Aphid Infestation and Predator Dynamics in Cultivated Ruta chalepensis: Evidence of Myzus persicae Adaptation and Natural Enemy Responses
Simple Summary
Abstract
1. Introduction
2. Material and Methods
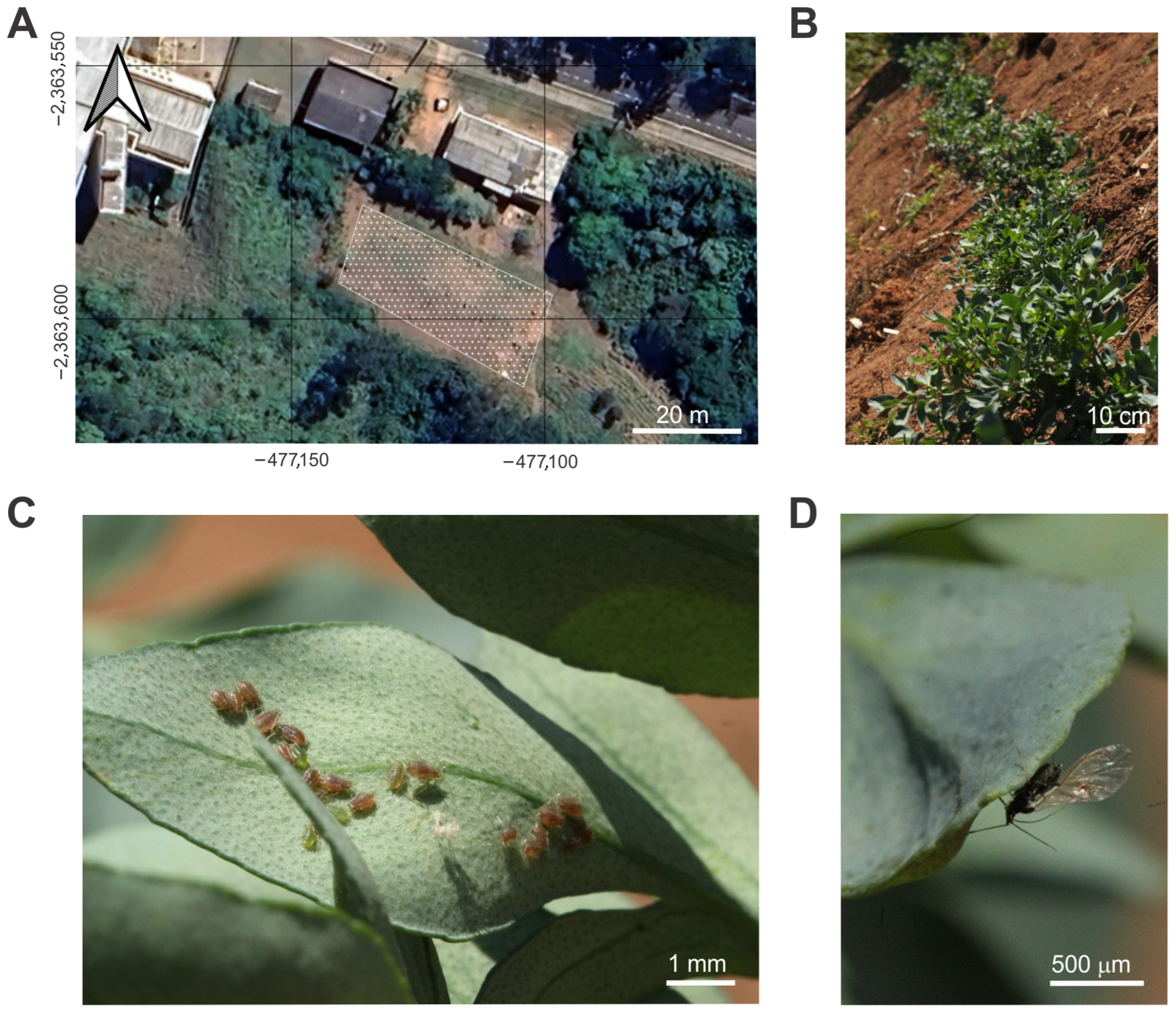
2.1. Study Place
2.2. Arthropod Sampling
2.3. Plant Identification
2.4. Aphid Identification
3. Results
3.1. Identification of Plant Species
3.2. Identification of Aphid Species
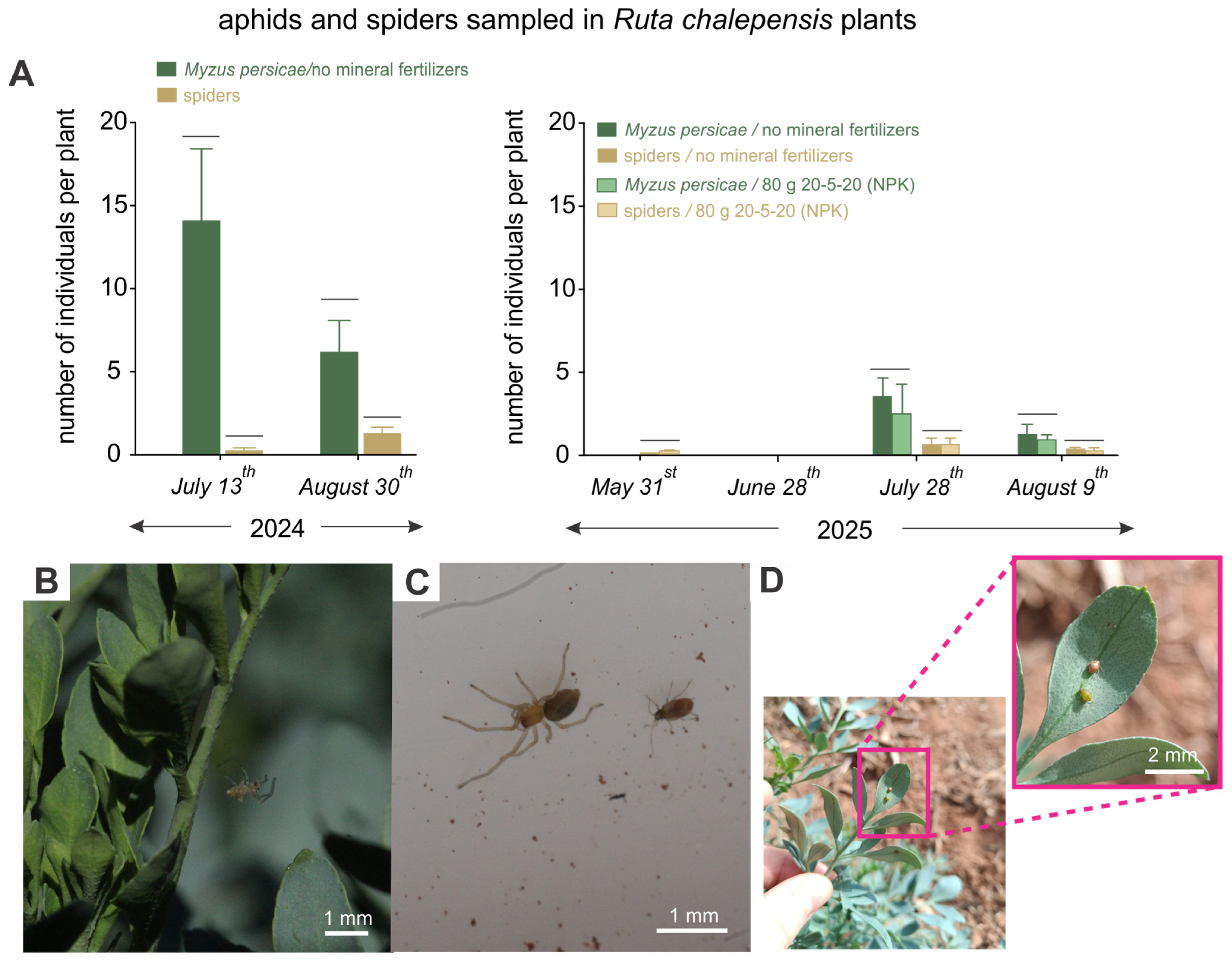
3.3. Sampling of Aphids and Predatory Spiders
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, A.; Gulshana, M.I.A. Taxonomy and medicinal uses on amaranthaceae family of Rajshahi, Bangladesh. Appl. Ecol. Environ. Sci. 2014, 2, 54–59. [Google Scholar] [CrossRef]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle marmelos. BioMed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef] [PubMed]
- Hammiche, V.; Azzouz, M. Les rues: Ethnobotanique, phytopharmacologie et toxicité. Phytothérapie 2013, 11, 22–30. [Google Scholar] [CrossRef]
- Perdomo, T.V.P.; Güisa, D.H.; López, J.P.M. Fusarium oxysporum as the causal agent of vascular wilt in Ruta graveolens L. in Colombia. Rev. Cienc. Agric. 2024, 41, e3240. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Alatar, A.A.; Faisal, M. In Vitro regeneration, phytochemical profiling and antioxidant activity in Ruta chalepensis plants established from alginate encapsulated synthetic seeds. S. Afr. J. Bot. 2023, 161, 575–585. [Google Scholar] [CrossRef]
- Althaher, A.R.; Oran, S.A.; Bustanji, Y.K. Phytochemical Analysis, In Vitro Assessment of Antioxidant Properties and Cytotoxic Potential of Ruta chalepensis L. Essential Oil. J. Essent. Oil-Bear. Plants 2020, 23, 1409–1421. [Google Scholar] [CrossRef]
- Degu, A.; Kefale, B.; Alemayehu, D.; Tegegne, G.T. Evaluation of the Antidiarrheal Activity of Hydromethanol Crude Extracts of Ruta chalepensis and Vernonia amygdalina in Mice. eCAM 2020, 2020, 8318713. [Google Scholar] [CrossRef]
- Szewczyk, A.; Marino, A.; Molinari, J.; Ekiert, H.; Miceli, N. Phytochemical Characterization, and Antioxidant and Antimicrobial Properties of Agitated Cultures of Three Rue Species: Ruta chalepensis, Ruta corsica, and Ruta graveolens. Antioxidants 2022, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, I.; Bettaieb Rebey, I.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani Tounsi, M. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef]
