Impacts of Climate Change and Human Activity on the Potential Distribution of Conogethes punctiferalis in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Points of C. punctiferalis
2.2. Environmental Variables
2.3. Model Analysis Optimization
2.4. Analysis of Spatial Pattern Change and Centroid Transfer
3. Results
3.1. Model Accuracy Evaluation and Variable Selection
3.2. Potential Distribution of C. punctiferalis Under Current Climate and Human Interference in the Current Period
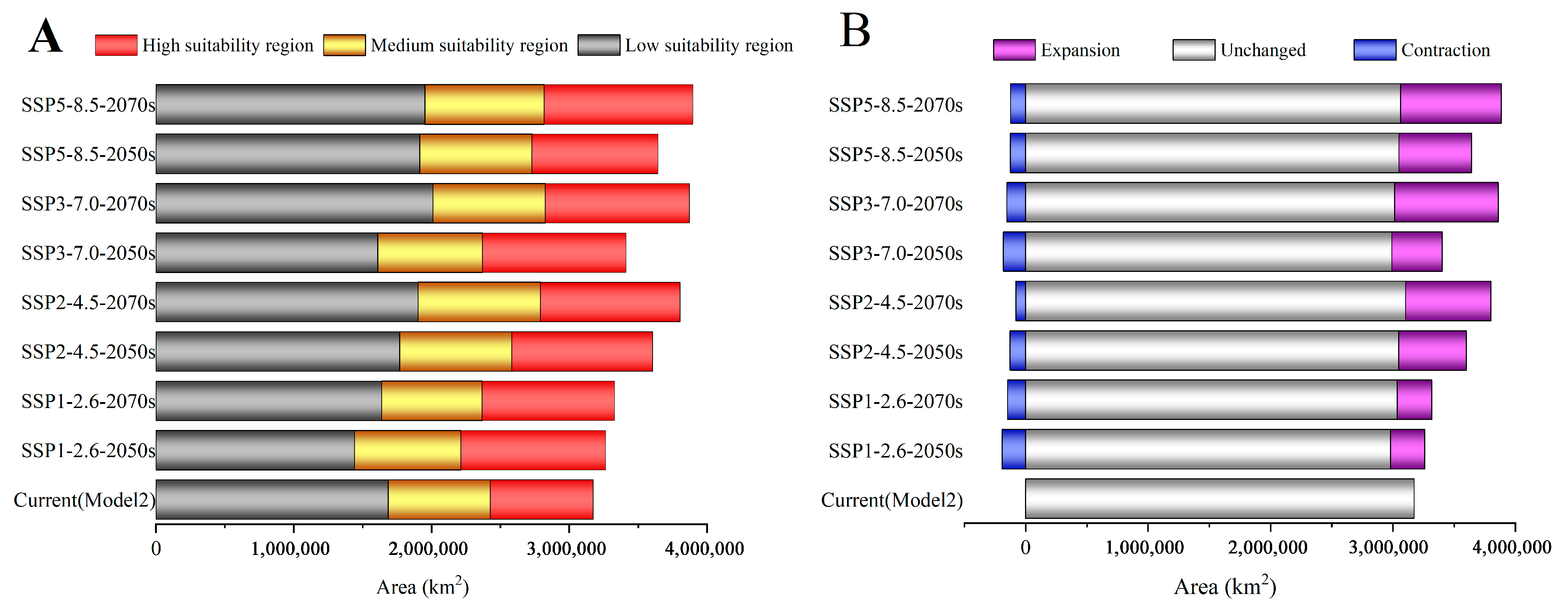
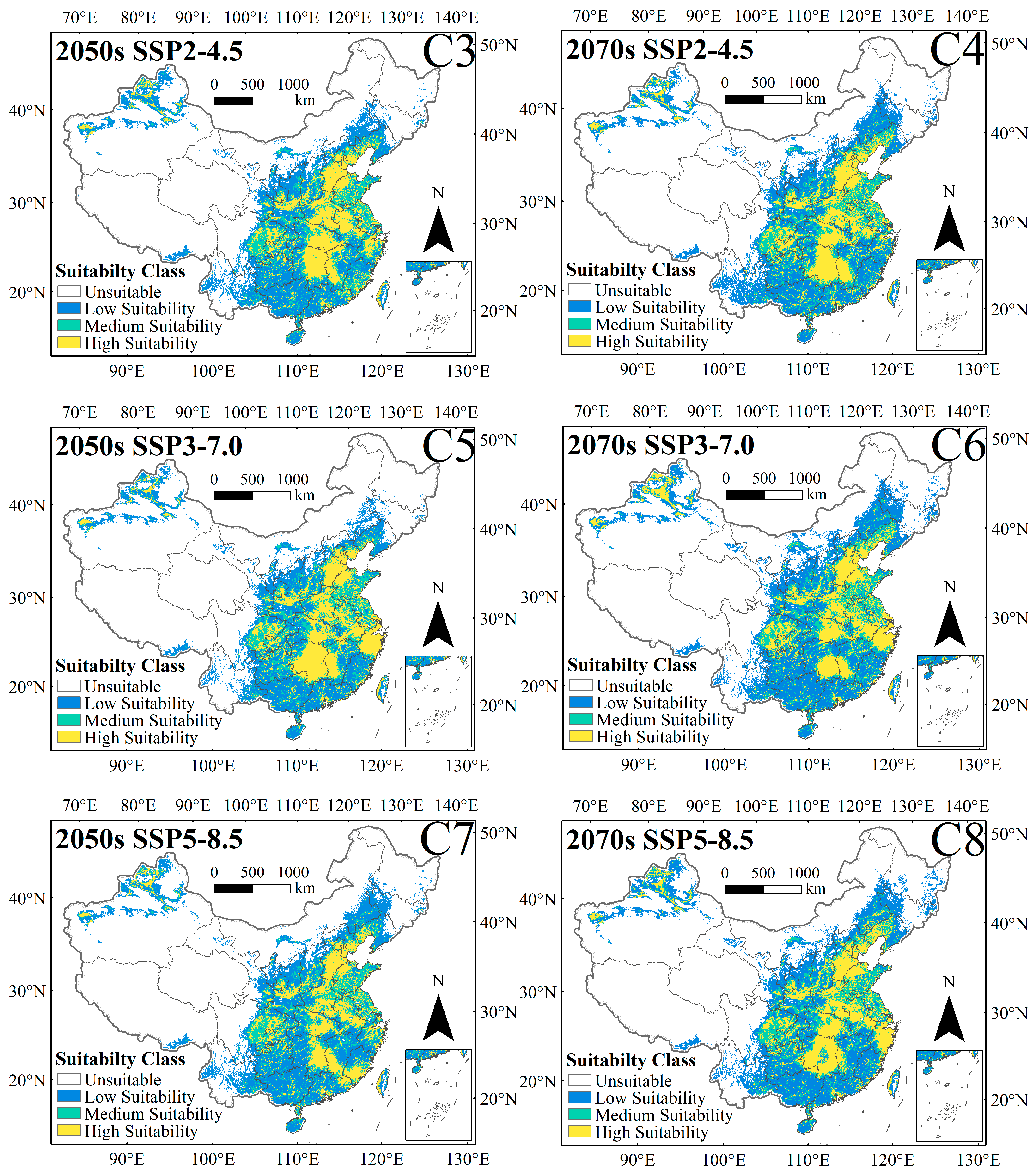
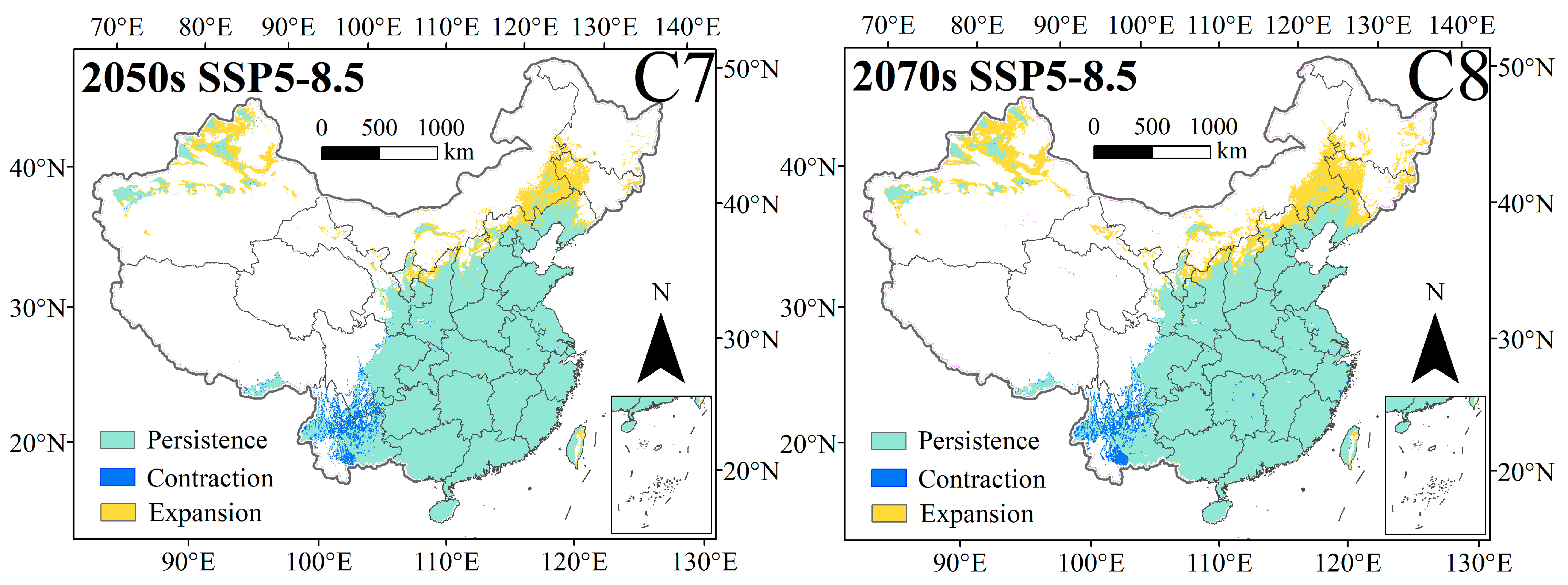
3.3. Changes in the Spatial Distribution Pattern of C. punctiferalis Under Different Climate Change Scenarios
3.4. Transfer of the Potential Distribution of C. punctiferalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanley, J.; Chandrasekaran, S.; Preetha, G. Conogethes punctiferalis (Lepidoptera: Pyralidae) its biology and field parasitization. Indian J. Agric. Sci. 2009, 79, 906–909. [Google Scholar]
- Wang, Z.; He, K.; Shi, J.; Ma, S. Reasons of increasing damage caused by Conogethes punctiferalis and the control strategy. Plant Prot. 2006, 32, 67–69. [Google Scholar]
- Kammar, V.; Sagar, D.; Chandel, R.; Shashank, P.; Chakravarthy, A.K. Development of species-specific marker and molecular diversity of Conogethes punctiferalis and Conogethes sahyadriensis (Lepidoptera: Crambidae). J. Environ. Biol. 2021, 42, 271–279. [Google Scholar] [CrossRef]
- Ramzan, M.; Pang, T.; Shi, L.; Naeem-Ullah, U.; Saeed, S.; Zhang, T.; Panhwar, W.A.; Zhang, Y.J. Bio-ecology and management approaches of yellow peach moth, Conogethes punctiferalis (Lepidoptera: Crambidae). Eur. J. Entomol. 2024, 121, 234–251. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, Y.; Li, Z.; Liu, X.G.; Liu, X.M.; Yao, S.Y.; Du, M.F.; An, S.H. Sublethal indoxacarb exposure alters pheromone production and ovarian development in the yellow peach moth, Conogethes punctiferalis. Pestic. Biochem. Physiol. 2025, 210, 106368. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yao, Y.; Gao, P.; Xiu, M.; Shi, C. Molecular evaluation of Conogethes punctiferalis (Guenée, 1854): Species status and intraspecific divergence. Zool. Syst. 2024, 49, 267–275. [Google Scholar]
