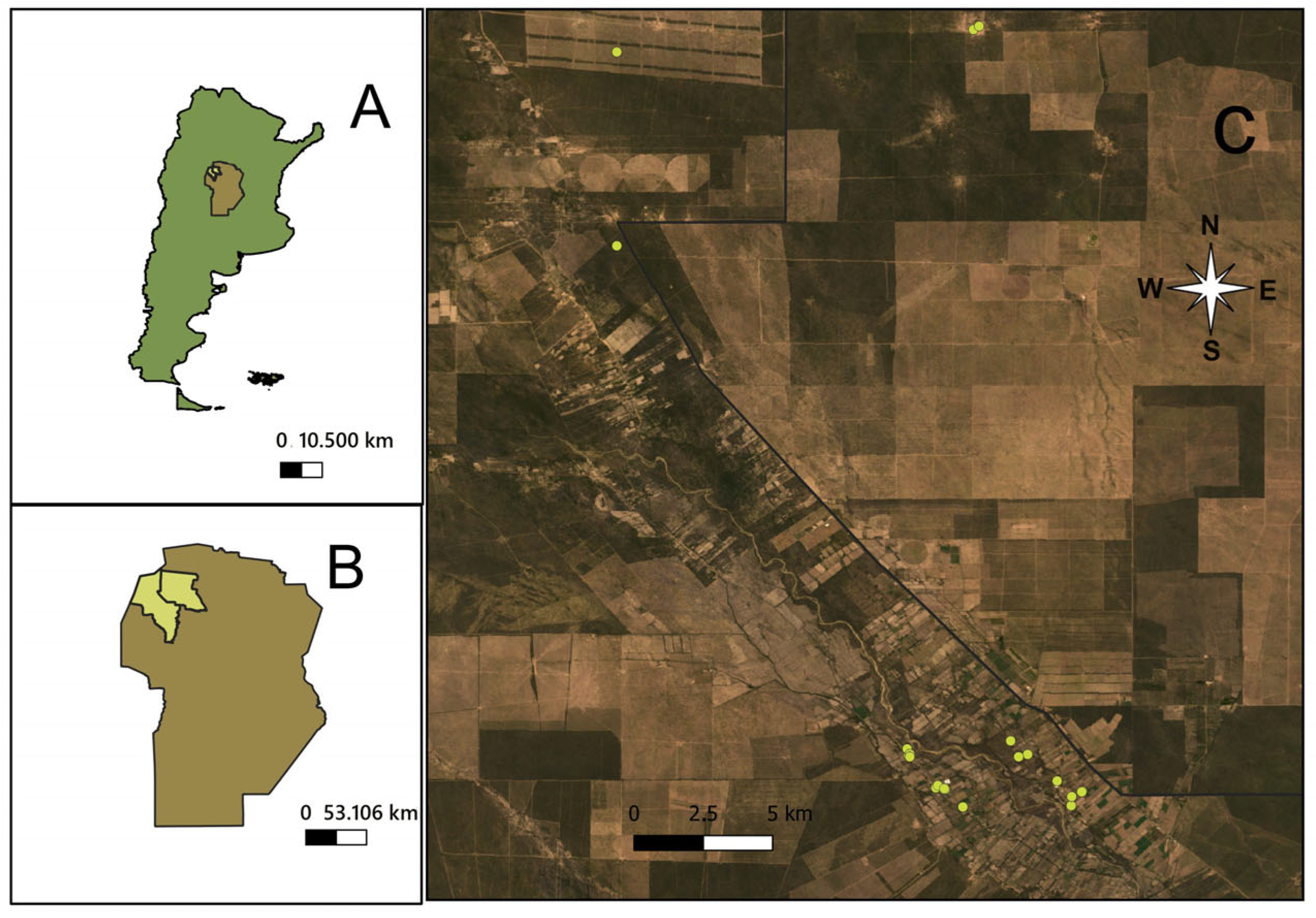
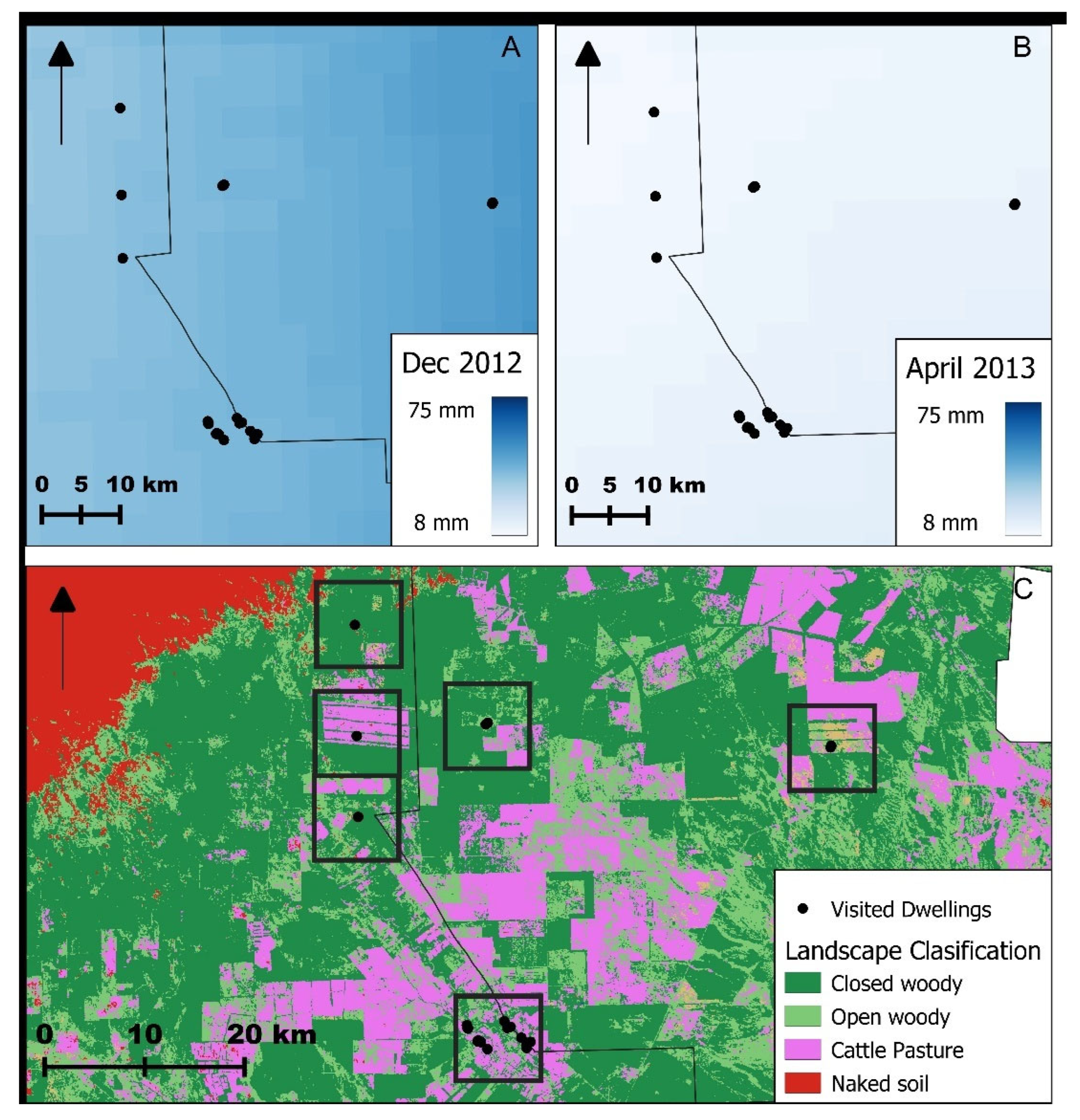
2.1. Study Area
2.2. Insects
2.3. Colorimetric Analysis
2.4. Nutritional Status
2.5. Linear Morphometric Measurements
2.6. Flight Performance Estimators
2.7. Climatic Variables and Vegetation Cover Estimation
2.8. Data Analysis
3. Results
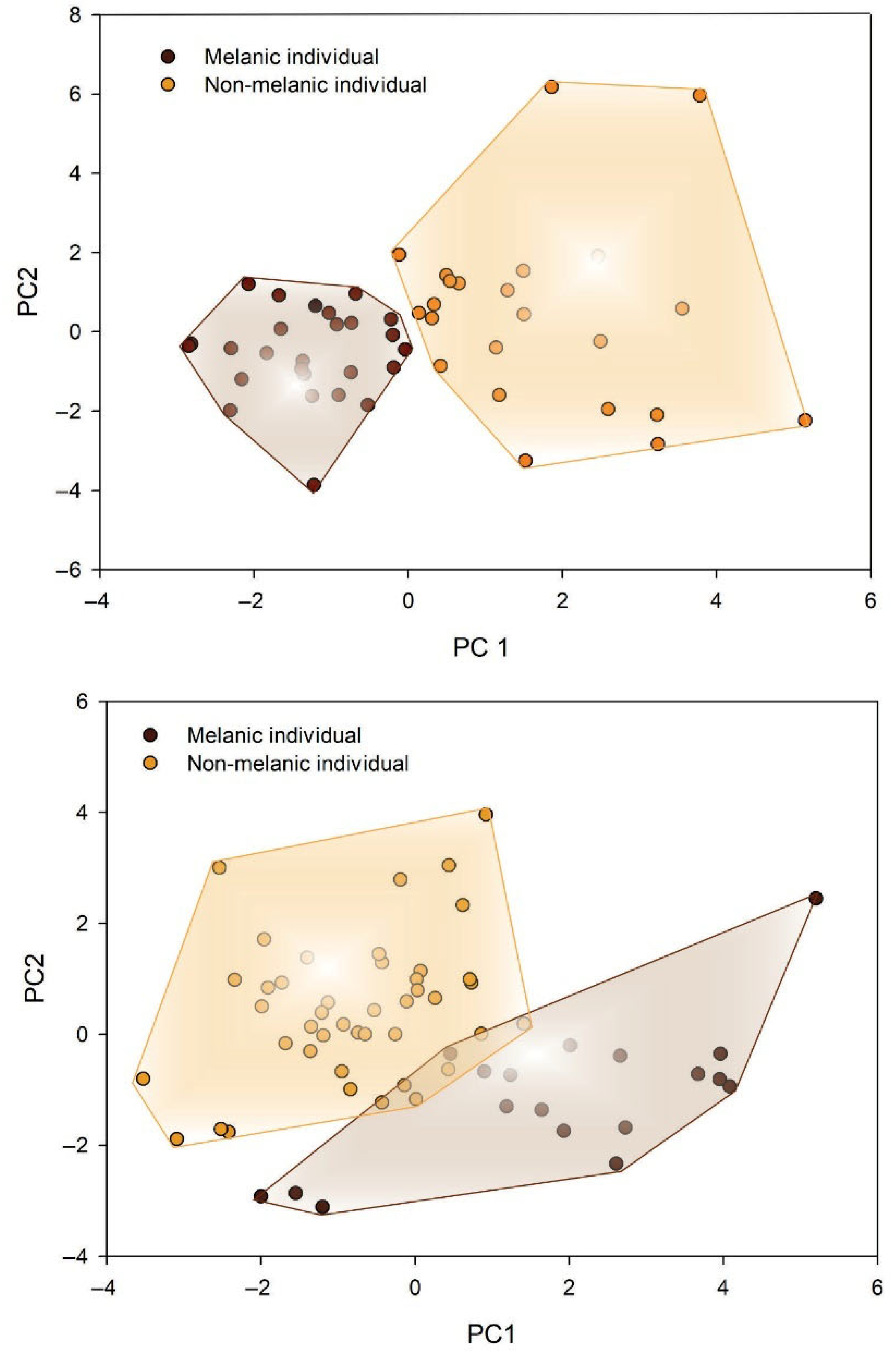
3.1. Colorimetric Analysis, Seasonal and Sex-Specific Variation in the Frequency of Chromatic Morphotypes
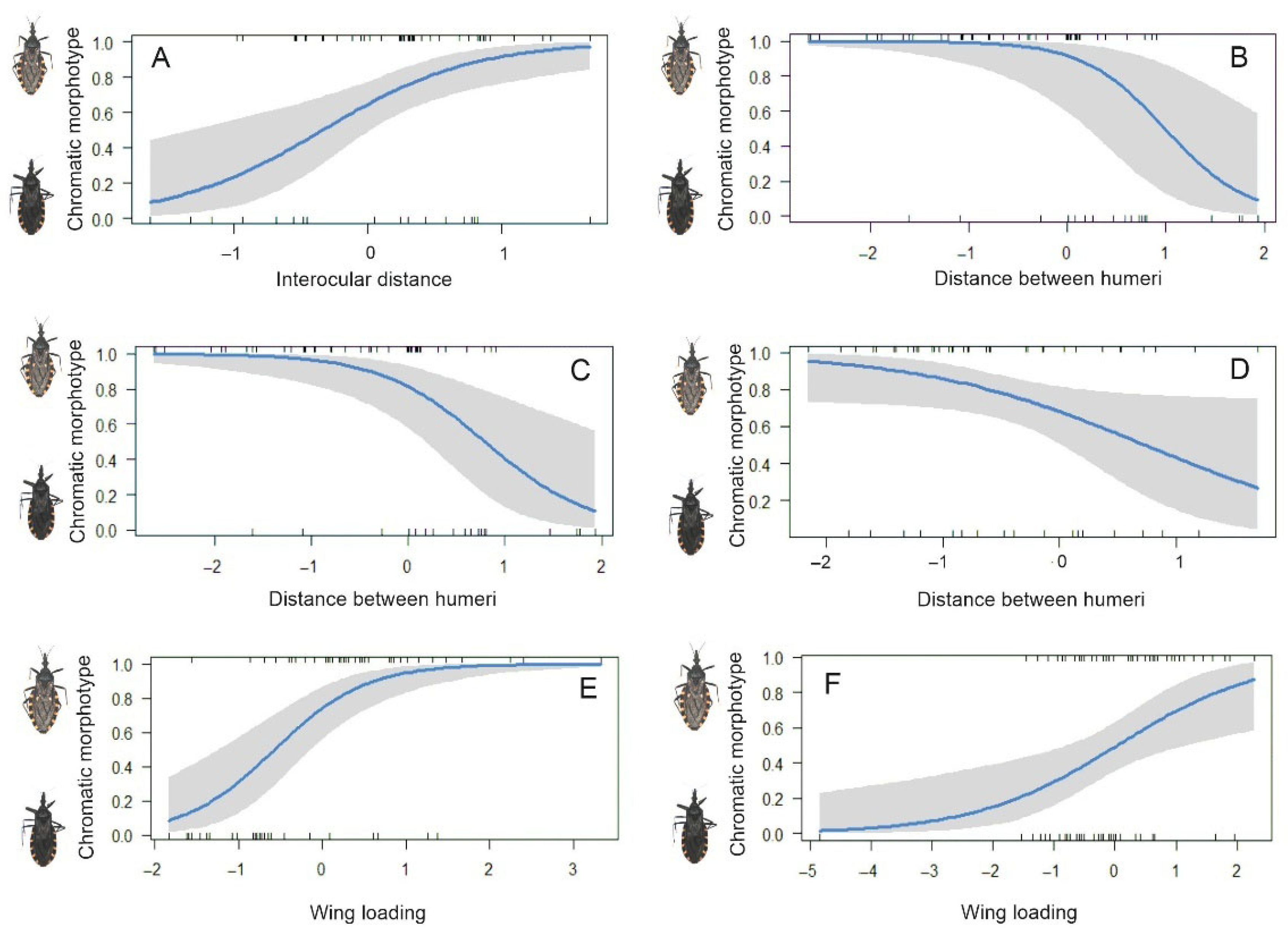
3.2. Morphometric Variation Explains Chromatic Morphotypes in a Season- and Sex-Dependent Manner
3.3. Nutritional Status Fails to Predict Chromatic Morphotypes Across Seasons
3.4. Wing Loading as a Significant Predictor of Chromatic Morphotype