- Abdel-Salam, E.M.; Faisal, M.; Alatar, A.A.; Qahtan, A.A.; Alam, P. Genome-wide transcriptome variation landscape in Ruta chalepensis organs revealed potential genes responsible for rutin biosynthesis. J. Biotechnol. 2021, 325, 43–56. [Google Scholar] [CrossRef]
- Freitas, F.A.M.; Lima, R. Um estudo bibliográfico sobre a Ruta graveolens L. (Rutaceae). Rev. Biodivers. 2021, 20, 111–120. [Google Scholar]
- Najem, M.; Bammou, M.; Bachiri, L.; Bouiamrine, E.H.; Ibijbijen, J.; Nassiri, L. Ruta chalepensis L. Essential Oil Has a Biological Potential for a Natural Fight against the Pest of Stored Foodstuffs: Tribolium castaneum Herbst. eCAM 2020, 2020, 5739786. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, R.S.; Alghamdi, A.S.; Althagafi, S.S. Morphological Identification of Aphid Species Infesting Some Ornamental plants in Taif Governorate. Egypt. Acad. J. Biol. Sci. A Entomol. 2016, 9, 15–35. [Google Scholar] [CrossRef]
- Holman, J. Host Plant Catalog of Aphids: Palaearctic Region; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs. Host Lists and Keys; John Wiley & Sons Ltd.: Chichester, UK, 2006; Volume I. [Google Scholar]
- Lima, C.H.; Sarmento, R.A.; Pereira, P.S.; Galdino, T.V.; Santos, F.A.; Silva, J.; Picanço, M.C. Feasible sampling plan for Bemisia tabaci control decision-making in watermelon fields. Pest Manag. Sci. 2017, 73, 2345–2352. [Google Scholar] [CrossRef]
- Lopes, M.C.; Farias, E.S.; Costa, T.L.; Arcanjo, L.P.; Santos, A.A.; Ribeiro, A.V.; Santos, R.C.; Picanço, M.C. Economic injury level and sequential sampling plan for Liriomyza huidobrensis management in tomato crops. Crop Prot. 2019, 124, 104848. [Google Scholar] [CrossRef]
- Wanderley, M.; Shepherd, G.; Giulietti, A.; Melhem, T. Flora Fanerogâmica do Estado de São Paulo; Fapesp: São Paulo, Brazil, 2002; Volume 2, pp. 281–308. [Google Scholar]
- El, E.N. Contribución al Estudio de Áfidos (Hemiptera: Aphididae); Universidad Nacional Agraria La Molina: Lima, Peru, 2018. [Google Scholar]
- Lozier, J.D.; Foottit, R.G.; Miller, G.L.; Mills, N.J.; Roderick, G.K. Molecular and morphological evaluation of the aphid genus Hyalopterus Koch (Insecta: Hemiptera: Aphididae), with a description of a new species. Zootaxa 2008, 1688, 1–19. [Google Scholar] [CrossRef]
