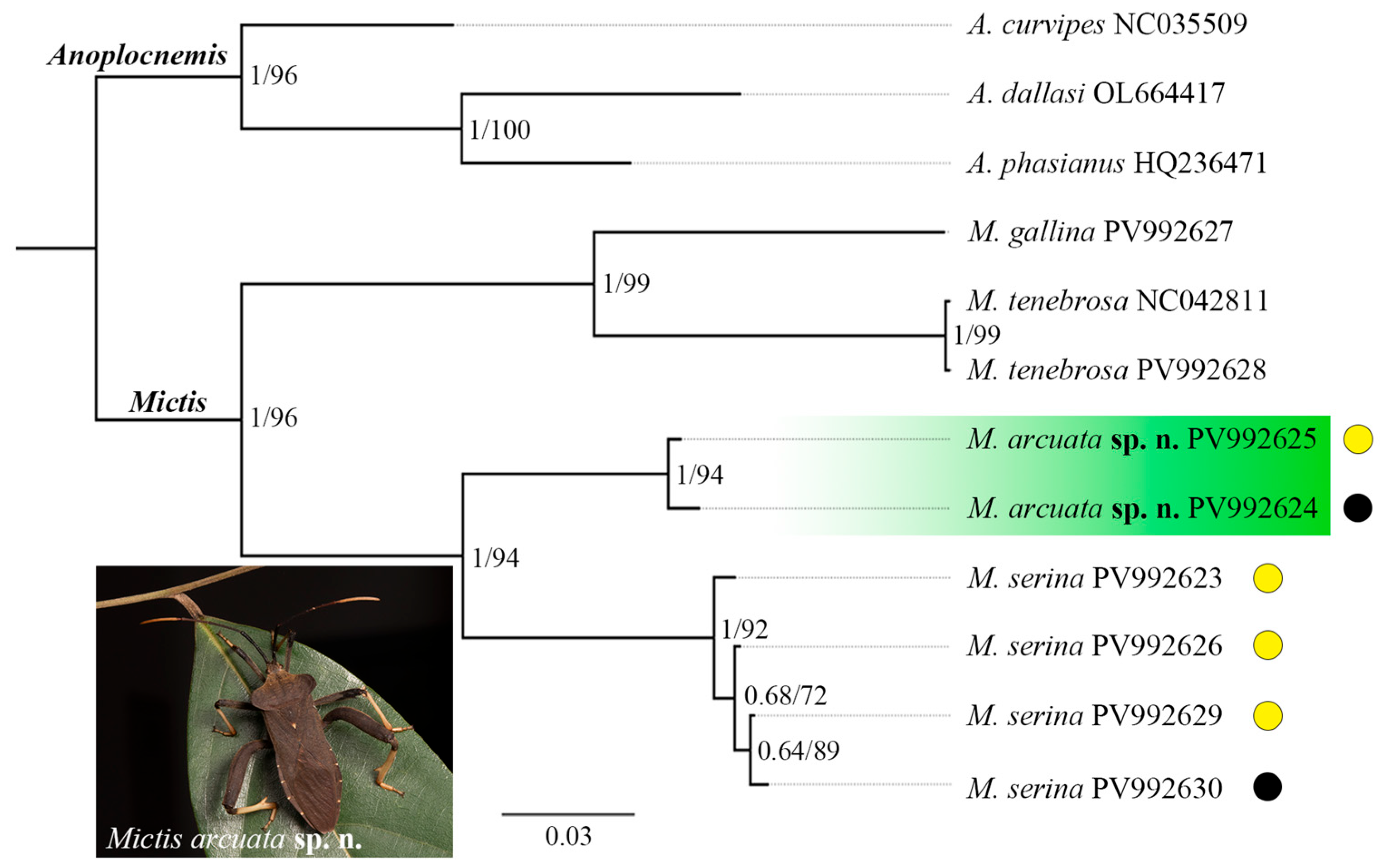
3.3. Taxonomy
Genus Mictis Leach, 1814
Chinese common name: 侎缘蝽属
(
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14 and
Figure 15)
Mictis Leach, 1814: 91. Type species by monotypy: Mictis crecifera Leach, 1814 (=profana Fabricius, 1803), Australia.
Myctis Leach, 1815: 121 (footnote). For Mictis Leach, 1814. Unjustified emendation or error.
Cerbus Hahn, 1831: 7, 14 (syn. Westwood, 1842: 4). Type species by subsequent designation (O’Shea & Schaefer, 1980: 229): Cerbus fulvicornis sensu Hahn, 1831 (=longicornis Westwood, 1842), Oriental Region.
Mictes Stål, 1856: 193 (subsequent misspelling).
Lygaeomictis Blöte, 1938: 294 (syn. O’Shea & Schaefer, 1980: 229). Type species by original designation: Lygaeomictis dilatipes Blöte, 1938, Timor.
Hsiao, 1977: 211–213, keyed six Chinese species.
Diagnosis. Body large, stout; head quadrate, preocular pits well developed, antennifers large, prominent, positioned in close proximity, projecting anteriorly beyond the tylus, antennae long, terete; pronotum moderately declivous, pronotum shape variable, posterior margin smooth; scutellum exhibiting faint transverse striations; labium reaching or nearly reaching middle coxae; all femora armed with distal spines, hind femora incrassate in male, dorsal margin smooth or slightly tubercles, ventral margin distinctly tubercles; hind tibiae usually dilated ventrally in male, often bearing a large tooth; common boundary of abdominal segments III and IV forming a usually prominent median tubercle; abdominal segment III with a pair of small to large of ventrolateral tubercles; female seventh sternite featuring an elevated plica forming a blunt angulation; paramere robust, terminating in a short curved apex.
Distribution. Oriental, Australian, and Afrotropical regions [
5,
6].
Mictis arcuata Xun, Jiang & Zhu sp. n.
Chinese common name: 宽肩侎缘蝽
ZooBankLSID: urn:lsid:zoobank.org:act: 159D25FD-CB32-484D-8A76-1F789D5FBE32
Type material. Holotype. ♂, China, Guangxi Zhuang Autonomous Region, Guilin City, Lingui District, Wantian Yao Ethnic Township, 25.5347° N, 110.0706° E, alt. 220 m, 9-IX-2024, Haofei Fan leg. (IZCAS). Paratypes. 1♀, China, Guangxi Zhuang Autonomous Region, Guilin City, Xing’an County, Gaozhai Village, 25.8555° N, 110.4852° E, alt. 492 m, 13-VII-2011, Hanyong Liu leg. (SHEM); 1♂, China, Guangxi Zhuang Autonomous Region, Guiping City, Junfeng Village, 23.0931° N, 110.2453° E, alt. 133 m, VII-2024, De Huang leg. (CHX); 1♂, China, Hunan Province, Yongzhou City, Jiangyong County, Yangjia, 24.9408° N, 111.0570° E, alt. 488 m, 27-IX-2025, Jianping Rong leg. (CHX); 1♂, China, Yunnan Province, Weixi Lisu Autonomous County, Xuelong Mountain, 27.1647° N, 99.3234° E, alt. 2 220 m, X-2023, Guirong Zhang leg. (CHX); 1♂1♀, Vietnam, Cao Bang Province, Cao Bang City, 22.6754° N, 106.2753° E, alt. 208 m, IV-2024, local villagers leg. (SHEM); 1♂, Vietnam, Ha Giang Province, Vi Xuyen district, 22.6631° N, 104.8275° E, alt. 586 m, VII-2025, local villagers leg. (CHX); 1♂1♀, Vietnam, Yen Bai Province, Muong Khuong District, Nam Co Commune, 21.8380° N, 104.2969° E, alt. 943 m, IV-2023, local villagers leg. (CMO, IZCAS).
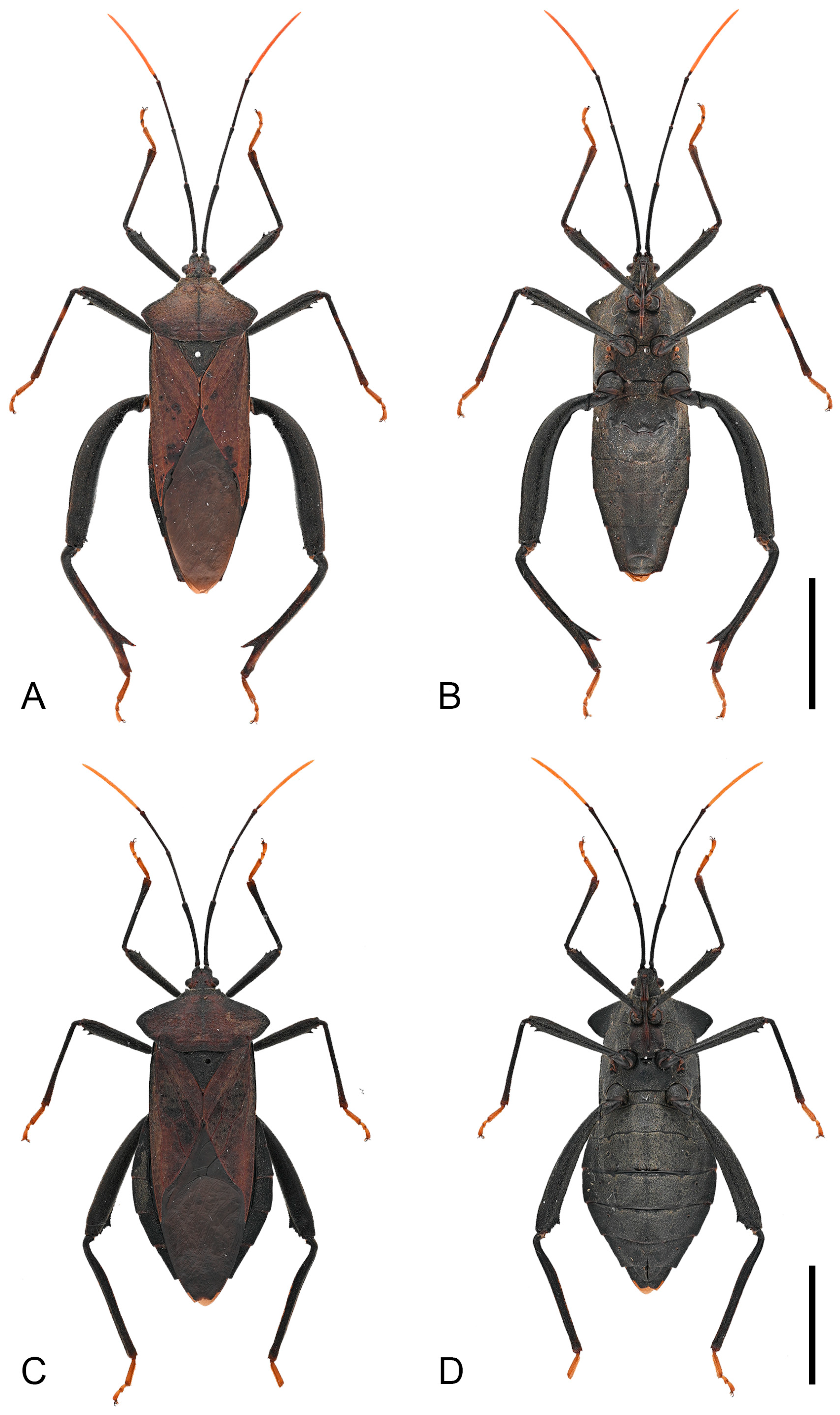
Diagnosis. Mictis arcuata sp. n. can be diagnosed from all other species by the following morphological characters: (1) body size large (28.2–31.7 mm); (2) dark-brown, integument with irregular, short, reddish-brown serration; (3) pronotum extremely extended laterally beyond lateral margins of corium base; (4) anterolateral borders arcuate, smooth, without serration; (5) humeral angles obtuse; (6) tibiae pale orange with dark-brown base to entirely dark-brown; (7) male hind tibia with a large spine at posterior half; (8) male with entire abdominal segments IV produced into a tubercle; (9) male posterior margin of segment IV slightly produced, overlapping anterior margin of segment V.