- He, Q.Y.; Fan, X.C.; Wang, S.X.; Chen, S.S.; Chen, J.X. Juvenile hormone inhibits adult cuticle formation in Drosophila melanogaster through Kr-h1/Dnmt2-mediated DNA methylation of Acp65A promoter. Insect Mol. Biol. 2024, 33, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.K.; Li, C.; Song, Y.Y.; Li, L.L.; Men, X. Population genetics of the yellow peach moth, Conogethes punctiferalis, reveals landscape-mediated genetic distribution in agroecosystems. Entomol. Gen. 2023, 43, 861–869. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, M.; Li, S.; Wang, S.; Zhao, Y.; Xu, Y.; Chen, Z.; Sun, J.; Kang, Z.; Liu, F. Comparison of gut transcriptome and bacterial composition of the yellow peach moth, Conogethes punctiferalis larvae associated with host plants adaptation. CABI Agric. Biosci. 2024, 5, 1–12. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, H.; Jiang, L.; Wen, J.; Zhang, W.; Sun, R. Effects of supplemental nutrients on ovarian development and oviposition in Conogethes punctiferalis (Lepidoptera: Crambidae). Environ. Entomol. 2023, 52, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kirchengast, G.; Pichler, M. A traceable global warming record and clarity for the 1.5 °C and well-below-2 °C goals. Commun. Earth Environ. 2025, 6, 402. [Google Scholar] [CrossRef]
- Traill, L.W.; Lim, M.L.M.; Sodhi, N.S.; Bradshaw, C.J.A. Mechanisms driving change: Altered species interactions and ecosystem function through global warming. J. Anim. Ecol. 2010, 79, 937–947. [Google Scholar] [CrossRef]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Change 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- Mao, X.J.; Zheng, H.S.; Liao, S.K.; Wei, H.J.; Lin, H.Y.; Wang, Q.; Chen, H. Predicting the potential distribution of Perina nuda under climate change in China. Entomol. Exp. Et Appl. 2024, 172, 738–750. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, L.; Yang, L.; He, H. Potential distribution of the weaver ant Oecophylla under current and future climate. Pest Manag. Sci. 2025, 81, 5324–5334. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Urbani, F.; D’Alessandro, P.; Frasca, R.; Biondi, M. Maximum entropy modeling of geographic distributions of the flea beetle species endemic in Italy (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Zool. Anz. 2015, 258, 99–109. [Google Scholar] [CrossRef]