3.5. Sex-Specific Environmental Associations of Melanic Morphotypes
4. Discussion
4.1. Seasonal and Sex-Specific Variation in the Frequency of Chromatic Morphotypes
4.2. Sex-Specific Environmental Associations of Melanic Morphotypes
4.3. Morphometric and Wing Loading Variations as Predictor of Chromatic Morphotypes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| W | Body weight |
| L | Total bod length |
| DH | Distance between humeri |
| AD | Anterocular distance |
| MID | Interocular distance |
| WL | Forewing length |
| MW | Maximum width |
| FA | Forewing area |
| MA | Membranous area |
| SA | Stiff area |
| WL | Wing loading |
| AR | Aspect ratio |
References
- Alevi, K.C.C.; Oliveira, J.; Rocha, D.S.; Galvão, C. Trends in taxonomy of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae): From Linnaean to integrative taxonomy. Pathogens 2021, 10, 1627. [Google Scholar] [CrossRef]
- FIOCRUZ (Fundação Instituto Oswaldo Cruz). National and International Reference Laboratory in Triatomine Taxonomy, Biological Collection; FIOCRUZ (Fundação Instituto Oswaldo Cruz): Rio de Janeiro, Brazil, 2025; Available online: https://www.ioc.fiocruz.br/en/lnirtt?num_for=2 (accessed on 10 September 2025).
- WHO (World Health Organization). Chagas Disease; WHO (World Health Organization): Cham, Switzerland, 2025. Available online: https://www.who.int/health-topics/chagas-disease#:~:text=Chagas%20disease%2C%20also%20known%20as,cruzi (accessed on 10 September 2025).