Description. Color and vestiture. Dark-brown; antennal segment III reddish-brown, and segment IV orange-brown; apex of scutellum pale orange; tibiae pale orange with dark-brown base to entirely dark-brown, tarsi pale orange-brown to pale brown (
Figure 3,
Figure 4 and
Figure 13A,B); body covered with short, suberect, reddish-brown setae.
Head. Quadrate, width across eyes greater than length; eyes small, flat; antenniferous tubercle large, prominent; antennae long, terete, relatively stout, relative lengths of antennal segments IV > I > II > III; labium reaching posterior margin of mesosternum.
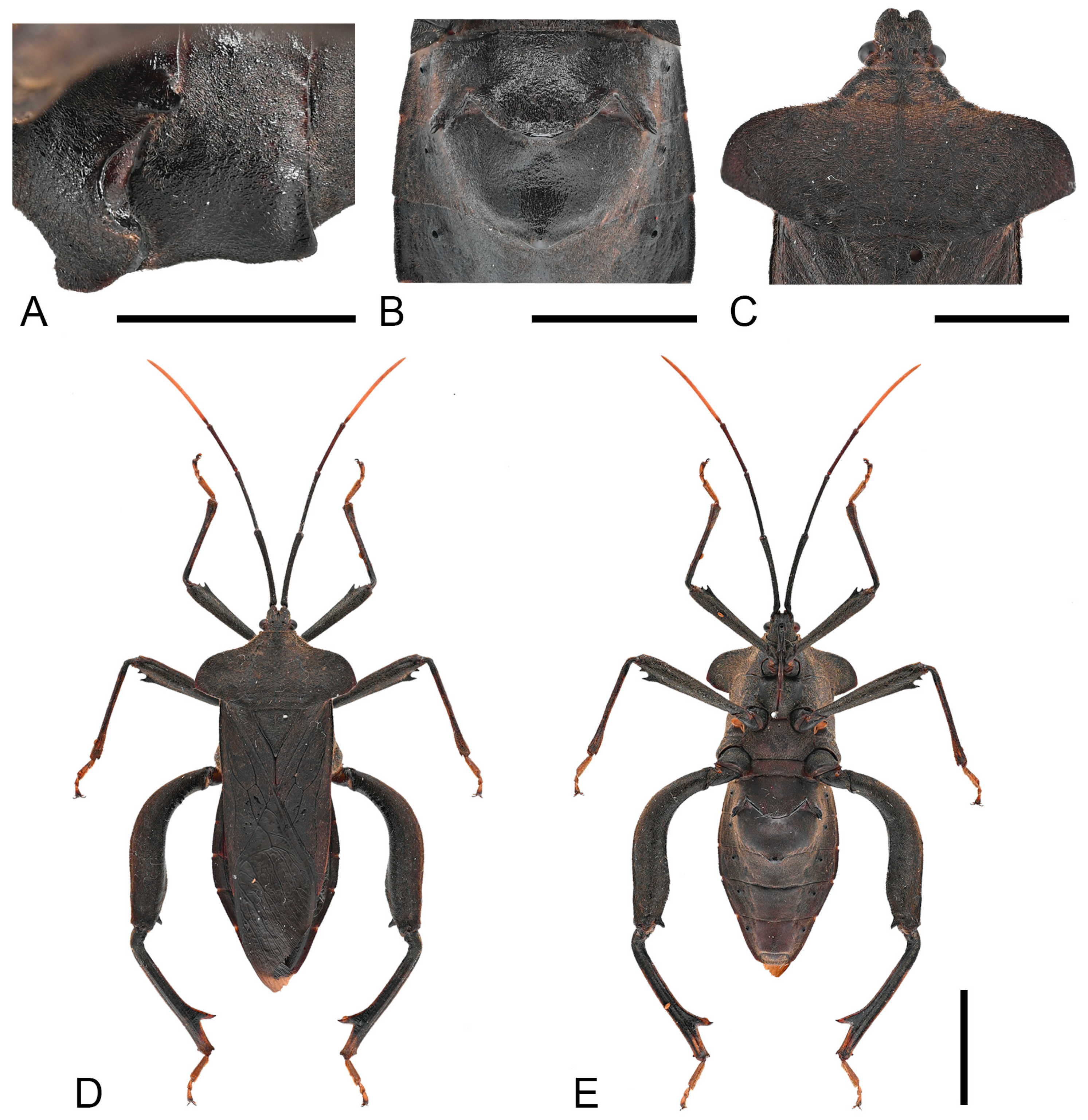
Thorax. Pronotum steeply declivent, extremely extended, beyond lateral margins of corium base, smooth, without tubercles; collar wide; anterolateral borders arcuate, smooth, without serration; humeral angles obtuse; posterolateral borders sinuate (
Figure 4C). Scutellum smooth, with obscure transverse striations; apex flat. Metapleura (both sides) each bearing a large tubercle. Hemelytra reaching the apex of the last abdominal segment.
Legs. Fore and mid femora smooth, slightly incrassate (both sexes), hind femora more incrassate (especially in male), all femora ventrally with two subdistal spines (male hind femur with only one distinct spine); male hind femora distinctly curved, basally with faint tubercles, apical swelling slightly wider than base; female hind femora smooth; male hind tibia with a large spine at posterior half (