- Khanghah, S.S.; Moameri, M.; Ghorbani, A.; Mostafazadeh, R.; Biswas, A. Modeling potential habitats and predicting habitat connectivity for Leucanthemum vulgare Lam. in northwestern rangelands of Iran. Environ. Monit. Assess. 2022, 194, 1–16. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Xiao, N.W.; Shen, M.; Li, J.S. Comparison between optimized MaxEnt and random forest modeling in predicting potential distribution: A case study with Quasipaa boulengeri in China. Sci. Total Environ. 2022, 842, 156867. [Google Scholar] [CrossRef]
- Li, R.Q. Protecting rare and endangered species under climate change on the Qinghai Plateau, China. Ecol. Evol. 2019, 9, 427–436. [Google Scholar] [CrossRef]
- Hu, H.C.; Zhou, H.W.; Li, Y.X.; Li, Y.Z.; Yan, Y.B.; Yang, J.; Chen, J.; Chen, Y.M.; Cui, D. The Involvement of human factors brings new findings for predicting global suitability habitat for Hyphantria cunea (Lepidoptera: Arctiidae). Ecol. Evol. 2025, 15, e71421. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pysek, P.; Thuiller, W.; Yesson, C.; Vilà, M. Protected areas offer refuge from invasive species spreading under climate change. Glob. Change Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef]
- Maiorano, L.; Chiaverini, L.; Falco, M.; Ciucci, P. Combining multi-state species distribution models, mortality estimates, and landscape connectivity to model potential species distribution for endangered species in human dominated landscapes. Biol. Conserv. 2019, 237, 19–27. [Google Scholar] [CrossRef]
- Marshall, F.E.; Banks, K.; Cook, G.S. Ecosystem indicators for Southeast Florida beaches. Ecol. Indic. 2014, 44, 81–91. [Google Scholar] [CrossRef]
- Hemmingmoore, H.; Aronsson, M.; Åkesson, M.; Persson, J.; Andrén, H. Evaluating habitat suitability and connectivity for a recolonizing large carnivore. Biol. Conserv. 2020, 242, 108352. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zheng, H.-Y.; Chang, R.; Gong, D.-F.; Yuan, G.-J.; Lu, S.-H.; Wang, X.-Y. Primary biological characteristics of an emerging major boring pest, Conogethes punctiferalis (Guenée) (Lepidoptera: Crambidae), on Juglans regia (Juglandales: Juglandaceae) in Taihang Mountains. Entomol. News 2022, 130, 296–307. [Google Scholar] [CrossRef]