- Remaudière, G.; Remaudiere, M. Catalogue of the World’s Aphididae; INRA: Versailles, France, 1997. [Google Scholar]
- Ali, J.; Bayram, A.; Mukarram, M.; Zhou, F.; Karim, M.F.; Hafez, M.M.A.; Mahamood, M.; Yusuf, A.A.; King, P.J.H.; Adil, M.F.; et al. Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions. Sustainability 2023, 15, 11150. [Google Scholar] [CrossRef]
- Dajic-Stevanovic, Z.; Pljevljakusic, D. Challenges and decision making in cultivation of medicinal and aromatic plants. In Medicinal and Aromatic Plants of the World: Scientific, Production, Commercial and Utilization Aspects; Springer: Dordrecht, The Netherlands, 2015; Volume I, pp. 145–164. [Google Scholar]
- Powell, G.; Tosh, C.R.; Hardie, J. Host Plant Selection By Aphids: Behavioral, Evolutionary, and Applied Perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Goggin, F.L. Plant–aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408. [Google Scholar] [CrossRef]
- Loxdale, H.D. Aspects, Including Pitfalls, of Temporal Sampling of Flying Insects, with Special Reference to Aphids. Insects 2018, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Loxdale, H.D.; Balog, A.; Biron, D.G. Aphids in focus: Unravelling their complex ecology and evolution using genetic and molecular approaches. Biol. J. Linn. Soc. 2020, 129, 507–531. [Google Scholar] [CrossRef]
- Crossley, M.S.; Smith, O.M.; Davis, T.S.; Eigenbrode, S.D.; Hartman, G.L.; Lagos-Kutz, D.; Halbert, S.E.; Voegtlin, D.J.; Moran, M.D.; Snyder, W.E. Complex life histories predispose aphids to recent abundance declines. Glob. Change Biol. 2021, 27, 4283–4293. [Google Scholar] [CrossRef] [PubMed]
- Kinyanjui, G.; Khamis, F.M.; Mohamed, S.; Ombura, L.O.; Warigia, M.; Ekesi, S. Identification of aphid (Hemiptera: Aphididae) species of economic importance in Kenya using DNA barcodes and PCR-RFLP-based approach. Bull. Entomol. Res. 2015, 106, 63–72. [Google Scholar] [CrossRef]
- Rebijith, K.B.; Asokan, R.; Kumar, N.K.K.; Krishna, V.; Chaitanya, B.N.; Ramamurthy, V.V. DNA barcoding and elucidation of cryptic aphid species (Hemiptera: Aphididae) in India. Bull. Entomol. Res. 2013, 103, 601–610. [Google Scholar] [CrossRef]