- Ceccarelli, S.; Balsalobre, A.; Medone, P.; Cano, M.E.; Gurgel Gonçalves, R.; Feliciangeli, D.; Vezzani, D.; Wisnivesky-Colli, C.; Gorla, D.E.; Marti, G.A.; et al. DataTri, a database of American triatomine species occurrence. Sci. Data 2018, 5, 180071. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Balsalobre, A.; Vicente, M.A.; Curtis-Robles, R.; Hamer, S.A.; Ayala Landa, J.M.; Rabinovich, J.E.; Martí, G.E. American triatomine species occurrences: Updates and novelties in the DataTri database. Gigabyte 2022, 31, 1–14. [Google Scholar] [CrossRef]
- Abrahan, L.; Gorla, D.; Catalá, S. Active dispersal of Triatoma infestans and other triatomines in the Argentinean arid Chaco before and after vector control interventions. J. Vector Ecol. 2016, 41, 90–96. [Google Scholar] [CrossRef]
- Villaseca, C.; Méndez, M.A.; Pinto, C.A.; Lemic, D.; Benítez, H.A. Unraveling the morphological variation of Triatoma infestans in the peridomestic habitats of Chuquisaca Bolivia: A geometric morphometric approach. Insects 2021, 12, 185. [Google Scholar] [CrossRef]
- Schofield, C.J.; Jannin, J.; Salvatella, R. The future of Chagas disease control. Trends Parasitol. 2006, 22, 583–588. [Google Scholar] [CrossRef]
- Gürtler, R.E. Sustainability of vector control strategies in the Gran Chaco Region: Current challenges and possible approaches. Mem. Inst. Oswaldo Cruz 2009, 104, 52–59. [Google Scholar] [CrossRef]
- Rojas de Arias, A.; Messenger, L.A.; Rolon, M.; Vega, M.C.; Acosta, N.; Villalba, C.; Marcet, P.L. Dynamics of Triatoma infestans populations in the Paraguayan Chaco: Population genetic analysis of household reinfestation following vector control. PLoS ONE 2022, 17, e0263465. [Google Scholar] [CrossRef]
- Pérez-Cascales, E.; Sossa-Soruco, V.; Brénière, S.F.; Depickère, S. Reinfestation with Triatoma infestans despite vigilance efforts in the municipality of Saipina, Santa Cruz, Bolivia: Situational description two months after fumigation. Acta Trop. 2020, 203, 105292. [Google Scholar] [CrossRef]
- Bedin, C.; Wilhelm, T.; Villela, M.M.; da Silva, G.C.C.; Riffel, A.P.K.; Sackis, P.; de Mello, F. Residual foci of Triatoma infestans infestation: Surveillance and control in Rio Grande do Sul, Brazil, 2001–2018. Rev. Soc. Bras. Med. Trop. 2021, 54, 325. [Google Scholar] [CrossRef]
- Cecere, M.C.; Gürtler, R.E.; Canale, D.; Chuit, R.; Cohen, J.E. The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev. Panam. Salud Publica 1997, 11, 273–279. [Google Scholar] [CrossRef]
- López, A.; Crocco, L.; Morales, G.; Catalá, S. Feeding frequency and nutritional status of peridomestic populations of Triatoma infestans from Argentina. Acta Trop. 1999, 73, 275–281. [Google Scholar] [CrossRef]
- Fronza, G.; Toloza, A.C.; Mougabure-Cueto, G.A.; Carbajo, A.E. Pyrethroid resistance distribution in Triatoma infestans and environmental association along the Argentine endemic zone. Acta Trop. 2024, 257, 107307. [Google Scholar] [CrossRef]
- Lardeux, F.; Depickère, S.; Aliaga, C.; Chavez, T.; Zambrana, L. Experimental control of Triatoma infestans in poor rural villages of Bolivia through community participation. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 150–158. [Google Scholar] [CrossRef]
- Crocco, L.B.; Nattero, J.; López, A.G.; Cardozo, M.; Soria, C.; Ortiz, V.; Rodríguez, C.S. Factors associated with the presence of triatomines in rural areas of south Argentine Chaco. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180357. [Google Scholar] [CrossRef]
- Cecere, M.C.; Vázquez-Prokopec, G.M.; Gürtler, R.E.; Kitron, U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am. J. Trop. Med. Hyg. 2004, 71, 803–810. [Google Scholar] [CrossRef]
- Matthysen, E. Multicausality of dispersal: A review. In Dispersal Ecology and Evolution; Clobert, J., Baguette, M., Benton, T.G., Bullock, J.M., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 3–18. ISBN 978-019-960-889-8. [Google Scholar]
- Renault, D. A review of the phenotypic traits associated with insect dispersal polymorphism, and experimental designs for sorting out resident and disperser phenotypes. Insects 2020, 11, 214. [Google Scholar] [CrossRef]
- Vázquez-Prokopec, G.M.; Ceballos, L.A.; Marcet, P.L.; Cecere, M.C.; Cardinal, M.V.; Kitron, U.; Gürtler, R.E. Seasonal variations in active dispersal of natural populations of Triatoma infestans in rural north-western Argentina. Med. Vet. Entomol. 2006, 20, 273–279. [Google Scholar] [CrossRef]
- Abrahan, L.B.; Gorla, D.E.; Catala, S.S. Dispersal of Triatoma infestans and other triatominae species in the arid Chaco of Argentina—Flying, walking or passive carriage? Mem. Inst. Oswaldo Cruz 2011, 106, 232–239. [Google Scholar] [CrossRef]
- McEwen, P.; Lehane, M.; Whitaker, C. The effect of adult population density on flight initiation in Triatoma infestans (Klug) (Hemiptera: Reduviidae). J. Appl. Entomol. 1993, 116, 321–325. [Google Scholar] [CrossRef]
- Di Iorio, O.; Gürtler, R.E. Seasonality and temperature-dependent flight dispersal of Triatoma infestans (Hemiptera: Reduviidae) and other vectors of Chagas disease in Western Argentina. J. Med. Entomol. 2017, 54, 1285–1292. [Google Scholar] [CrossRef]
- Lobbia, P.A.; Mougabure-Cueto, G. Active dispersal in Triatoma infestans (Klug,1834) (Hemiptera: Reduviidae: Triatominae): Effects of nutritional status, the presence of a food source and the toxicological phenotype. Acta Trop. 2020, 204, 105345. [Google Scholar] [CrossRef]