- Ramzan, M.; Shi, L.F.; Pang, T.Y.; Chen, X.Z.; Li, R.A.; Almaary, K.S.; Zhang, Y.J. Influence of different temperatures and diets on the life cycle of invasive species Conogethes punctiferalis. Bull. Entomol. Res. 2025, 115, 12–20. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.; Shera, P.S.; Siraj, M.; Sandhu, R.K.; Maredia, K. Evaluation of augmentative biological control options against fruit and shoot borer, Conogethes punctiferalis (Guenée) (Lepidoptera: Crambidae) in guava in India. Egypt. J. Biol. Pest Control 2023, 33, 1–8. [Google Scholar] [CrossRef]
- Kim, K.; Baek, S.; Kim, M.J.; Jung, J.K.; Jung, C.; Lee, J.H. Efficiency of chemical and organic pesticides for Conogethes punctiferalis (Lepidoptera: Crambidae) in commercial chestnut and walnut fields. J. Asia-Pac. Entomol. 2022, 25, 101897. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.Y.; Jin, Z.Y.; Li, J.Y.; Xue, D.R.; Tong, Y.; Zhang, A.H.; Du, Y.L. Penicillium-infected apples benefit larval development of Conogethes punctiferalis via alterations of their gut bacteria community and gene expression. J. Agric. Food Chem. 2024, 72, 7774–7783. [Google Scholar] [CrossRef]
- Jing, D.P.; Prabu, S.; Zhang, T.T.; Bai, S.X.; He, K.L.; Zhang, Y.J.; Wang, Z.Y. Revealing the difference of α-amylase and CYP6AE76 gene between polyphagous Conogethes punctiferalis and oligophagous C. pinicolalis by multiple-omics and molecular biological technique. Bmc Genom. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- An, E.; Zhang, Y.; Yao, S.Y. Bifenthrin at sublethal concentrations suppresses mating and laying of female Conogethes punctiferalis by regulating sex pheromone biosynthesis and JH signals. J. Agric. Food Chem. 2024, 72, 22908–22917. [Google Scholar] [CrossRef]
- Zhang, Y.; She, Z.L.; He, R.L.; Yao, S.Y.; Li, X.; Liu, X.G.; Yin, X.M.; Wei, J.Z.; Du, M.F.; An, S.H. The Ca2+/CaN/ACC and cAMP/PKA/HK signal pathways are required for PBAN-mediated sex pheromone biosynthesis in Conogethes punctiferalis. J. Integr. Agric. 2024, 23, 2735–2751. [Google Scholar] [CrossRef]
- Xin, X.G.; Wu, T.W.; Zhang, J.; Zhang, F.; Li, W.P.; Zhang, Y.W.; Lu, Y.X.; Fang, Y.J.; Jie, W.H.; Zhang, L.; et al. Introduction of BCC models and its participation in CMIP6. Clim. Change Res. 2019, 15, 533–539. [Google Scholar]