- Suganthi, M.; Abirami, G.; Jayanthi, M.; Kumar, K.A.; Karuppanan, K.; Palanisamy, S. A method for DNA extraction and molecular identification of Aphids. MethodsX 2023, 10, 102100. [Google Scholar] [CrossRef]
- Altesor, P.; González, A. Preference and performance of the generalist aphid Myzus persicae on closely and distantly related plant species. Èntomol. Exp. Appl. 2023, 171, 754–764. [Google Scholar] [CrossRef]
- Welch, K.D.; Whitney, T.D.; Harwood, J.D. Non-pest prey do not disrupt aphid predation by a web-building spider. Bull. Entomol. Res. 2015, 106, 91–98. [Google Scholar] [CrossRef]
- Mathers, T.C.; Chen, Y.; Kaithakottil, G.; Legeai, F.; Mugford, S.T.; Baa-Puyoulet, P.; Bretaudeau, A.; Clavijo, B.; Colella, S.; Collin, O.; et al. Rapid transcriptional plasticity of duplicated gene clusters enables a clonally reproducing aphid to colonise diverse plant species. Genome Biol. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Michalko, R.; Pekár, S.; Dul’a, M.; Entling, M.H. Global patterns in the biocontrol efficacy of spiders: A meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 1366–1378. [Google Scholar] [CrossRef]
- Fallahpour, F.; Ghorbani, R.; Nassiri-Mahallati, M.; Hosseini, M. Plant fertilization helps plants to compensate for aphid damage, positively affects predator efficiency and improves canola yield. J. Pest Sci. 2020, 93, 251–260. [Google Scholar] [CrossRef]
- Braham, M.; Boulahia-Kheder, S.; Kahia, M.; Nouira, S. Aphids and citrus responses to nitrogen fertilization. J. Saudi Soc. Agric. Sci. 2023, 22, 374–383. [Google Scholar] [CrossRef]
- Nascimento, P.M. Produção da Arruda (Ruta graveolens L.) Cultivada com Resíduo Orgânico com cama de Frango; Universidade Federal da Grande Dourado: Mato Grosso do Sul, Brasil, 2023. [Google Scholar]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2007; pp. 87–113. [Google Scholar]
- Szewczyk, A.; Grabowski, M.; Zych, D. Ruta chalepensis L. In Vitro Cultures as a Source of Bioactive Furanocoumarins and Furoquinoline Alkaloids. Life 2023, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Alatar, A.A.; Qahtan, A.A.; Faisal, M. Eco-friendly synthesis of silver nanoparticles: Unraveling their influence on callus proliferation, secondary metabolite production, and antioxidant activities in Ruta chalepensis L. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 157, 55. [Google Scholar] [CrossRef]