- Cote, J.; Clobert, J.; Fitze, P.S. Mother–offspring competition promotes colonization success. Proc. Natl. Acad. Sci. USA 2007, 104, 9703–9708. [Google Scholar] [CrossRef]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef]
- Hernández, M.L.; Dujardin, J.P.; Gorla, D.E.; Catalá, S.S. Can body traits, other than wings, reflect the flight ability of Triatominae bugs? Rev. Soc. Bras. Med. Trop. 2015, 48, 682–691. [Google Scholar] [CrossRef]
- Hernández, M.L.; Espinoza, J.; Gomez, M.; Gorla, D. Morphological changes associated with brachypterous Triatoma guasayana (Hemiptera, Reduviidae) and their relationship with flight. Int. J. Trop. Insect Sci. 2020, 40, 413–421. [Google Scholar] [CrossRef]
- Ribak, G.; Barkan, S.; Soroker, V.; Gurka, R. The aerodynamics of flight in an insect flight-mill. PLoS ONE 2017, 12, e0186441. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, S.; Galante, E.; Micó, E. Sex specificity of dispersal behaviour and flight morphology varies among tree hollow beetle species. Mov. Ecol. 2022, 10, 41. [Google Scholar] [CrossRef]
- Hernández, M.L.; Abrahan, L.B.; Dujardin, J.P.; Gorla, D.E.; Catalá, S.S. Phenotypic variability and population structure of peridomestic Triatoma infestans in rural areas of the arid Chaco (Western Argentina): Spatial influence of macro-and microhabitats. Vector Borne Zoonotic Dis. 2011, 11, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Nattero, J.; Malerba, R.; Rodríguez, C.S.; Crocco, L. Phenotypic plasticity in response to food source in Triatoma infestans (Klug 1834) (Hemiptera, Reduviidae: Triatominae). Infect. Genet. Evol. 2013, 19, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Nattero, J.; Cecere, M.C.; Gürtler, R.E. Temporal variations of fluctuating asymmetry in wing size and shape of Triatoma infestans populations from northwest Argentina. Infect. Genet. Evol. 2015, 56, 133–142. [Google Scholar] [CrossRef]
- Nattero, J.; Piccinali, R.V.; Fiad, F.G.; Cano, F.; Carbajal de la Fuente, A.L. Relationship between flight muscle dimorphism and wing morphometry in Triatoma infestans (Klug, 1834) (Hemiptera, Reduviidae, Triatominae). Front. Ecol. Evol. 2023, 11, 1211219. [Google Scholar] [CrossRef]
- Fiad, F.G.; Cardozo, M.; Rodríguez, C.S.; Hernandez, M.L.; Crocco, L.B.; Gorla, D.E. Ecomorphological variation of the Triatoma guasayana wing shape in semi-arid Chaco region. Acta Trop. 2022, 232, 106488. [Google Scholar] [CrossRef]
- Fiad, F.G.; Cardozo, M.; Nattero, J.; Gigena, G.V.; Gorla, D.E.; Rodríguez, C.S. Association between environmental gradient of anthropization and phenotypic plasticity in two species of triatomines. Parasites Vectors 2024, 17, 169. [Google Scholar] [CrossRef]
- Gigena, G.V.; Rodríguez, C.S.; Fiad, F.G.; Hernández, M.L.; Carbajal-de-la-Fuente, A.L.; Piccinali, R.V.; Sánchez Casaccia, P.; Rojas de Arias, A.; Lobbia, P.; Abrahan, L.; et al. Phenotypic variability in traits related to flight dispersal in the wing dimorphic species Triatoma guasayana. Parasites Vectors 2023, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Verly, T.; Fiad, F.G.; Carbajal-de-la-Fuente, A.L.; Pita, S.; Piccinali, R.V.; Lobbia, P.A.; Sánchez Casaccia, P.; Rojas de Arias, A.; Cavallo, M.J. Bug off or bug out: Mapping flight secrets of Triatoma garciabesi (Hemiptera: Reduviidae) through climate, geography, and greenery. Front. Insect Sci. 2025, 5, 1532298. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-de-la-Fuente, A.L.; Piccinali, R.V.; Porcasi, X.; Marti, G.A.; de Arias, A.R.; Abrahan, L.; Cano Suárez, F.; Medina, G.; Provecho, Y.; Cortez, M.R.; et al. Variety is the spice: The role of morphological variation of Triatoma infestans (Hemiptera, Reduviidae) at a macro-scale. Acta Trop. 2024, 256, 107239. [Google Scholar] [CrossRef]
- Verly, T.; Pita, S.; Carbajal-de-la-Fuente, A.L.; Burgueño-Rodríguez, G.; Piccinali, R.V.; Fiad, F.G.; Ríos, N.; Panzera, F.; Lobbia, P.; Sánchez Cassacia, P.; et al. Relationship between genetic diversity and morpho-functional characteristics of flight-related traits in Triatoma garciabesi (Hemiptera: Reduviidae). Parasites Vectors 2024, 17, 145. [Google Scholar] [CrossRef]
- True, J.R. Insect melanism: The molecules matter. Trends Ecol. Evol. 2003, 18, 640–647. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Whitten, M.M.A.; Kryukov, V.Y.; Yaroslavtseva, O.N.; Grizanova, E.V.; Greig, C.; Mukherjee, K.; Vilcinskas, A.; Mitkovets, P.V.; Gluppy, G.G.; et al. More than a colour change: Insect melanism, disease resistance and fecundity. Proc. R. Soc. B 2013, 280, 20130584. [Google Scholar] [CrossRef] [PubMed]
- Watt, W.B. Adaptive significance of pigment polymorphism in Colias butterflies. I: Variation of melanin in relation to thermoregulation. Evolution 1968, 22, 437–458. [Google Scholar] [CrossRef]
- de Jong, P.W.; Gussekloo, S.W.S.; Brakefeld, P.M. Differences in thermal balance, body temperature and activity between non-melanic and melanic two-spot ladybird beetles (Adalia bipunctata) under controlled conditions. J. Exp. Biol. 1996, 199, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Noireau, F.; Flores, R.; Vargas, F. Trapping sylvatic triatominae (Reduviidae) in hollow trees. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Noireau, F.; Flores, R.; Gutierrez, T.; Abad-Franch, F.; Flores, E.; Vargas, F. Natural ecotopes of Triatoma infestans dark morph and other sylvatic triatomines in the Bolivian Chaco. Trans. R. Soc. Trop. Med. Hyg. 2000, 94, 23–27. [Google Scholar] [CrossRef]