- Liu, Y.B.; Li, Y.Y.; Wang, R.; Guo, L.Z.; Ji, Y.; Chen, Y.H.; Hao, L.F.; Lin, K.J. Impacts of human activity and climate change on the suitable habitats for Xanthium spinosum in China. Plants 2025, 14, 306. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Wang, P.; Xie, G.; Wang, W. Assessing the potential global distribution of Monochamus sutor (Coleoptera: Cerambycidae) under the influence of climate change and human activities based on Maximum Entropy model. J. Econ. Entomol. 2025, 118, 1174–1187. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; James, C.; Pruinská, A.; Hrtensteiner, S.; Thomas, H.; Ougham, H. Analysis of the chlorophyll catabolism pathway in leaves of an introgression senescence mutant of Lolium temulentum. Phytochemistry 2004, 65, 1231–1238. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Santos-Hernández, A.F.; Monterroso-Rivas, A.I.; Granados-Sánchez, D.; Villanueva-Morales, A.; Santacruz-Carrillo, M. Projections for Mexico’s tropical rainforests considering ecological niche and climate change. Forests 2021, 12, 119. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Peerj 2017, 5, e4095. [Google Scholar] [CrossRef]
- Amat, M.E.; Vargas, P.; Gómez, J.M. Effects of human activity on the distribution and abundance of an endangered Mediterranean high-mountain plant (Erysimum penyalarense). J. Nat. Conserv. 2013, 21, 262–271. [Google Scholar] [CrossRef]
- Boyer, S.; Rivault, C. Impact of human activity on the distribution of native and non-native cockroach species (Dictyoptera) in La Reunion and Mayotte. Bull Entomol Res 2006, 96, 399–406. [Google Scholar] [CrossRef]
- Yu, P.J.; Han, D.L.; Liu, S.W.; Wen, X.; Huang, Y.X.; Jia, H.T. Soil quality assessment under different land uses in an alpine grassland. Catena 2018, 171, 280–287. [Google Scholar] [CrossRef]
- Rehman, M.; Liu, J.; Johnson, A.C.; Dada, T.E.; Gurr, G.M. Organic mulches reduce crop attack by sweetpotato weevil (Cylas formicarius). Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Zhu, G.D.; Xia, N.N.; Xue, M.; Li, Z.P.; Zhao, H.P.; Qu, C. Effects of colored polyethylene film mulch on pest populations, plant growth and yield of peanut in Northern China. J. Asia-Pac. Entomol. 2022, 25, 101944. [Google Scholar] [CrossRef]