- Pym, A.; Umina, P.A.; Reidy-Crofts, J.; Troczka, B.J.; Matthews, A.; Gardner, J.; Hunt, B.J.; van Rooyen, A.R.; Edwards, O.R.; Bass, C. Overexpression of UDP-glucuronosyltransferase and cytochrome P450 enzymes confers resistance to sulfoxaflor in field populations of the aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2022, 143, 103743. [Google Scholar] [CrossRef]
- Scott, J.G. Insect UDP-glycosyltransferases and xenobiotic metabolism. Pestic. Biochem. Physiol. 2026, 216, 106731. [Google Scholar] [CrossRef]
- Jiang, W.; Cheng, Q.; Lu, C.; Chen, W.; Zhao, D.; He, Y. Different Host Plants Distinctly Influence the Adaptability of Myzus persicae (Hemiptera: Aphididae). Agriculture 2022, 12, 2162. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, E.E.; Nascimento, T.F.; Francesco, L.S.; Svacina, T.; León, C.; Gomes, C.N.; Jumbo, L.O.V.; González Armijos, M.J.; Vilca Mallqui, K.S.; Smagghe, G. Aphid Infestation and Predator Dynamics in Cultivated Ruta chalepensis: Evidence of Myzus persicae Adaptation and Natural Enemy Responses. Insects 2025, 16, 1088. https://doi.org/10.3390/insects16111088
Oliveira EE, Nascimento TF, Francesco LS, Svacina T, León C, Gomes CN, Jumbo LOV, González Armijos MJ, Vilca Mallqui KS, Smagghe G. Aphid Infestation and Predator Dynamics in Cultivated Ruta chalepensis: Evidence of Myzus persicae Adaptation and Natural Enemy Responses. Insects. 2025; 16(11):1088. https://doi.org/10.3390/insects16111088
Chicago/Turabian StyleOliveira, Eugênio E., Tarciza F. Nascimento, Leonardo S. Francesco, Thiago Svacina, César León, Carlos N. Gomes, Luis O. Viteri Jumbo, Maria José González Armijos, Karina S. Vilca Mallqui, and Guy Smagghe. 2025. "Aphid Infestation and Predator Dynamics in Cultivated Ruta chalepensis: Evidence of Myzus persicae Adaptation and Natural Enemy Responses" Insects 16, no. 11: 1088. https://doi.org/10.3390/insects16111088
APA StyleOliveira, E. E., Nascimento, T. F., Francesco, L. S., Svacina, T., León, C., Gomes, C. N., Jumbo, L. O. V., González Armijos, M. J., Vilca Mallqui, K. S., & Smagghe, G. (2025). Aphid Infestation and Predator Dynamics in Cultivated Ruta chalepensis: Evidence of Myzus persicae Adaptation and Natural Enemy Responses. Insects, 16(11), 1088. https://doi.org/10.3390/insects16111088