- Ceballos, L.A.; Piccinali, R.V.; Berkunsky, I.; Kitron, U.; Gürtler, R.E. First finding of melanic sylvatic Triatoma infestans (Hemiptera: Reduviidae) colonies in the Argentine Chaco. J. Med. Entomol. 2009, 46, 1195–1202. [Google Scholar] [CrossRef]
- Lobbia, P.A.; Alvarez, R.; Picollo, M.I.; Mougabure-Cueto, G. First record of domestic colonies of the dark chromatic variant of Triatoma infestans (Hemiptera: Reduviidae). Rev. Soc. Entomol. Argent. 2019, 78, 33–37. [Google Scholar] [CrossRef]
- Nattero, J.; Carbajal-de-la-Fuente, A.L.; Piccinali, R.V.; Cardozo, M.; Rodríguez, C.S.; Crocco, L.B. Characterization of melanic and non-melanic forms in domestic and peridomestic populations of Triatoma infestans (Hemiptera: Reduviidae). Parasites Vectors 2020, 13, 47. [Google Scholar] [CrossRef]
- Martínez, A.; Olmedo, R.A.; Carcavallo, R.U. Una nueva subespecie Argentina de Triatoma infestans. Chagas 1987, 4, 479–480. [Google Scholar]
- Monteiro, F.A.; Pérez, R.; Panzera, F.; Dujardin, J.-P.; Galvão, C.; Rocha, D.; Noireau, F.; Schofield, C.; Beard, C.B. Mitochondrial DNA variation of Triatoma infestans populations and its implication on the specific status of T. melanosoma. Mem. Inst. Oswaldo Cruz 1999, 94 (Suppl. S1), 229–238. [Google Scholar] [CrossRef]
- Moreno, M.; Moretti, E.; Basso, B.; Frias, M.; Catalá, S.; Gorla, D. Seroprevalence of Trypanosoma cruzi infection and vector control activities in rural communities of the southern Gran Chaco (Argentina). Acta Trop. 2010, 113, 257–262. [Google Scholar] [CrossRef]
- Moreno, M.L.; Hoyos, L.; Cabido, M.; Catalá, S.S.; Gorla, D.E. Exploring the association between Trypanosoma cruzi infection in rural communities and environmental changes in the southern Gran Chaco. Mem. Inst. Oswaldo Cruz 2012, 107, 231–237. [Google Scholar] [CrossRef]
- Cardozo, M.; Fiad, F.G.; Crocco, L.B.; Gorla, D.E. Triatominae of the semi-arid Chaco in Central Argentina. Acta Trop. 2021, 224, 106158. [Google Scholar] [CrossRef]
- Yeo, M.; Acosta, N.; Llewellyn, M.; Sánchez, H.; Adamson, S.; Miles, G.A.; López, E.; González, N.; Patterson, J.S.; Gaunt, M.W.; et al. Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillos hosts of Trypanosoma cruzi II, including hybrids. Int. J. Parasitol. 2005, 35, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Cabido, M.; Zeballos, S.R.; Zak, M.; Carranza, M.L.; Giorgis, M.A.; Cantero, J.J.; Acosta, A.T.R. Native woody vegetation in central Argentina: Classification of Chaco and Espinal forests. Appl. Veg. Sci. 2018, 21, 298–311. [Google Scholar] [CrossRef]
- Cabido, M.R.; Zak, M.R. Vegetación del Norte de Córdoba; Secretaría de Agricultura y Recursos Renovables de la Provincia de Córdoba, Agencia Córdoba Ambiente: Córdoba, Argentina, 1999. [Google Scholar]
- Ministerio de Salud de la Nación. Guía para el Control Vectorial de la Enfermedad de Chagas. 2015. Available online: https://www.argentina.gob.ar/sites/default/files/2021/07/guia_vectorial_chagas.pdf (accessed on 10 September 2025).
- Lent, H.; Wygodzinsky, P. Revision of Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 123–520. [Google Scholar]
- Van Belleghem, S.V.; Papa, R.; Ortiz-Zuazaga, H.; Hendrickx, F.; Jiggins, C.D.; McMillan, O.W. Patternize: An R package for quantifying colour pattern variation. Methods Ecol. Evol. 2018, 9, 390–398. [Google Scholar] [CrossRef]
- Ceballos, L.A.; Vazquez-Prokopec, G.M.; Cecere, M.C.; Marcet, P.L.; Gurtler, R.E. Feeding rates, nutritional status and fight dispersal potential of peridomestic populations of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2005, 95, 149–159. [Google Scholar] [CrossRef]
- Lehane, M.J.; Schofield, C.J. Flight initiation in Triatoma infestans (Klug) (Hemiptera: Reduviidae). Bull. Entomol. Res. 1982, 72, 497–510. [Google Scholar] [CrossRef]
- Betts, C.R.; Wootton, R.J. Wing shape and flight behavior in butterflies (Lepidoptera: Papilionoidea and Hesperioidea): A preliminary analysis. J. Exp. Biol. 1988, 138, 271–288. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Dobrowski, S.Z.; Parks, S.A.; Hegewisch, K.C. Terraclimate, a high-resolution global dataset of monthly climate and climatic water balance from 1958–2015. Sci. Data 2018, 5, 170191. [Google Scholar] [CrossRef]
- de la Vega, G.J.; Medone, P.; Ceccarelli, S.; Rabinovich, J.; Schilman, P.E. Geographical distribution, climatic variability and thermo-tolerance of Chagas disease vectors. Ecography 2015, 38, 851–860. [Google Scholar] [CrossRef]
- Pereira, J.M.; Almeida, P.S.D.; Sousa, A.V.D.; Paula, A.M.D.; Machado, R.B.; Gurgel-Gonc<monospace>̧</monospace>alves, R. Climatic factors influencing triatomine occurrence in Central-West Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 335–341. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical Maps (Version 4). 2024. Available online: https://www.fragstats.org (accessed on 23 October 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org (accessed on 23 October 2025).
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed-Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-10. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 23 October 2025).