Figure 13A,B).
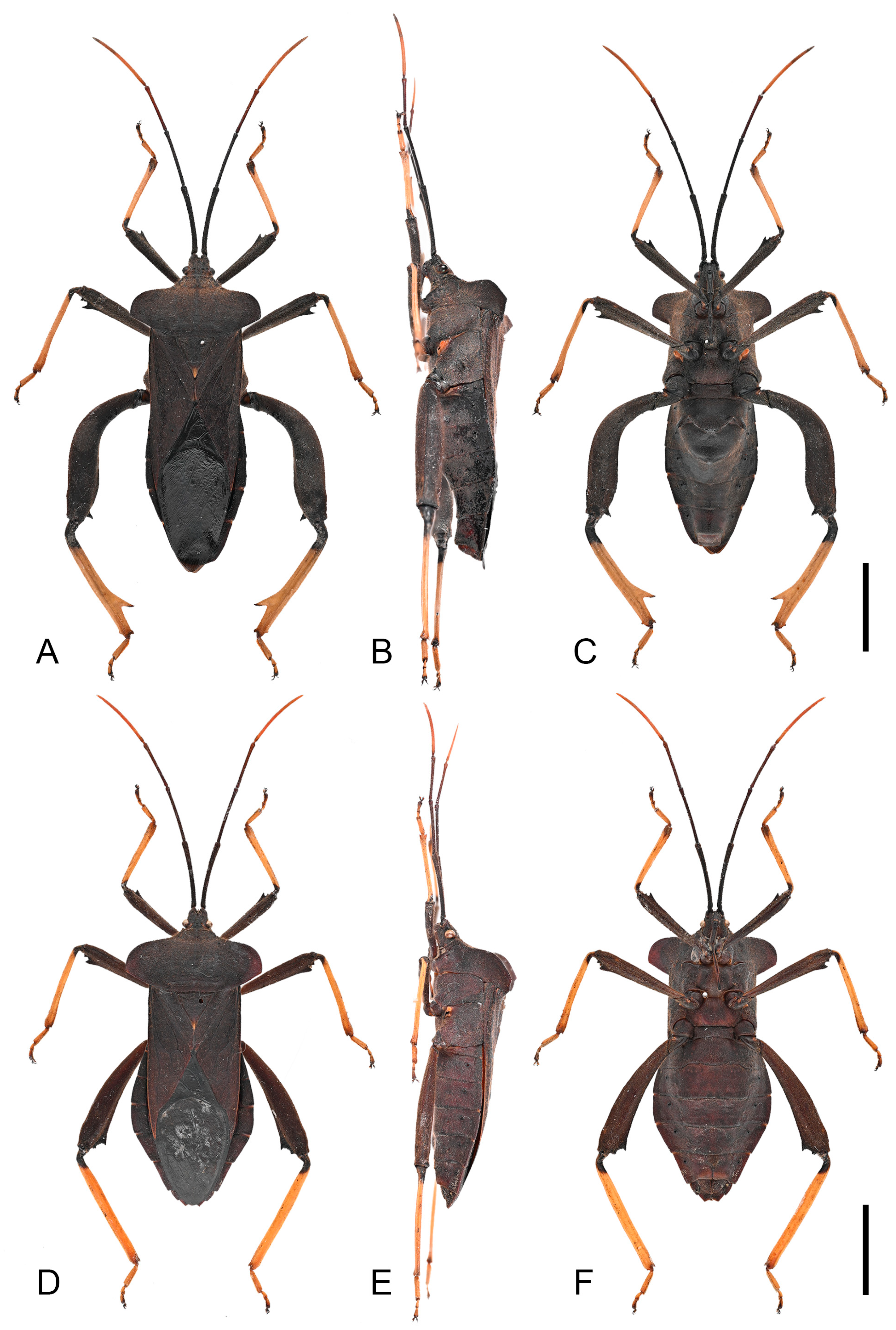
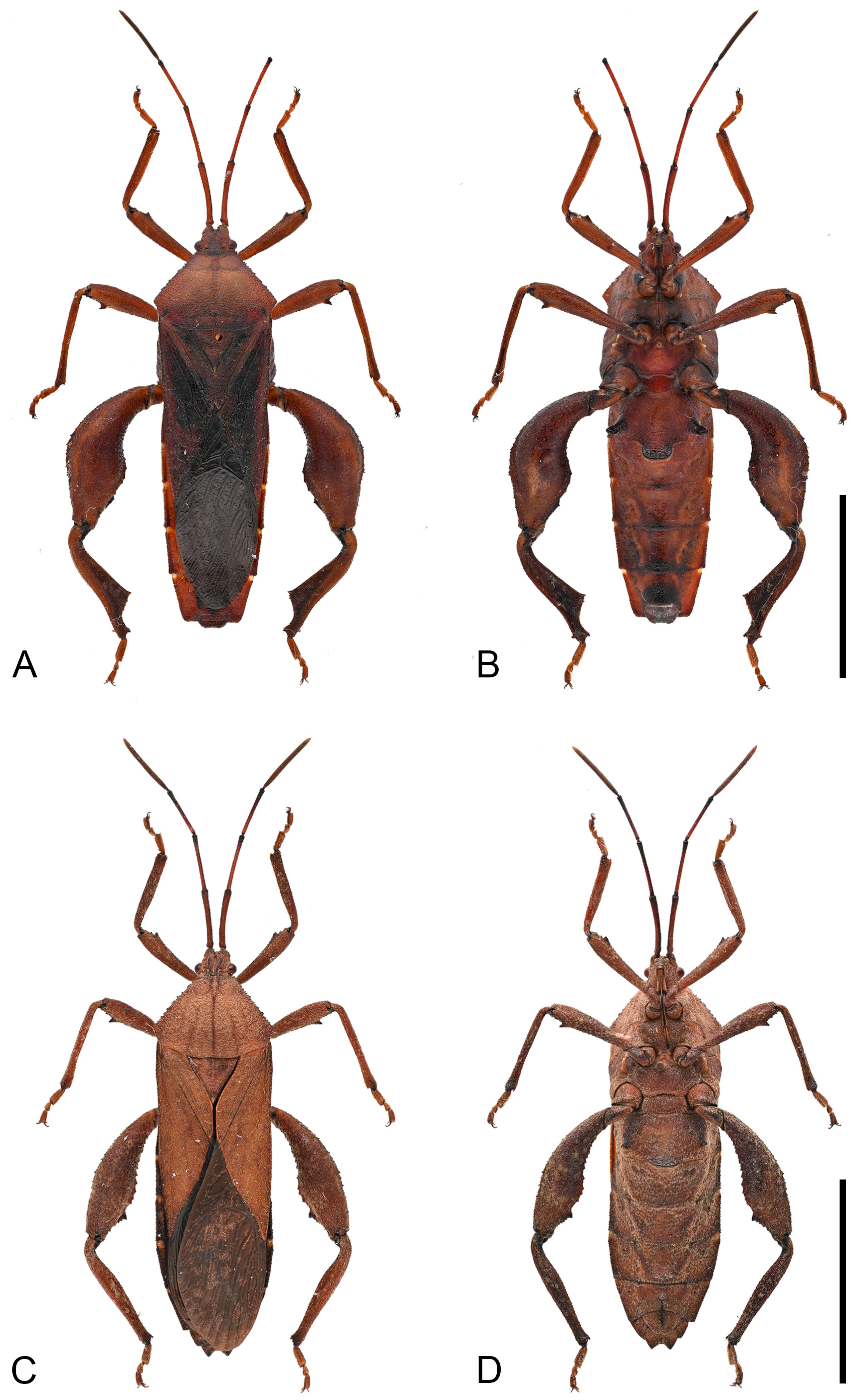
Figure 3.
Habitus of Mictis arcuata sp. n. (A–C), male, holotype (Lingui, Guangxi, China); (D–F), female, paratype (Yen Bai, Vietnam); (A,D), dorsal; (B,E), lateral; (C,F), ventral. Scales in 10 mm.
Figure 3.
Habitus of Mictis arcuata sp. n. (A–C), male, holotype (Lingui, Guangxi, China); (D–F), female, paratype (Yen Bai, Vietnam); (A,D), dorsal; (B,E), lateral; (C,F), ventral. Scales in 10 mm.
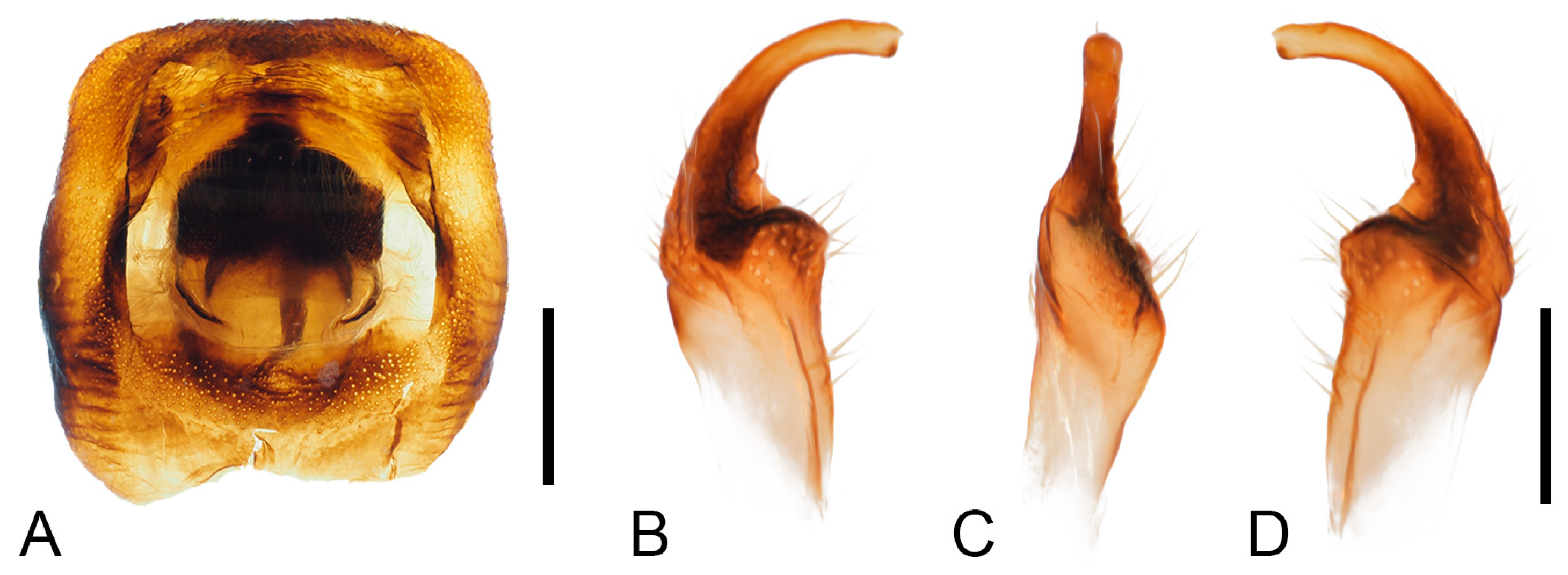
Male genitalia. Male genitalia capsule opening nearly square, with small golden setae; ventral rim broadly and shallowly depressed; cuplike sclerite small, broadly rounded. Parameres small, base stout, apical 1/3 evenly curved, with small, narrow, curved tridentate tip; central base to apical part with several large setae; external surface of base with deep, wide, transverse grooves (
Figure 5).
Measurements [in mm, ♂(n = 4)/♀(n = 3)]. BL: 28.2–30.6/28.9–31.7; HL: 1.9–2.2/1.8–2.4; HW: 3.1–3.4/3.2–3.3; IS: 1.1–1.3/1.1–1.2; AS I: 6.4–7.2/6.3–7.3; AS II: 5.5–6.1/4.8–6.9; AS III: 4.5–5.2/4.3–5.3; AS IV: 7.0–7.4/7.0–7.3; PL: 6.4–7.7/5.9–7.7; PW: 12.9–14.1/12.0–14.9; SL: 3.5–4.5/4.0–4.6; SW: 3.8–4.2/4.4–4.5.
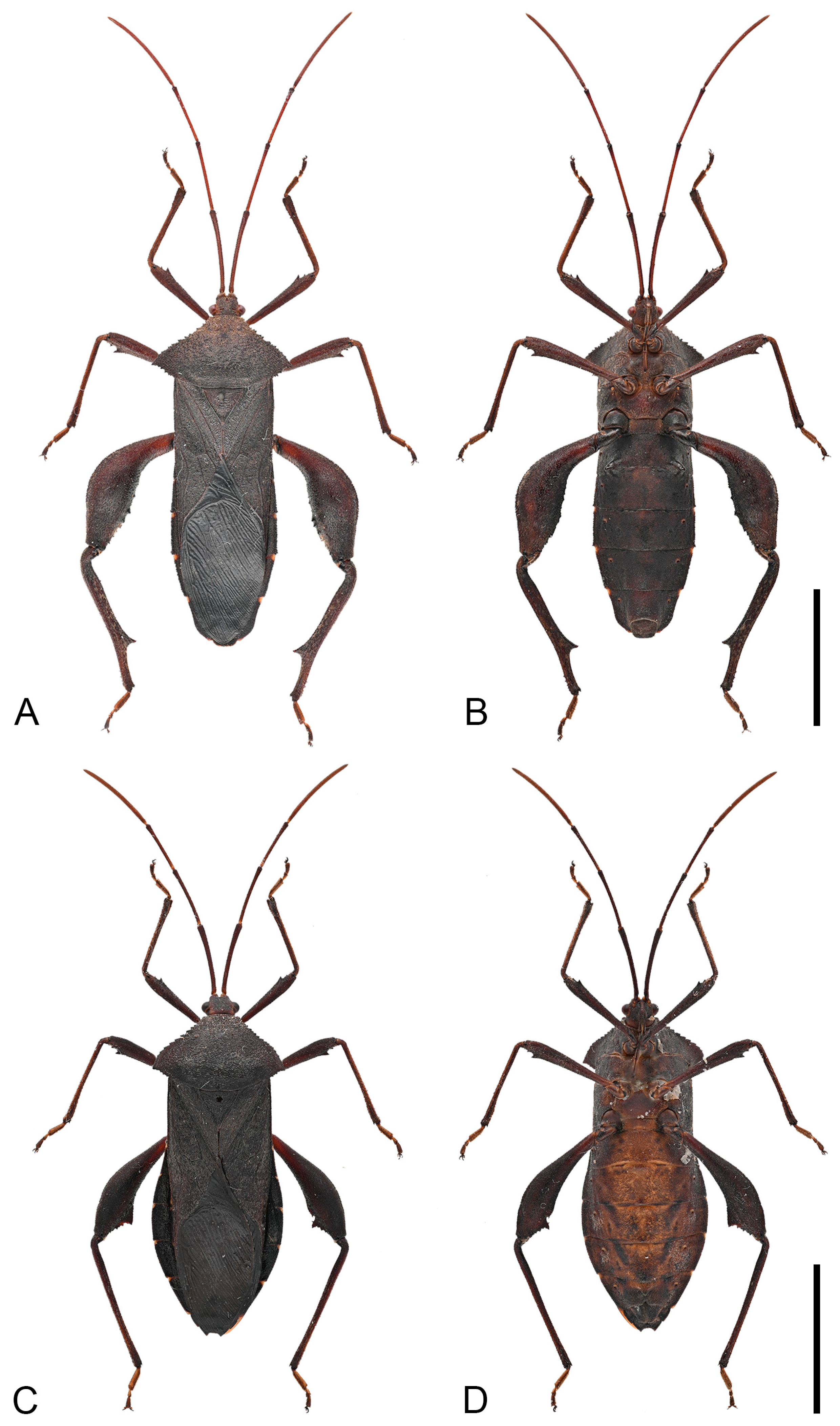
Figure 4.