- Dal Zotto, G.; Costaz, T.P.M.; Pesavento, G.; van Rozen, K.; Helsen, H.H.M.; Gotta, P.; Cavagna, B.; Ciampitti, M.; Mori, N. Effectiveness of mulches in preventing Popillia japonica (Coleoptera: Scarabaeidae) oviposition in nursery potted plants. J. Econ. Entomol. 2025, 118, 1589–1598. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Chaudhary, M.A.; Ali, M.I.; Hussain, A.; Ali, I. On tree fruit bagging influences quality of Guava Harvested at different maturity stages during summer. Int. J. Agric. Biol. 2014, 16, 543–549. [Google Scholar]
- Frank, D.L. Evaluation of fruit bagging as a pest management option for direct pests of apple. Insects 2018, 9, 178. [Google Scholar] [CrossRef]
- Expósito, A.; García, S.; Giné, A.; Escudero, N.; Sorribas, F.J. Cucumis metuliferus reduces Meloidogyne incognita virulence against the Mi1.2 resistance gene in a tomato-melon rotation sequence. Pest Manag. Sci. 2019, 75, 1902–1910. [Google Scholar] [CrossRef]
- Kim, D.H.; Wade, T.; Brym, Z.; Ogisma, L.; Bhattarai, R.; Bai, X.; Bhadha, J.; Her, Y. Assessing the agricultural, environmental, and economic effects of crop diversity management: A comprehensive review on crop rotation and cover crop practices. J. Environ. Manag. 2025, 387, 125833. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Sarkar, B.C.; Hossain, M.M.; Mian, M.Y.; Rajotte, E.G.; Muniappan, R.; O’Rourke, M.E. Comparison of biorational management approaches against mango fruit fly (Bactrocera dorsalis Hendel) in Bangladesh. Crop Prot. 2020, 135, 104807. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Z.; Ning, T.; Mi, Q.; Zhang, X.; Zhang, S.; Liu, Z. Colored polyethylene film mulches on weed control, soil conditions and peanut yield. Plant Soil Environ. 2015, 61, 79–85. [Google Scholar] [CrossRef]
- Hue, S.M.; Low, M.Y. An insight into sweet potato weevils management: A review. Psyche: A J. Entomol. 2015, 2015, 849560. [Google Scholar] [CrossRef]
- Yuan, X.; Li, R.D.; Tang, G.L.; Yang, S.L.; Deng, J. Enhancing sugarcane yield and weed control sustainability with degradable film mulching. Plants-Basel 2025, 14, 2521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, P.; Xie, G.L.; Wang, W.K. Evaluating the impact of climate change and human activities on the potential distribution of pine wood Nnematode (Bursaphelenchus xylophilus) in China. Forests 2024, 15, 1253. [Google Scholar] [CrossRef]