- Gurevitz, J.M.; Kitron, U.; Gürtler, R.E. Temporal dynamics of flight muscle development in Triatoma infestans (Hemiptera: Reduviidae). J. Med. Entomol. 2009, 46, 1021–1024. [Google Scholar] [CrossRef]
- Marcet, P.L.; Mora, M.S.; Cutrera, A.P.; Jones, L.; Gürtler, R.E.; Kitron, U.; Dotson, E. Genetic structure of Triatoma infestans populations in rural communities of Santiago del Estero, northern Argentina. Infect. Genet. Evol. 2008, 8, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Gaspe, M.S.; Schachter-Brodie, J.; Gurevitz, J.M.; Kitron, U.; Gürtler, R.E.; Dujardin, J.-P. Microgeographic spatial structuring of Triatoma infestans (Hemiptera: Reduviidae) populations using wing geometric morphometry in the argentine Chaco. J. Med. Entomol. 2012, 49, 504–514. [Google Scholar] [CrossRef]
- Piccinali, R.V.; Gaspe, M.S.; Nattero, J.; Gürtler, R.E. Population structure and migration in Triatoma infestans (Hemiptera: Reduviidae) from the Argentine Chaco: An integration of genetic and morphometric data. Acta Trop. 2023, 247, 107010. [Google Scholar] [CrossRef]
- McEwen, P.K.; Lehane, M.J. Relationships between flight initiation and oviposition in Triatoma infestans (Klug) (Hem., Reduviidae). J. Appl. Entomol. 1994, 117, 217–223. [Google Scholar] [CrossRef]
- Lehane, M.J.; McEwan, P.K.; Whitaker, C.J.; Schofield, C.J. The role of temperature and nutritional dependence in the flight initiation of Triatoma infestans. Acta Trop. 1992, 52, 27–38. [Google Scholar] [CrossRef]
- Gonçalves, T.C.; de Oliveira, E.; Dias, L.S.; Almeida, M.D.; Nogueira, W.O.; Pires, F.D. An investigation on the ecology of Triatoma vitticeps (Stal, 1859) and its possible role in the transmission of Trypanosoma cruzi, in the locality of Triunfo, Santa Maria Madalena municipal district, state of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 1998, 93, 711–717. [Google Scholar] [CrossRef]
- Minoli, S.A.; Lazzari, C.R. Take-off activity and orientation of triatomines (Heteroptera: Reduviidae) in relation to the presence of artificial lights. Acta Trop. 2006, 97, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, J.M.; Ceballos, L.A.; Kitron, U.; Gürtler, R.E. Flight initiation of Triatoma infestans (Hemiptera: Reduviidae) under natural climatic conditions. J. Med. Entomol. 2006, 43, 143–150. [Google Scholar] [CrossRef]
- Noireau, F.; Dujardin, J.P. Biology of triatominae. In American Trypanosomiasis: Chagas Disease One Hundred Years of Research; Telleria, J., Tibayrenc, M., Eds.; Elsevier: Burlington, VT, USA, 2010; pp. 149–162. [Google Scholar]
- Leite, G.R.; dos Santos, C.B.; Falqueto, A. Influence of the landscape on dispersal of sylvatic triatomines to anthropic habitats in the Atlantic Forest. J. Biogeogr. 2010, 38, 651–663. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P.; Liu, C. Modelling species distributions in Britain: A hierarchical integration of climate and land-cover data. Ecography 2004, 27, 285–298. [Google Scholar] [CrossRef]
- Luoto, M.; Virkkala, R.; Heikkinen, R.K. The role of land cover in bioclimatic models depends on spatial resolution. Glob. Ecol. Biogeogr. 2007, 16, 34–42. [Google Scholar] [CrossRef]
- Franklin, J. Predictive vegetation mapping: Geographic modelling of biospatial patterns in relation to environmental gradients. Prog. Phys. Geog. 1995, 19, 474–499. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimatic envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Vazquez-Prokopec, G.M.; Spillmann, C.; Zaidenberg, M.; Gürtler, R.E.; Kitron, U. Spatial heterogeneity and risk maps of community infestation by Triatoma infestans in rural Northwestern Argentina. PLoS Negl. Trop. Dis. 2012, 6, e1788. [Google Scholar] [CrossRef]
- Carmona-Galindo, V.D.; Sheppard, C.C.; Bastin, M.L.; Kehrig, M.R.; Marín-Recinos, M.F.; Choi, J.J.; Castañeda de Abrego, V. Chromatic and morphological differentiation of Triatoma dimidiata (Hemiptera: Reduviidae) with land use diversity in El Salvador. Pathogens 2021, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Sandon, L.; Weinberg, D.; Espinosa, M.O.; Abril, M.O.; Chuit, R.; Porcasi, X.; Periago, M.V. Association between landscape transformation and the Chagas disease vector dynamics in a rural area with continuous surveillance and control. Parasites Vectors 2025, 18, 203. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Brown, K.S.; Brown, G.G. Habitat alteration and species loss in Brazilian forests. In Tropical Deforestation and Species Extinction; Whitmore, T.C., Sayer, J.A., Eds.; Chapman and Hall: London, UK, 1992; pp. 119–142. [Google Scholar]
- Gottdenker, N.L.; Calzada, J.E.; Saldaña, A.; Carroll, C.R. Association of anthropogenic land use change and increased abundance of the Chagas disease vector Rhodnius pallescens in a rural landscape of Panama. Am. J. Trop. Med. Hyg. 2011, 84, 70–77. [Google Scholar] [CrossRef]
- Majerus, M.E.N. Melanism: Evolution in Action; Oxford Academic: Oxford, UK, 1998. [Google Scholar]
- Safranek, L.; Riddiford, L.M. The biology of the black larval mutant of the tobacco hornworm. Manduca sexta. J. Insect Physiol. 1975, 21, 1931–1938. [Google Scholar] [CrossRef]
- Almeida, C.E.; Oliveira, H.L.; Correia, N.; Dornak, L.L.; Gumiel, M.; Neiva, V.L.; Harry, M.; Mendonça, V.J.; Costa, J.; Galvão, C. Dispersion capacity of Triatoma sherlocki, Triatoma juazeirensis and laboratory-bred hybrids. Acta Trop. 2012, 122, 71–79. [Google Scholar] [CrossRef]
- Dujardin, J.P.; Bermudez, H.; Casini, C.; Schofeld, C.J.; Tibayrenc, M. Metric differences between sylvatic and Triatoma infestans (Heteroptera: Reduviidae) in Bolivia. J. Med. Entomol. 1997, 34, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, J.P.; Forgues, G.; Torrez, M.; Martínez, E.; Córdoba, C.; Gianella, A. Morphometrics of domestic Panstrongylus rufotuberculatus in Bolivia. Ann. Trop. Med. Parasitol. 1998, 92, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Casaccia, P.; Carbajal-de-la-Fuente, A.L.; Rojas-de-Arias, A.; Piccinali, R.V.; Nattero, J. Use of colorimetric analysis to study the origin of reinfestant Triatoma infestans in a rural community of the Paraguayan Chaco. In II Congress of the Latin American Society for Vector Ecology; 2019; p. 64. Available online: https://congresos.unlp.edu.ar/lasove/wp-content/uploads/sites/45/2023/03/ABSTRACT-BOOK-LA-SOVE-2022_final-version.pdf (accessed on 23 October 2025).