Detailed habitus of Mictis arcuata sp. n. (A–C), male, holotype (Lingui, Guangxi, China); (D,E), male, paratype (Weixi, Yunnan, China), black-tibia type (with dark-brown tibiae); (A), abdominal segments III and IV, lateral; (B), abdominal segments III–IV, ventral; (C), pronotum; (D), dorsal; (E), ventral. Scales in 10 mm.
Figure 4.
Detailed habitus of Mictis arcuata sp. n. (A–C), male, holotype (Lingui, Guangxi, China); (D,E), male, paratype (Weixi, Yunnan, China), black-tibia type (with dark-brown tibiae); (A), abdominal segments III and IV, lateral; (B), abdominal segments III–IV, ventral; (C), pronotum; (D), dorsal; (E), ventral. Scales in 10 mm.
Figure 5.
Male genitalia of Mictis arcuata sp. n., male, holotype (Lingui, Guangxi, China). (A), genital capsule, dorsal; (B–D), right paramere in their different aspects, photos by Hanqiang Wang. (A), scale in 1 mm; (B–D), scale in 0.5 mm.
Figure 5.
Male genitalia of Mictis arcuata sp. n., male, holotype (Lingui, Guangxi, China). (A), genital capsule, dorsal; (B–D), right paramere in their different aspects, photos by Hanqiang Wang. (A), scale in 1 mm; (B–D), scale in 0.5 mm.
Etymology. The specific epithet is the Latin adjective arcuatus (-a, -um) referring to the arcuate anterolateral borders of the pronotum.
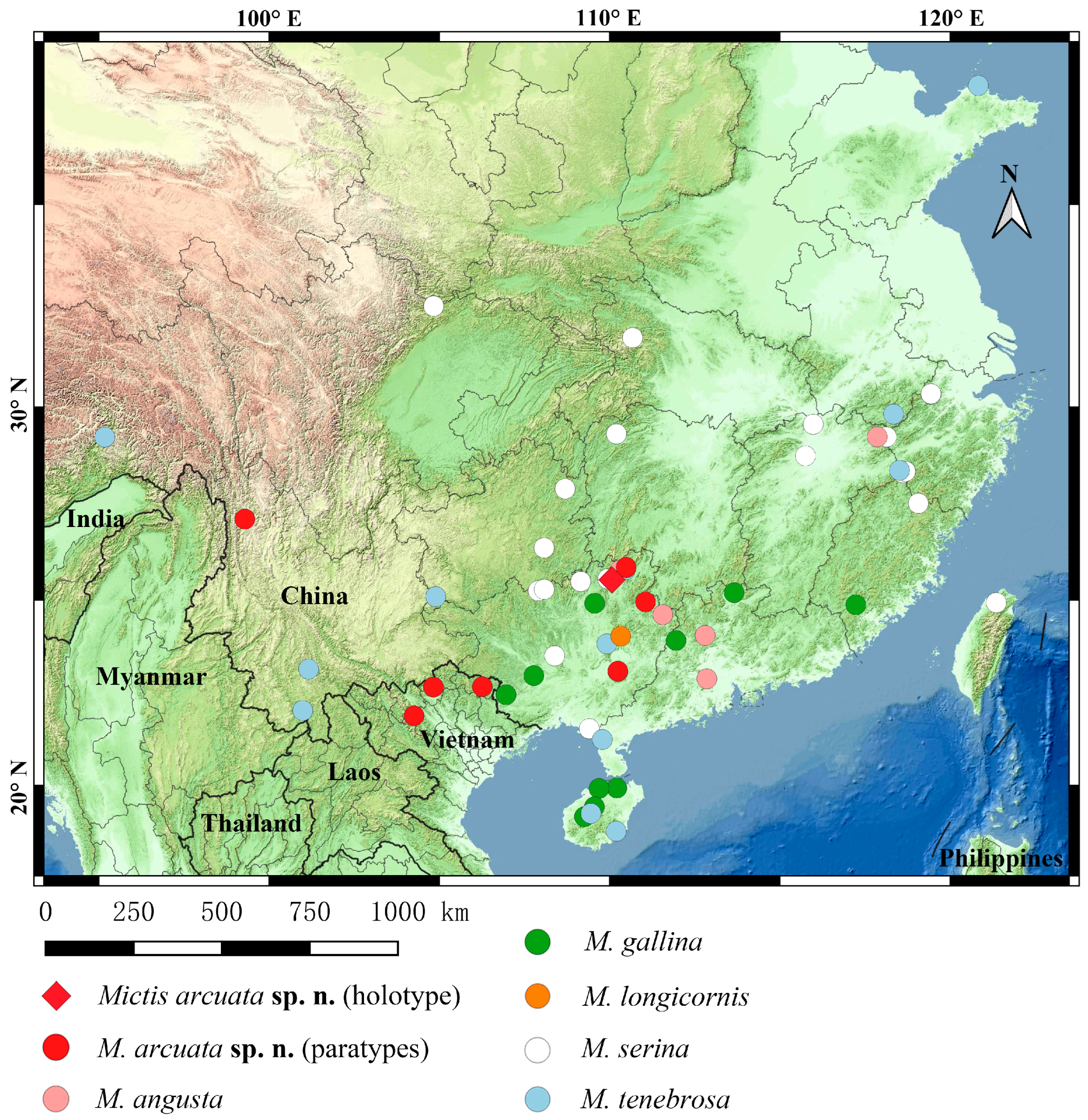
Distribution (Figure 2). China: Guangxi (Guilin, Guiping), Hunan (Jiangyong), Yunnan (Weixi); Vietnam: (Cao Bang, Yen Bai).
Host plant. Lauraceae.
Remarks. Examination of specimens revealed chromatic variation in tibiae: some individuals exhibit pale orange tibiae with dark-brown base, while others possess entirely dark-brown tibiae, occurring sympatrically (
Figure 13A,B). Morphological studies demonstrated no discernible differences beyond tibial coloration, including male genital structures (
Figure 3 and
Figure 4). Combined with the result of the molecular study, given their high morphological consistency and minimal genetic divergence, we propose these variants as conspecific.
Mictis angusta Hsiao, 1965
Chinese common name: 狭侎缘蝽
Mictis angusta Hsiao, 1965: 423, 432. HT: ♂, China, Jiangxi, Wuyuan; NKUM.
Type material. Holotype. ♂, China, Jiangxi Province, Wuyuan County, VII-1934, Yu tse Hong leg. (NKUM).
Additional material examined. 1♂, China, Guangdong Province, Foshan City, Gaoming District, Hecheng Subdistrict, 22.8983° N, 112.8569° E, alt. 12 m, VII-2023, Hansheng Qu leg. (CHX); 1♀, China, Guangdong Province, Qingyuan City, Qingxin District, Jintan Town, 24.0594° N, 112.8090° E, alt. 66 m, 20-IV-2024, Jiancheng Lei leg. (CHX); 1♀, same locality as above (Qingxin District, Jintan Town), 7-V-2025, Jiancheng Lei leg. (CHX); 2♂1♀, China, Guangxi Zhuang Autonomous Region, Guilin City, Xing’an County, Yong’an Village, 25.7949° N, 110.3056° E, alt. 388 m, 10-VII-2009, Jianhua Huang leg. (SHEM); 1♀, China, Guangxi Zhuang Autonomous Region, Hezhou City, Pingui District, Gupo Mountain, 24.6145° N, 111.5642° E, alt. 676 m, VII-2011, intern students of Guangxi Normal University leg. (SHEM).
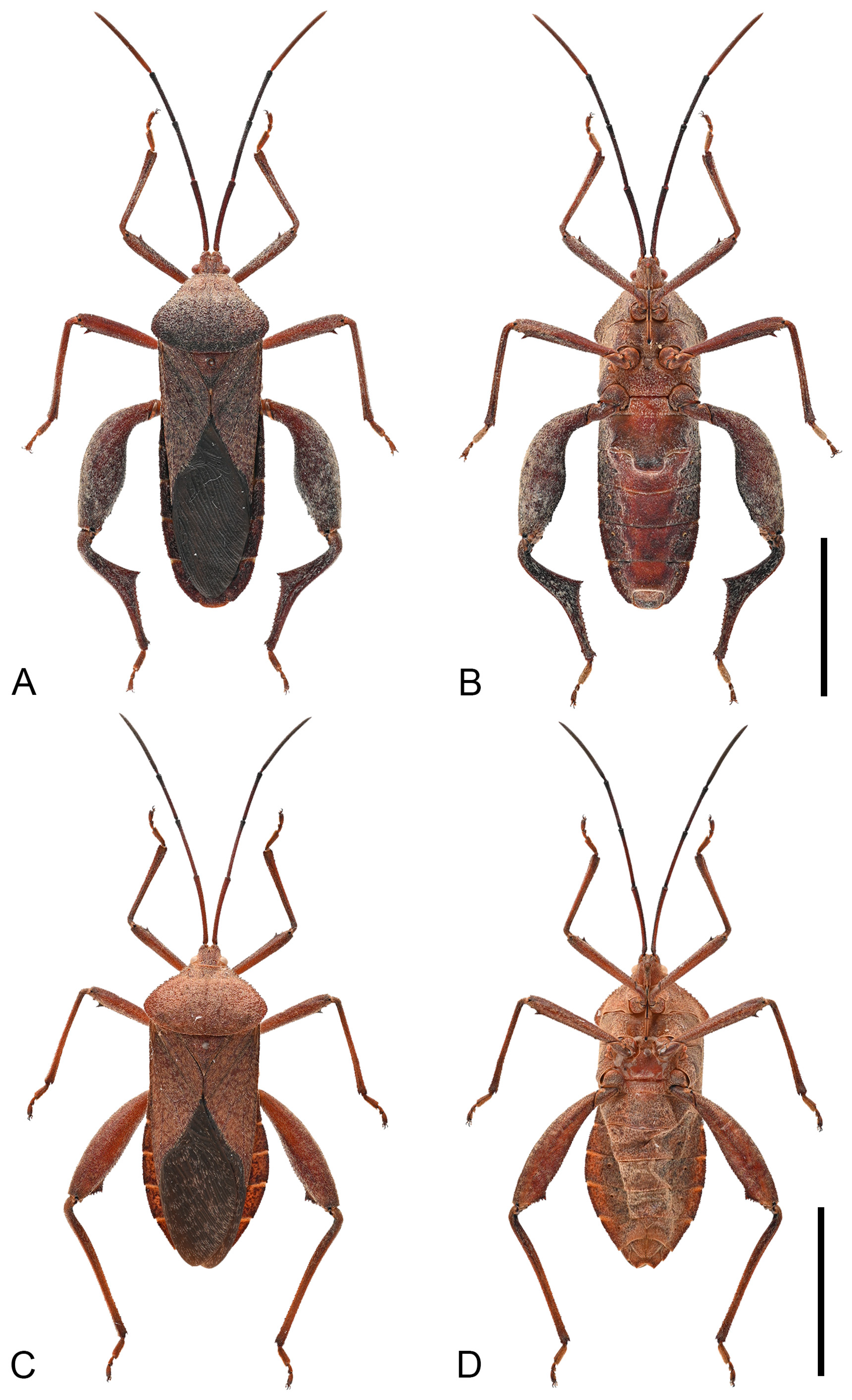
Diagnosis. Mictis angusta can be diagnosed from all other species by the following morphological characters: (1) body size small (19.3–22.2 mm), narrow; (2) chestnut-brown, integument with yellowish-brown setae; (3) humeral angles barely extended, obtuse; (4) anterolateral borders obliquely straight and slightly serrate; (5) male hind tibia with a large leaf-like spine at posterior half; (6) male with common boundary of abdominal segments III and IV produced into a large median tubercle; (7) female abdominal segment III with a pair of small ventrolateral tubercles.