- Hellmann, J.J.; Grundel, R.; Hoving, C.; Schuurman, G.W. A call to insect scientists: Challenges and opportunities of managing insect communities under climate change. Curr. Opin. Insect Sci. 2016, 17, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological flora of the british isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Kueffer, C. Plant invasions in the anthropocene. Science 2017, 358, 724–725. [Google Scholar] [CrossRef]
- Ma, B.B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Azrag, A.G.A.; Pirk, C.W.W.; Yusuf, A.A.; Pinard, F.; Niassy, S.; Mosomtai, G.; Babin, R. Prediction of insect pest distribution as influenced by elevation: Combining field observations and temperature-dependent development models for the coffee stink bug, Antestiopsis thunbergii (Gmelin). PLoS ONE 2018, 13, e0199569. [Google Scholar] [CrossRef] [PubMed]
- Azrag, A.G.A.; Obala, F.; Tonnang, H.E.Z.; Hogg, B.N.; Ndlela, S.; Mohamed, S.A. Predicting the impact of climate change on the potential distribution of the invasive tomato pinworm Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelechiidae). Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- dos Santos, L.A.; Mendes, M.F.; Krüger, A.P.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.F.; Peng, L.F.; He, Z.Q.; Lu, Y.Y.; Wang, F. Potential distribution of two invasive pineapple pests under climate change. Pest Manag. Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef]
- Wei, X.J.; Xu, D.P.; Liu, Q.W.; Wu, Y.H.; Zhuo, Z.H. Predicting the potential distribution range of Batocera horsfieldi under CMIP6 climate change using the MaxEnt model. J. Econ. Entomol. 2024, 117, 187–198. [Google Scholar] [CrossRef]
- Xu, L.R.; Ni, X.Z.; Wang, Z.Y.; He, K.L. Effects of photoperiod and temperature on diapause induction in Conogethes punctiferalis (Lepidoptera: Pyralidae). Insect Sci. 2014, 21, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Yang, H.; Luo, W.; Wang, M.T.; Lu, X.L.; Huang, T.T.; Zhao, J.P.; Li, Q. Predicting the potential distribution of the Asian citrus psyllid, Diaphorina citri (Kuwayama), in China using the MaxEnt model. Peerj 2019, 7, e7323. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.X.; Shen, L.Q.; Liu, R.; Wang, G.; Zhang, A.P.; Chen, L.; Zhang, Y.Z.; Zhang, X.Y.; Qi, J.; Wang, C.L.; et al. Summer habitat suitability of wild alpine musk deer based on MaxEnt model. Acta Ecol. Sin. 2023, 43, 441–448. [Google Scholar] [CrossRef]