- Rolón, M.; Vega, M.C.; Román, F.; Gómez, A.; Rojas de Arias, A. First report of colonies of sylvatic Triatoma infestans (Hemiptera: Reduviidae) in the Paraguayan Chaco, using a trained dog. PLoS Negl. Trop. Dis. 2011, 5, e1026. [Google Scholar] [CrossRef] [PubMed]


| Sampling Periods | Number of Adults Collected | Sexes | Melanic Morphotype | Non-Melanic Morphotype |
|---|---|---|---|---|
| Beginning of the warm season (December 2012) | 105 | 50 females 55 males | 15 females 28 males | 35 females 27 males |
| End of the warm season (April 2013) | 61 | 28 females 33 males | 12 females 6 males | 16 females 27 males |
| Sex | Variable | Estimate (β) | SE | z-Value | p-Value | OR (Odds Ratio) | 95% CI (OR) | p-Value (OR) |
|---|---|---|---|---|---|---|---|---|
| Female | Interocular distance | 0.980 | 0.550 | 0.564 | 0.081 | 3.314 | 1.12–12.28 | 0.046 |
| Anterocular distance | −0.920 | 0.516 | 1.741 | 0.082 | 0.424 | 0.13–1.19 | 0.115 | |
| Membranous portion area | −2.737 | 2.717 | 0.981 | 0.327 | 0.204 | 0.00–52.78 | 0.593 | |
| Distance humeri | −0.910 | 0.601 | 1.478 | 0.140 | 0.563 | 0.14–2.07 | 0.389 | |
| Male | Interocular distance | 1.107 | 0.527 | 2.050 | 0.040 | 2.754 | 0.98–9.08 | 0.068 |
| Forewing length | 0.904 | 0.816 | 1.082 | 0.279 | 0.692 | 0.21–2.13 | 0.565 | |
| Forewing width | 0.506 | 0.702 | 0.703 | 0.482 | 0.722 | 0.22–2.13 | 0.565 | |
| Membranous portion area | 4.718 | 4.517 | 1.019 | 0.482 | 22.87 | 0.01–3.55 | 0.473 | |
| Distance humeri | −1.287 | 0.550 | 2.286 | 0.022 | 0.251 | 0.07–0.71 | 0.016 |
| Sex | Variable | Estimate (β) | SE | z-Value | p-Value | OR (Odds Ratio) | 95% CI (OR) | p-Value (OR) |
|---|---|---|---|---|---|---|---|---|
| Female | Interocular distance | −0.016 | 0.590 | 0.027 | 0.9788 | 1.039 | 0.32–3.87 | 0.949 |
| Anterocular distance | 0.191 | 0.730 | 0.250 | 0.803 | 1.975 | 0.33–20.98 | 0.482 | |
| Forewing length | −0.516 | 0.792 | 0.621 | 0.534 | 0.373 | 0.02–2.55 | 0.392 | |
| Distance humeri | −2.171 | 0.909 | 2.276 | 0.023 | 0.082 | 0.00–0.43 | 0.033 | |
| Male | Interocular distance | 2.504 | 1.463 | 1.651 | 0.099 | 22.688 | 1.62–21.48 | 0.082 |
| Anterocular distance | 0.634 | 1.529 | 0.401 | 0.688 | 0.766 | 0.03–0.52 | 0.024 | |
| Forewing length | 1.872 | 1.188 | 1.510 | 0.131 | 7.498 | 0.64–26.27 | 0.183 | |
| Distance humeri | −1.977 | 0.938 | 2.028 | 0.043 | 0.104 | 0.01–0.52 | 0.024 |
| Sex | Variable | Estimate (β) | Std. Error | z-Value | p-Value | OR (Odds Ratio) | 95% CI (OR) | p-Value (OR) |
|---|---|---|---|---|---|---|---|---|
| Female | Wing loading | 1.829 | 0.440 | 4.091 | 0.000 | 6.353 | 2.87–17.15 | 0.000 |
| Aspect ratio | 0.086 | 0.300 | 0.281 | 0.779 | 1.512 | 0.56–4.12 | 0.402 | |
| Sampling | 0.173 | 0.630 | 0.271 | 0.787 | 2.343 | 0.31–19.04 | 0.406 | |
| Male | Wing loading | 0.844 | 0.304 | 2.705 | 0.007 | 2.341 | 1.35–4.61 | 0.006 |
| Aspect ratio | −0.079 | 0.372 | 2.322 | 0.203 | 0.385 | 0.09–1.06 | 0.094 | |
| Sampling | −0.242 | 1.207 | 0.198 | 0.198 | 0.785 | 0.04–7.01 | 0.841 |
| Variable | Chi-Square Value | F | p-Value | Variable | Chi-Square Value | F | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Female | pr | 0.011 | 0.433 | 0.528 | Male | pr | 0.117 | 5.942 | 0.025 |
| tmean | 0.014 | 0.564 | 0.489 | tmean | 0.078 | 3.955 | 0.060 | ||
| CWV | 0.002 | 0.083 | 0.806 | CWV | 0.001 | 0.009 | 0.931 | ||
| OWV | 0.074 | 2.964 | 0.102 | OWV | 0.005 | 0.264 | 0.590 | ||
| CP | 0.066 | 2.621 | 0.118 | CP | 0.166 | 7.441 | 0.004 | ||
| NS | 0.015 | 0.612 | 0.466 | NS | 0.028 | 1.427 | 0.267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, E.V.; Fiad, F.G.; Gigena, G.V.; López, A.G.; Piccinali, R.V.; Carbajal-de-la-Fuente, A.L.; Rodríguez, C.S.; Nattero, J. Environmental and Dispersal-Related Drivers of Color Morph Distribution in Triatoma infestans (Klug, 1834) (Hemiptera, Reduviidae). Insects 2025, 16, 1103. https://doi.org/10.3390/insects16111103
Díaz EV, Fiad FG, Gigena GV, López AG, Piccinali RV, Carbajal-de-la-Fuente AL, Rodríguez CS, Nattero J. Environmental and Dispersal-Related Drivers of Color Morph Distribution in Triatoma infestans (Klug, 1834) (Hemiptera, Reduviidae). Insects. 2025; 16(11):1103. https://doi.org/10.3390/insects16111103
Chicago/Turabian StyleDíaz, Erika V., Federico G. Fiad, Gisel V. Gigena, Ana G. López, Romina V. Piccinali, Ana Laura Carbajal-de-la-Fuente, Claudia S. Rodríguez, and Julieta Nattero. 2025. "Environmental and Dispersal-Related Drivers of Color Morph Distribution in Triatoma infestans (Klug, 1834) (Hemiptera, Reduviidae)" Insects 16, no. 11: 1103. https://doi.org/10.3390/insects16111103
APA StyleDíaz, E. V., Fiad, F. G., Gigena, G. V., López, A. G., Piccinali, R. V., Carbajal-de-la-Fuente, A. L., Rodríguez, C. S., & Nattero, J. (2025). Environmental and Dispersal-Related Drivers of Color Morph Distribution in Triatoma infestans (Klug, 1834) (Hemiptera, Reduviidae). Insects, 16(11), 1103. https://doi.org/10.3390/insects16111103