Measurements [in mm, ♂(n = 4)/♀(n = 7)]. BL: 19.3–22.2/19.4–21.4; HL: 1.2–1.5/1.2–1.4; HW: 2.1–2.6/2.1–2.3; IS: 0.8–0.9/0.8–0.9; AS I: 3.0–3.8/2.7–3.3; AS II: 2.7–3.3/2.5–3.2; AS III: 2.3–2.9/2.2–2.5; AS IV: 2.9–3.7/2.5–3.4; PL: 3.9–5.1/4.6–4.9; PW: 5.6–7.0/6.0–6.9; SL: 2.0–2.4/2.1–2.3; SW: 2.3–2.6/2.3–2.7.
Distribution (Figure 2). China: Fujian (Shaowu) [
23], Guangdong (Foshan, Qingyuan), Guangxi (Guilin, Hezhou), Jiangxi (Wuyuan), Taiwan [
24].
Remarks. This species was originally described in 1965 based on a single male specimen, with no female records reported until now [
25]. This study documents the female specimens of this species for the first time, revealing several distinctive morphological characteristics in females. Notably, females exhibit a small median tubercle at common boundary of abdominal segments III and IV, along with a pair of small ventrolateral tubercles on abdominal segment III (
Figure 6C). These features contrast with most
Mictis species, where females typically have smooth ventral abdominal surfaces without tubercles.
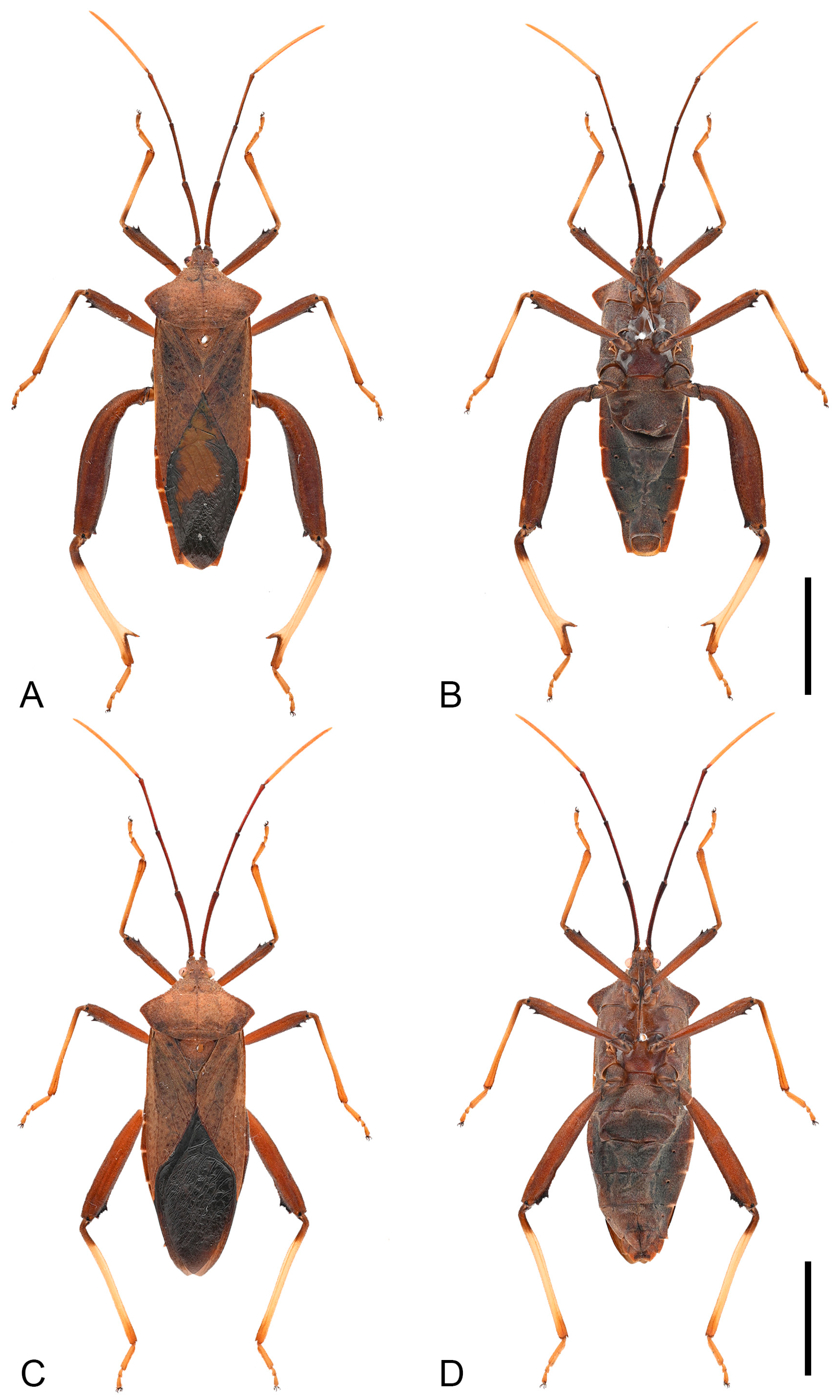
Figure 6.
Habitus of Mictis angusta. (A,B), male (Gaoming, Guangdong, China); (C,D), female, (Qingxin, Guangdong, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 6.
Habitus of Mictis angusta. (A,B), male (Gaoming, Guangdong, China); (C,D), female, (Qingxin, Guangdong, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Mictis gallina Dallas, 1852
Chinese common name: 锐肩侎缘蝽.
Mictis gallina Dallas, 1852: 403. Syntypes: Bangladesh, Sylhet (as Silhet); BMNH.
Type material. Lectotype (here designated). ♂, Indian Subcontinent, Bangladesh, Sylhet (as Silhet), 23.5000° N, 91.6667° E (BMNH 884212). Paralectotype (here designated). ♀, same data as lectotype (BMNH 884213).
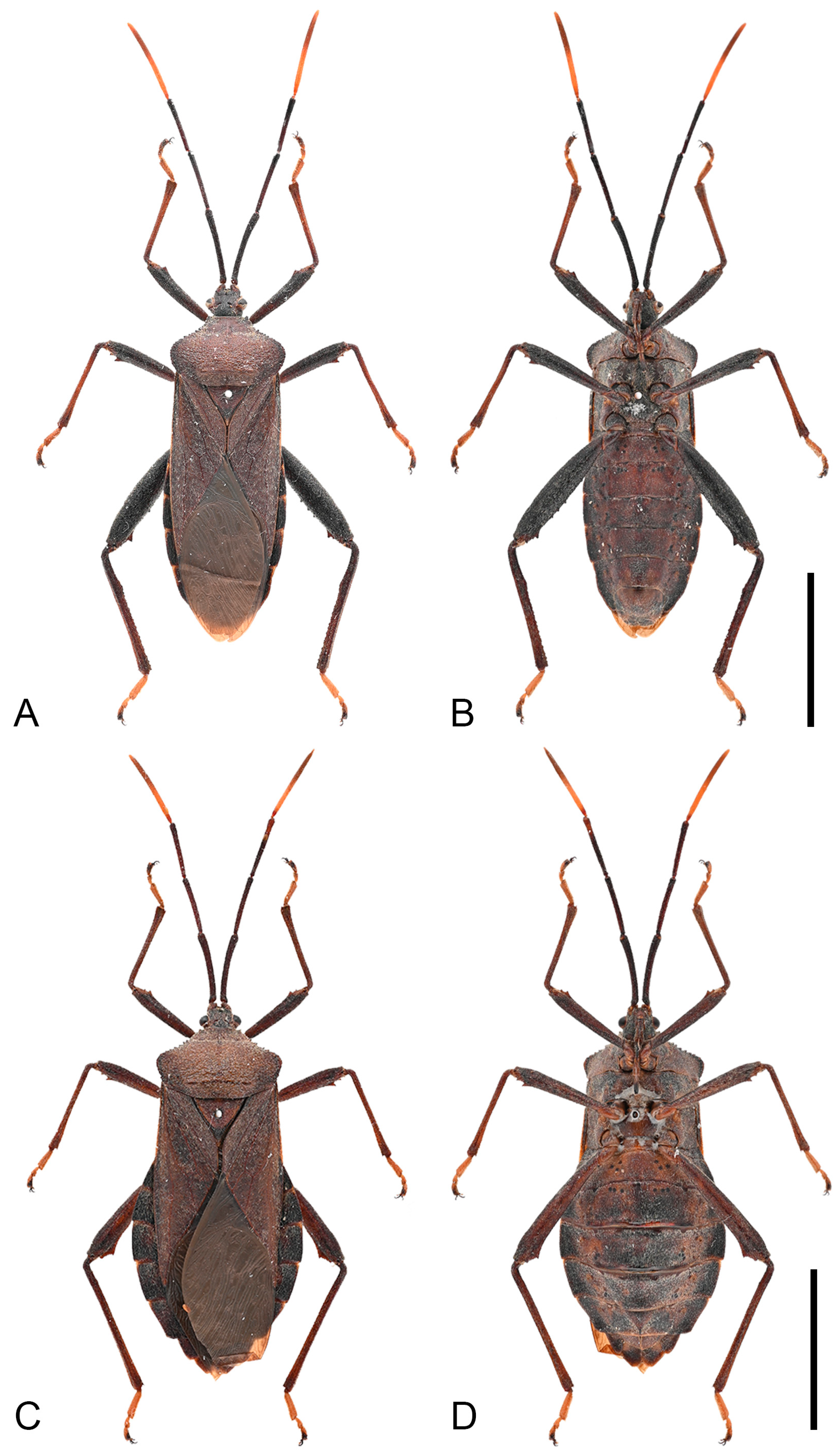
Figure 7.
Habitus of Mictis gallina. (A,B), male (Xiuying, Hainan, China); (C,D), female, (Rongan, Guangxi, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 7.
Habitus of Mictis gallina. (A,B), male (Xiuying, Hainan, China); (C,D), female, (Rongan, Guangxi, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Additional material examined. 2♀, China, Fujian Province, Zhangzhou City, Nanjing County, Hexi Town, 24.8763° N, 117.2321° E, alt. 220 m, 12-V-1962, Yongcheng Lin leg. (SHEM); 1♂, China, Guangdong Province, Shaoguan City, Renhua County, Jimageng Village, 25.1969° N, 113.6591° E, alt. 505 m, 11-V-2025, Han Wan leg. (CHX); 1♂, China, Guangdong Province, Zhaoqing City, Fengkai County, Yidou Mountain, 23.9269° N, 111.9531° E, alt. 537 m, 22-V-2023, Hengjing Liang leg. (CZZ); 1♂1♀, China, Guangxi Zhuang Autonomous Region, Liuzhou City, Rongan County, Qiaoban Village, 24.9172° N, 109.5622° E, alt. 456 m, 20-V-2023, Changlai Luo leg. (CHX); 1♂1♀, China, Guangxi Zhuang Autonomous Region, Chongzuo City, Longzhou County, Nonggang, 22.4679° N, 106.9608° E, alt. 261 m, 18–23-VII-1995, Xianwei Liu, Weinian Zhang & Xingbao Jin leg. (SHEM); 4♂4♀, same locality as above (Longzhou County, Nonggang), 11–15-VII-2013, Weibing Zhu & Haiguang Zhang leg. (SHEM); 1♂1♀, China, Guangxi Zhuang Autonomous Region, Nanning City, Long’an County, Gutan Village, 22.9830° N, 107.7772° E, alt. 99 m, 17-X-2024, Yunneng Fan leg. (CHX); 2♀, China, Hainan Province, Baisha Li Autonomous County, Bawangling National Nature Reserve, 19.1346° N, 109.2619° E, alt. 780 m, 24-IV-2017, Xianwei Liu leg. (SHEM); 1♀, China, Hainan Province, Danzhou City, Nanfeng Town, 7-VII-1932, F. K. To leg. (NKUM); 1♂, China, Hainan Province, Haikou City, Xiuying District, Shishan Town, Leiqiong Haikou Volcanic Geopark, 19.9254° N, 110.2140° E, alt. 191 m, 2-VII-2022, Michael Zelun Lee leg. (CHX); 1♂, China, Hainan Province, Lingao City, 23–25-V-1932, F. K. To leg. (NKUM).
Diagnosis. Mictis gallina can be diagnosed from all other species by the following morphological characters: (1) body size medium (21.3–26.6 mm); (2) reddish-brown to dark-brown; (3) humeral angles extended, pointed, with serrate margins; (4) anterolateral borders obliquely straight and distinct serrate; (5) male hind tibia with a large spine at posterior half; (6) male hind femora serrate along ventral margin; (7) male without median abdominal tubercle, only ventrolateral tubercles present.
Measurements [in mm, ♂(n = 3)/♀(n = 4)]. BL: 22.4–26.6/21.3–23.2; HL: 1.3–1.7/1.3–1.5; HW: 2.5–2.8/2.5–2.7; IS: 1.0–1.1/0.9–1.0; AS I: 5.3–6.2/4.9–5.2; AS II: 4.2–5.2/4.1–4.6; AS III: 3.6–4.4/3.2–4.0; AS IV: 5.4–7.1/5.5–6.2; PL: 5.4–6.9/5.3–5.8; PW: 8.1–10.5/7.8–8.7; SL: 2.2–2.6/2.3–2.8; SW: 2.8–3.2/3.1–3.5.
Distribution (Figure 2). China: Fujian (Zhangzhou), Guangdong (Meizhou, Zhaoqing) [
23], Guangxi (Liuzhou, Chongzuo, Nanning), Hainan (Baisha, Danzhou, Haikou, Lingao), Hong Kong [
26], Jiangxi [
23], Yunnan (Hekou) [
23]; Bangladesh; India [
2]; Myanmar [
2].
Mictis longicornis Westwood, 1842
Chinese common name: 长角侎缘蝽
Myctis longicornis Westwood, 1842: 4, 11–12. Syntypes: Jawa; UMO. Mictis longicornis, Stål, 1873: 45 (cataloged).
Mictis conjunctus Herrich-Schäffer, 1850: 247 (Syn. Stål, 1873: 45). Syntype: without locality; lost.
Mictis tuberosa Hsiao, 1965: 423, 432. HT: ♂, China, Guangxi, Yaoshan; NKUM. Hsiao, 1977: 212 (listed); O’Shea & Schaefer, 1980: 231 (listed); Dolling, 2006: 93 (listed). Syn. n.
Type material. Mictis longicornis Westwood, 1842. Lectotype (here designated). ♂, Jawa (UMO HEMI 0299.1). Paralectotype (here designated). ♀, same data as lectotype (UMO HEMI 0299.2).
Mictis tuberosa Hsiao, 1965. Holotype. 1♂, China, Guangxi Zhuang Autonomous Region, Yaoshan County (NKUM).
Additional material examined. 1♂1♀, Malaysia, Sabah, Ranau District, Poring Hot Spring, 6.0568° N, 116.7055° E, alt. 553 m, 21-II-2024, Haoran Gao & Zixu Yin leg. (CHX).
Diagnosis. Mictis longicornis can be diagnosed from all other species by the following morphological characters: (1) body size medium; (2) reddish-brown, integument with yellowish-brown setae; (3) humeral angles extended, pointed, with serrate margins; (4) anterolateral borders obliquely straight with distinct serration; (5) male hind tibia with a large spine at posterior half; (6) male hind femora serrate along ventral margin; (7) male with common boundary of abdominal segments III and IV produced into a large thumb-like median tubercle.
Distribution (Figure 2). China: Guangxi (Yaoshan); Indonesia [
2]; Malaysia [
2], Philippines [
2].
Figure 8.
Type specimens of Mictis species. (A–D) Mictis tuberosa, male, holotype (Yaoshan, Guangxi, China); (E,F) Mictis longicornis, male, lectotype (Jawa); (A,E) dorsal; (B) lateral; (C) ventral; (D,F) labels. The Chinese text in (D) indicates ‘holotype, identified by Tsai-Yu Hsiao’. (A–C) scale in 10 mm; (E) scale in 1 mm. (A–D) photos by Hanqiang Wang, ©NKUM; (E,F) photos by Laurence Livermore, from Coreoidea Species File Version 5.0/5.0, published under CC BY-SA 4.0 International Licence, ©UMO.
Figure 8.
Type specimens of Mictis species. (A–D) Mictis tuberosa, male, holotype (Yaoshan, Guangxi, China); (E,F) Mictis longicornis, male, lectotype (Jawa); (A,E) dorsal; (B) lateral; (C) ventral; (D,F) labels. The Chinese text in (D) indicates ‘holotype, identified by Tsai-Yu Hsiao’. (A–C) scale in 10 mm; (E) scale in 1 mm. (A–D) photos by Hanqiang Wang, ©NKUM; (E,F) photos by Laurence Livermore, from Coreoidea Species File Version 5.0/5.0, published under CC BY-SA 4.0 International Licence, ©UMO.
Remarks. Original description of
Mictis tuberosa compared it only with
M. gallina, with no reference to
M. longicornis [
25]. Our examination of the holotypes of
M. tuberosa and
M. longicornis confirms their morphological identity: diagnostic traits such as hind femoral and median abdominal tubercles are entirely congruent (
Figure 8). However,
M. longicornis is restricted to the Malay Peninsula and Southeast Asian islands, while
M. tuberosa was purportedly collected in northern Indochina. Notably, no subsequent records of
M. tuberosa have been confirmed from this region since its original description in 1965, raising questions about the accuracy of its reported collection site. This discrepancy necessitates verification through targeted fieldwork in Indochina. Given currently no difference in potential specific importance, we formally relegate
Mictis tuberosa to a subjective junior synonym of
Mictis longicornis.
Mictis serina Dallas, 1852
Chinese common name: 黄胫侎缘蝽
Mictis serina Dallas, 1852: 403. Syntype: ♀, China; BMNH.
Mictis serina fuscipes Hsiao, 1963: 311, 337 (as color variety of Mictis serina; subspecies upgraded to species by Hsiao, 1964: 9, 17); O’Shea & Schaefer, 1980: 231 (listed). HT: ♂, Sichuan, Mt Emei; IZACS. Mictis fuscipes, Hsiao, 1977: 212 (listed); Dolling, 2006: 92, 93 (listed). Syn. n.
Type material. Mictis serina Dallas, 1852. Lectotype (here designated). ♀, China (BMNH 884224).
Mictis fuscipes Hsiao, 1963. Holotype. ♂, China, Sichuan, Mt Emei (IZCAS 222018).
Figure 9.
Holotype of Mictis fuscipes. (A–C), male, holotype (Emei, Sichuan, China); (A), dorsal; (B), lateral; (C), labels, The non-English term in (C) indicates collection details: Mont. Emei, 6-VI-1955, Keren Huang & Gentao Jin. Scale in mm. Photos by Laurence Livermore, from Coreoidea Species File Version 5.0/5.0, published under CC BY-SA 4.0 International Licence, ©IZCAS.
Figure 9.
Holotype of Mictis fuscipes. (A–C), male, holotype (Emei, Sichuan, China); (A), dorsal; (B), lateral; (C), labels, The non-English term in (C) indicates collection details: Mont. Emei, 6-VI-1955, Keren Huang & Gentao Jin. Scale in mm. Photos by Laurence Livermore, from Coreoidea Species File Version 5.0/5.0, published under CC BY-SA 4.0 International Licence, ©IZCAS.
Additional material examined. 1♂1♀, China, Guangxi Zhuang Autonomous Region, Beihai City, Tieshangang District, Longmen Village, 21.5478° N, 109.4026° E, alt. 27 m, 15-VI-2023, Jianxie Liu leg. (CHX); 2♂, China, Guangxi Zhuang Autonomous Region, Chongzuo City, Longzhou County, Nonggang, 22.4679° N, 106.9608° E, alt. 261 m, 18–23-VII-1995, Xianwei Liu, Weinian Zhang & Xingbao Jin leg. (SHEM); 5♂1♀, same locality as above (Longzhou County, Nonggang), 13-VII-2013, Weibing Zhu & Haiguang Zhang leg. (SHEM); 3♂, China, Guangxi Zhuang Autonomous Region, Guiping City, Junfeng Village, 23.0931° N, 110.2453° E, alt. 133 m, VII-2024, De Huang leg. (CHX); 1♀, China, Guangxi Zhuang Autonomous Region, Guilin City, Lingui District, Anjiangping, 25.5614° N, 109.9455° E, alt. 1314 m, 25-V-2023, Feiyu Duan leg. (CHX); 2♀, China, Guangxi Zhuang Autonomous Region, Guilin City, Xing’an County, Gaozhai Village, 25.8555° N, 110.4852° E, alt. 492 m, 13-VII-2011, Hanyong Liu leg. (SHEM); 1♂, China, Guangxi Zhuang Autonomous Region, Hezhou City, Pingui District, Gupo Mountain, 24.6145° N, 111.5642° E, alt. 676 m, 4-VII-2009, Jianhua Huang leg. (SHEM); 1♂1♀, China, Guangxi Zhuang Autonomous Region, Liuzhou City, Rongshui Miao Autonomous County, Hongshui Township, Liangshuang Village, 25.4928° N, 109.1519° E, alt. 570 m, 7-VI-2023, local villagers leg. (CHX); 1♀, China, Guangxi Zhuang Autonomous Region, Nanning City, Wuming District, Damingshan Mountain, 23.5145° N, 108.3879° E, alt. 850 m, VII–IX-2012, collector unknown (SHEM); 2♂, China, Guizhou Province, Leishan County, Wukai Village, 26.3784° N, 108.0780° E, alt. 858 m, 7-VII-1988, Zuyao Liu leg. (SHEM); 3♀, China, Guizhou Province, Libo County, Maolan National Nature Reserve, Weng’ang Township, 25.2474° N, 107.9053° E, alt. 851 m, 24–26-VII-2015, Weibing Zhu leg. (SHEM); 3♂2♀, China, Guizhou Province, Tongren City, Jiangkou County, Fanjing Mountain, 27.9136° N, 108.6941° E, alt. 1 701 m, 12–15-VII-1988, Zuyao Liu leg. (SHEM); 1♂1♀, China, Hubei Province, Shennongjia Forestry District, Yangri Town, 31.7446° N, 110.6760° E, alt. 986 m, 14–15-VII-1983, Gentao Jin & Zuyao Liu leg. (SHEM); 1♂1♀, China, Hunan Province, Zhangjiajie City, Zhushitou Forest Farm, 29.3214° N, 110.2039° E, alt. 995 m, 13-VI-1988, Zuyao Liu leg. (SHEM); 1♀, China, Jiangxi Province, Jiujiang City, Lushan District, Guling Town, 17-VIII-1935, O. Piel leg. (NKUM); 1♂1♀, China, Jiangxi Province, Nanchang City, Xinjian District, Meiling National Forest Park, Yueliangwan, 28.7601° N, 115.7529° E, alt. 146 m, 9-V-2023, Tianxiao Feng leg. (CZZ); 1♂, China, Taiwan Province, New Taipei City, Sanxia District, 24.9318° N, 121.3575° E, alt. 92 m, 23-V-2025, Zhenfeng Jian leg. (CHX); 1♂, China, Zhejiang Province, Hangzhou City, Lin’an District, Tianmu Mountain, 25-VII-1936, collector unknown (NKUM); 1♂, same locality as above (Lin’an District, Tianmu Mountain), 3-VIII-1962, Zhizi Chen leg. (SHEM); 1♀, same locality as above (Lin’an District, Tianmu Mountain), 14-VIII-1962, Gentao Jin leg. (SHEM); 1♂, China, Zhejiang Province, Lishui City, Qingyuan County, Wuligen, 27.5360° N, 119.0685° E, alt. 643 m, 12–20-VIII-1996, Weinian Zhang & Xingbao Jin leg. (SHEM); 1♂, China, Zhejiang Province, Jiangshan City, Shuangxikou Town, Laofoyan, 28.3619° N, 118.6889° E, alt. 643 m, 4-VII-2017, Hanqiang Wang leg. (SHEM); 1♂, China, Zhejiang Province, Jiangshan City, Zhou Village, 28.3264° N, 118.6175° E, alt. 643 m, 11-VIII-2016, Damin Zhang, Weibing Zhu & Xianwei Liu leg. (SHEM); 1♀, China, Zhejiang Province, Quzhou City, Kaihua County, Gutian Mountain, 29.2456° N, 118.1345° E, alt. 502 m, 15–17-VII-2012, Hanqiang Wang, Li Dai & Xianwei Liu leg. (SHEM); 1♂, China, Sichuan, Guangyuan City, Qingcuan County, Tangjiahe National Nature Reserve, Gongnong Village, 32.5282° N, 104.8342° E, alt. 1 112 m, 25-VII-2024, Zixu Yin leg. (EANU).
Diagnosis. Mictis serina can be diagnosed from all other species by the following morphological characters: (1) body size large (19.8–28.1 mm); (2) reddish-brown to dark-brown, integument with yellowish-brown setae; (3) pronotum slightly expanded beyond base of corium; (4) anterolateral borders curved, smooth, barely serrate; (5) humeral angles obtuse; (6) tibiae pale orange with dark-brown base to entirely dark-brown; (7) male hind tibia with a large spine at posterior half; (8) male with common boundary of abdominal segments III and IV produced into a large thumb-like median tubercle.
Measurements [in mm, ♂(n = 8)/♀(n = 8)]. BL: 19.8–27.4/23.4–28.1; HL: 1.3–2.1/1.3–1.8; HW: 2.5–2.8/2.6–2.8; IS: 0.8–1.0/0.9–1.0; AS I: 4.2–5.9/4.5–5.4; AS II: 3.9–5.0/3.4–5.0; AS III: 3.4–4.5/3.3–4.2; AS IV: 5.5–7.1/5.7–6.7; PL: 4.1–5.9/5.3–6.1; PW: 7.0–9.6/8.9–11.1; SL: 2.4–3.2/2.9–3.8; SW: 2.6–4.0/3.3–4.0.
Distribution (Figure 2). China: Fujian (Fuzhou, Shaowu) [
23], Guangdong (Meizhou, Qingyuan, Zhaoqing) [
23], Guangxi (Beihai, Chongzuo, Guiping, Guilin, Hezhou, Liuzhou, Nanning), Guizhou (Leishan, Libo, Tongren), Hong Kong [
26], Hubei (Shennongjia), Hunan (Zhangjiajie), Jiangxi (Jiujiang, Wuyuan) [
23], Sichuan (Emeishan, Guangyuan, Yaan) [
23], Taiwan, Zhejiang (Hangzhou, Lishui, Jiangshan, Quzhou).
Remarks. Hsiao, in 1963, established the subspecies
Mictis serina fuscipes based on comparison with
Mictis serina, distinguishing it by its slightly darker body coloration, uniformly black tibiae, and orange tarsi (
Figure 9) [
27]. In 1964, he elevated this subspecies to species rank as
Mictis fuscipes, primarily based on tibial coloration [
28]. However, after examining a large number of specimens, we found that tibial color exhibits continuous variation. Individuals with different tibial coloration can be collected from the same locality, and apart from tibial color, all other morphological features are identical (
Figure 10,
Figure 11 and
Figure 13F,G). These findings suggest that body and tibial coloration are more likely to represent individual variation rather than interspecific differences. According to the results of DNA barcode research, the genetic evidence further supports the interpretation that coloration differences reflect intraspecific variation. Based on both morphological and molecular evidence, we formally relegate
Mictis fuscipes to a subjective junior synonym of
Mictis serina.
Figure 10.
Habitus of Mictis serina. (A,B), male (Tieshangang, Guangxi, China); (C,D), female, (Tieshangang, Guangxi, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 10.
Habitus of Mictis serina. (A,B), male (Tieshangang, Guangxi, China); (C,D), female, (Tieshangang, Guangxi, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 11.
Habitus of Mictis serina, black-tibia type (with dark-brown tibiae). (A,B), male (Tieshangang, Guangxi, China); (C,D), female, (Tieshangang, Guangxi, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 11.
Habitus of Mictis serina, black-tibia type (with dark-brown tibiae). (A,B), male (Tieshangang, Guangxi, China); (C,D), female, (Tieshangang, Guangxi, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Mictis tenebrosa Fabricius, 1787
Chinese common name: 曲胫侎缘蝽
Cimex tenebrosus Fabricius, 1787: 288. Syntypes: E India; BMNH, ZMUC. Coreus tenebrosus, Fallén, 1814: 7 (generic placement). Myctis tenebrosus, Billberg, 1820: 69 (generic placement). Anisoscelis tenebrosus, Latreille, 1829: 197 (generic placement); Mictis tenebrosus, Amyot & Serville, 1843: 190 (generic placement).
Coreus (Cerbus) umbilicatus Burmeister, 1834: 296 (Syn. Stål, 1873: 45). Syntype: multiple unsexed adults; ZMHB. Cerbus umbilicatus, Burmeister, 1835: 341 (generic placement). Mictis umbilicatus, Herrich-Schäffer, 1853:121, 128 (generic placement).
Mycis fasciatus Westwood, 1842: 11 (Syn. Stål, 1873: 47, suspected; Distant, 1901: 327). Syntypes: ♂, ♀, China; UMO. Mictis fasciatus, Dallas, 1852: 404 (cataloged). Mictis fasciata, Dohrn, 1859: 24 (cataloged).
Mictis nigricornis Dallas, 1852: 400 (Syn. Stål, 1873: 47, suspected; Distant, 1902: 344). Syntypes: ♀, Bangladesh, Silhet [=Sylhet]; BMNH.
Figure 12.
Habitus of Mictis tenebrosa. (A,B), male (Jinghong, Yunnan, China); (C,D), female, (Suixi, Guangdong, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 12.
Habitus of Mictis tenebrosa. (A,B), male (Jinghong, Yunnan, China); (C,D), female, (Suixi, Guangdong, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Type material. Lectotype (here designated). ♂, Indian Subcontinent, India, East India (ZMUC 00101285). Paralectotypes (here designated). 1 unsexed adult, same data as lectotype (ZMUC 00101286); unsexed adult more than 1, same data as lectotype (BMNH).
Additional material examined. 1♂1♀, China, Anhui Province, Huangshan City, Tunxi District, 12-VIII-1985, Zuyao Liu leg. (SHEM); 1♂1♀, China, Guangdong Province, Zhanjiang City, Suixi County, Gangmen Town, 21.2518° N, 109.7904° E, alt. 25 m, 30-V-2023, Dexi Kong leg. (CHX); 3♀, China, Guangxi Zhuang Autonomous Region, Guilin City, Xing’an County, Gaozhai Village, 25.8555° N, 110.4852° E, alt. 492 m, 13-VII-2011, Hanyong Liu leg. (SHEM); 2♂4♀, China, Guangxi Zhuang Autonomous Region, Guilin City, Xing’an County, Tongren Village, 25.7952° N, 110.4579° E, alt. 327 m, 2-VII-2006, Yousong Zhang & Zhongcheng Pan leg. (SHEM); 1♀, China, Guangxi Zhuang Autonomous Region, Hezhou City, Pingui District, Gupo Mountain, 24.6145° N, 111.5642° E, alt. 676 m, 6-VII-2009, Jianhua Huang leg. (SHEM); 2♂, China, Guangxi Zhuang Autonomous Region, Laibin City, Jinxiu Yao Autonomous County, Hualu Village, 25-IV-1982, Zuyao Liu & Xingbao Jin leg. (SHEM); 1♂, China, Guizhou Province, Xingyi City, 11-VIII-1958, Daoying Bi & Zhizi Chen leg. (SHEM); 1♂, China, Hainan Province, Baisha Li Autonomous County, 24-III-1959, Gentao Jin leg. (SHEM); 3♂, China, Hainan Province, Wanning City, Xinglong Town, 14-X-1957, Zhizi Chen leg. (SHEM); 1♂1♀, China, Shandong Province, Yantai City, Penglai District, 26-VII-1983, Daoying Bi leg. (SHEM); 1♂, China, Xizang Autonomous Region, Mêdog County, Beibeng Town, 28-VII-1977, Jianyi Wu leg. (SHEM); 1♂, China, Yunnan Province, Puer City, Mohei Town, 6-IV-1982, Gentao Jin leg. (SHEM); 1♂, China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Jinuo Mountain, Jinuo Ethnic Township, 22.0406° N, 100.9900° E, alt. 1 073 m, 24-III-2023, Haoran Gao leg. (CHX); 3♂2♀, China, Zhejiang Province, Quzhou City, Jiangshan City, Xianxialing, 11-V-1984, Zuyao Liu & Shensheng Zheng leg. (SHEM).
Diagnosis. Mictis tenebrosa can be diagnosed from all other species by the following morphological characters: (1) body size medium (16.7–22.7 mm); (2) chestnut-brown, integument densely covered with creamy-white setae; (3) humeral angles barely extended, obtuse; (4) anterolateral borders obliquely straight and slightly serrate; (5) male hind tibia with a large leaf-like spine at midpoint; (6) male with common boundary of abdominal segments III and IV produced into a large median tubercle.
Measurements [in mm, ♂(n = 9)/♀(n = 5)]. BL: 16.7–22.7/20.1–21.3; HL: 1.2–1.7/1.3–1.6; HW: 2.1–2.5/2.3–2.4; IS: 0.8–1.0/0.8–1.0; AS I: 2.7–4.9/3.5–4.4; AS II: 3.1–4.3/3.1–4.0; AS III: 2.4–3.8/2.6–3.6; AS IV: 3.6–5.3/4.0–5.1; PL: 4.1–5.8/5.1–5.6; PW: 5.9–8.2/7.6–8.1; SL: 2.2–3.0/2.3–2.8; SW: 2.4–3.3/2.6–3.2.
Distribution (Figure 2). China: Anhui (Huangshan), Fujian (Fuzhou, Jianou) [
23], Guangdong (Xinyi, Zhanjiang) [
23], Guangxi (Guilin, Hezhou, Laibin, Liuzhou, Yulin) [
23], Guizhou (Xingyi), Hainan (Baisha), Hong Kong [
26], Hunan (Xiangtan) [
23], Jiangxi (Guixi, Shangrao) [
23], Shandong (Yantai), Sichuan (Emeishan) [
23], Xizang (Mêdog), Yunnan (Jinghong, Puer), Zhejiang (Quzhou, Shaoxing, Zhoushan) [
23]; India [
2]; Indonesia [
2]; Malaysia [
2].
Figure 13.
Morphological features of Mictis spp., males. (A–H), left hind tibia; (I–N), line drawing of abdominal segments III–V, lateral; (A,I), M. arcuata sp. n.; (B), M. arcuata sp. n., black-tibia type (with dark-brown tibiae); (C,J), M. angusta; (D,K), M. gallina; (E,L), M. longicornis; (F,M), M. serina; (G), M. serina, black-tibia type; (H,N), M. tenebrosa. Scales in 10 mm.
Figure 13.
Morphological features of Mictis spp., males. (A–H), left hind tibia; (I–N), line drawing of abdominal segments III–V, lateral; (A,I), M. arcuata sp. n.; (B), M. arcuata sp. n., black-tibia type (with dark-brown tibiae); (C,J), M. angusta; (D,K), M. gallina; (E,L), M. longicornis; (F,M), M. serina; (G), M. serina, black-tibia type; (H,N), M. tenebrosa. Scales in 10 mm.
Key to the genus Mictis from China| 1. | Humeral angles distinctly extended, male hind tibial spine large and spine-like…….2 |
| - | Humeral angles barely extended, male hind tibial spine small, angulate......................5 |
| 2. | Humeral angles extended pointed, with serrate margins…….........................................3 |
| - | Humeral angles extended rounded…….............................................................................4 |
| 3. | Apex of antennal segment IV pale, male with common boundary of abdominal
segments III and IV produced into a large median tubercle.................Mictis longicornis |
| - | Antennal segment IV uniformly colored, male without median abdominal tubercle,
only ventrolateral tubercles present................................................................Mictis gallina |
| 4. | Pronotum extremely extended laterally, the lateral extension beyond the margins of
the corium base greater than head width; male with entire abdominal segment IV
produced into a large median tubercle...............................................Mictis arcuata sp. n. |
| - | Pronotum moderately extended laterally, the lateral extension beyond the margins
of the corium base less than head width; male with common boundary of abdominal
segments III and IV not produced into a large median tubercle..................Mictis serina |
| 5. | Body narrow, antennae about 3/5 of body length, male hind tibial spine inserted at
2/3 of tibia........................................................................................................Mictis angusta |
| - | Body moderately broad, antennae about 4/5 of body length, male hind tibial spine
inserted at midpoint of tibia........................................................................Mictis tenebrosa |
Ochrochira falloui Reuter, 1888, comb. n.
Chinese common name: 北京赭缘蝽
Mictis falloui Reuter, 1888: 65. Syntypes: Beijing (as Pekin); MNHN. Lethierry & Se-verin, 1894: 9 (cataloged); Oshanin, 1906: 177 (cataloged); Oshanin, 1912: 20 (cataloged); Kiritshenko, 1916: 48, 57–59 (cataloged; description); Hsiao, 1964 (noted); O’Shea & Schaefer, 1980: 231 (listed); Dolling, 2006: 92 (listed).
Type material. Lectotype (here designated). ♀, China, Beijing (as Pekin), 39.9000° N, 116.3830° E (MNHN-EH-EH22406).
Additional material examined. 1♂, China, Beijing, Changping District, Nankou Town, Huyu Natural Scenic Area, 40.2813° N, 116.1320° E, alt. 646 m, 1-VI-2023, Michael Zelun Lee leg. (CHX); 2♀, China, Beijing, Fangshan District, Puwa Township, 37.7838° N, 115.5374° E, alt. 1 180 m, 11-VI-2023, Michael Zelun Lee leg. (CHX); 1♀, China, Beijing, Mentougou District, Qingshui Town, Baihua Mountain, 39.8395° N, 115.5786° E, alt. 550 m, 23-VI-2024, Yan Liu leg. (CHX).
Figure 14.
Type specimen of
Ochrochira falloui comb. n. (
A–
D), female, lectotype (Beijing, China); (
A), dorsal; (
B), lateral; (
C), ventral; (
D), labels. Scales in 10 mm. Photos from [
29], published under CC BY-NC-ND 4.0 International Licence, ©MNHN.
Figure 14.
Type specimen of
Ochrochira falloui comb. n. (
A–
D), female, lectotype (Beijing, China); (
A), dorsal; (
B), lateral; (
C), ventral; (
D), labels. Scales in 10 mm. Photos from [
29], published under CC BY-NC-ND 4.0 International Licence, ©MNHN.
Diagnosis. Ochrochira falloui can be diagnosed from all other species by the following morphological characters: (1) body size medium (19.4–21.2 mm); (2) dark-brown, antennal segment IV yellowish-brown with a dark stripe near the distal end; (3) humeral angles barely extended, obtuse; (4) anterolateral borders slightly serrate; (5) female hind femora smooth, without tubercles; (6) male hind femora incrassate, with tubercles on anterior and posterior surfaces; (7) abdominal dorsum entirely black; (8) abdominal segments III with six black spots; (9) abdomen smooth, without tubercles.
Figure 15.
Habitus of Ochrochira falloui comb. n. (A,B), male (Changping, Beijing, China); (C,D), female, (Fangshan, Beijing, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Figure 15.
Habitus of Ochrochira falloui comb. n. (A,B), male (Changping, Beijing, China); (C,D), female, (Fangshan, Beijing, China); (A,C), dorsal; (B,D), ventral. Scales in 10 mm.
Measurements [in mm, ♂(n = 1)/♀(n = 1)]. BL: 19.4/21.2; HL: 1.9/1.9; HW: 2.5/2.6; IS: 0.7/0.7; AS I: 4.0/3.9; AS II: 3.7/3.5; AS III: 2.9/2.8; AS IV: 4.9/4.8; PL: 3.9/4.2; PW: 6.7/7.2; SL: 2.0/2.1; SW: 2.6/3.1.
Distribution. China: Beijing.
Remarks. The original description of this species was based on a single female specimen collected from Beijing, China, and was placed in the genus
Mictis (
Figure 14). Our study examined both male and female specimens collected from the type locality and compared them with the type specimen (
Figure 14 and
Figure 15). The female specimens exhibited identical morphological characters to the original description, including abdominal segments III with six black spots, confirming that our material represents the published
Mictis falloui. However, the male morphology shows critical diagnostic differences from
Mictis: abdomen smooth, without tubercles and hind tibia with a large spine. Conversely, male hind femora with tubercles on anterior and posterior surfaces align with the morphological characteristics of
Ochrochira. Based on this evidence, we formally transfer this species to the genus
Ochrochira.