- Wang, Z.C.; Kang, Y.K.; Wang, Y.; Tan, Y.C.; Yao, B.H.; An, K.; Su, J.H. Himalayan Marmot (Marmota himalayana) redistribution to high latitudes under climate change. Animals 2023, 13, 2736. [Google Scholar] [CrossRef]
- Xu, Q.W.; Zhang, X.J.; Li, J.X.; Ren, J.R.; Ren, L.L.; Luo, Y.Q. Pine Wilt Disease in northeast and northwest China: A comprehensive risk review. Forests 2023, 14, 174. [Google Scholar] [CrossRef]
- Michalak, J.L.; Lawler, J.J.; Roberts, D.R.; Carroll, C. Distribution and protection of climatic refugia in North America. Conserv. Biol. 2018, 32, 1414–1425. [Google Scholar] [CrossRef]
- Mudereri, B.T.; Kimathi, E.; Chitata, T.; Moshobane, M.C.; Abdel-Rahman, E.M. Landscape-scale biogeographic distribution analysis of the whitefly, Bemisia tabaci (Gennadius, 1889) in Kenya. Int. J. Trop. Insect Sci. 2021, 41, 1585–1599. [Google Scholar] [CrossRef]
- Yan, X.B.; Guo, Y.X.; Zhou, H.; Wang, K. Effect of geographical factors on genetic variation of Elymus nutans indigenous in the Qinghai-Tibetan Plateau. Acta Bot. Boreali-Occident. Sin. 2007, 27, 328–333. [Google Scholar]
- Umina, P.A.; Weeks, A.R.; Kearney, M.R.; Mckechnie, S.W.; Hoffmann, A.A. A Rapid shift in a classic clinal pattern in drosophila reflecting climate change. Science 2005, 308, 691–693. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Blackwell Publ. Ltd. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest insects and climate change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Eickermann, M.; Junk, J.; Rapisarda, C. Climate change and insects. Insects 2023, 14, 678. [Google Scholar] [CrossRef]
- Sun, S.X.; Zhang, Y.; Huang, D.Z.; Wang, H.; Cao, Q.; Fan, P.X.; Yang, N.; Zheng, P.M.; Wang, R.Q. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar] [CrossRef]
- Guan, J.Y.; Li, M.Y.; Ju, X.F.; Lin, J.; Wu, J.G.; Zheng, J.H. The potential habitat of desert locusts is contracting: Predictions under climate change scenarios. Peerj 2021, 9, e12311. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
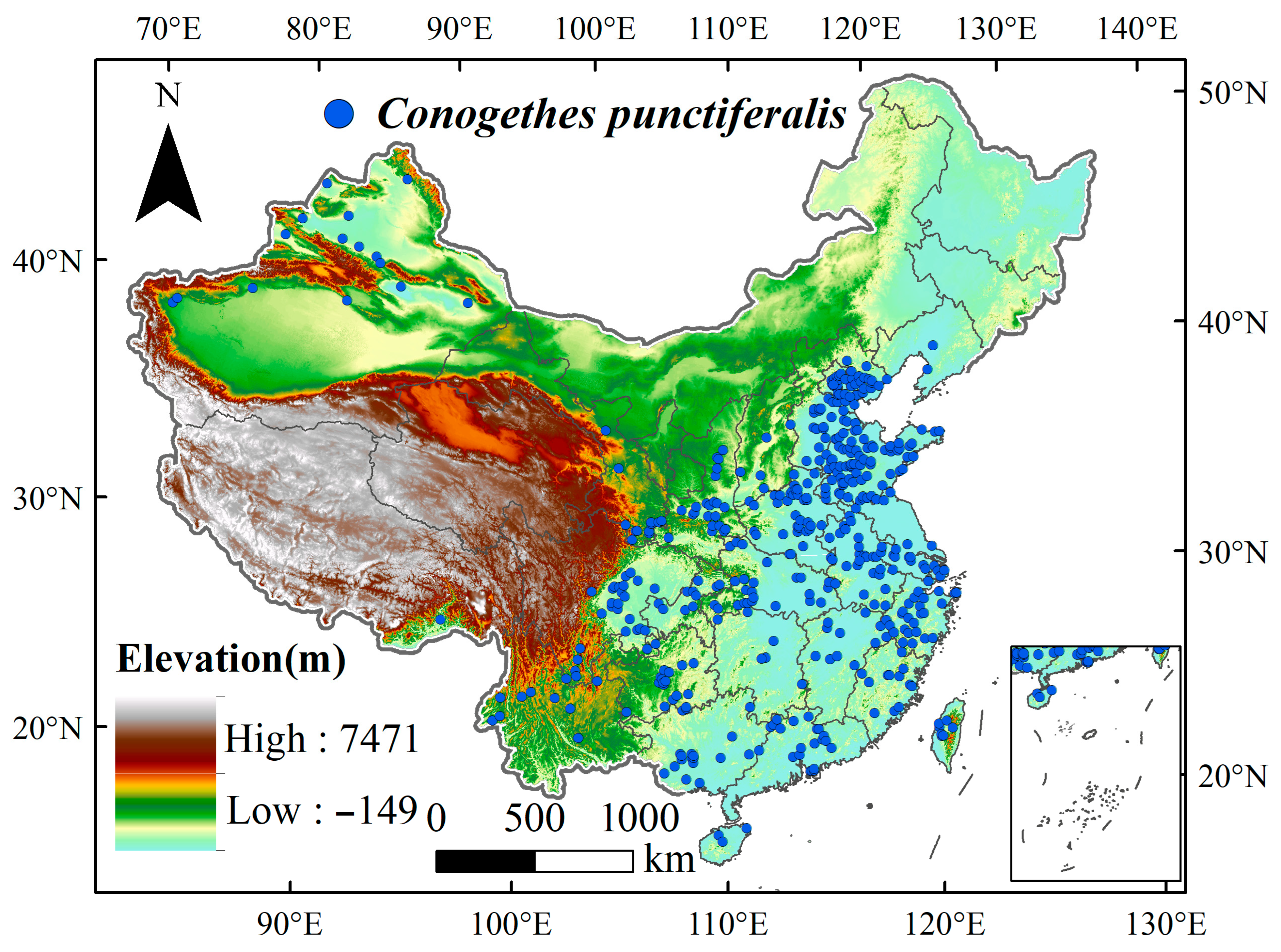


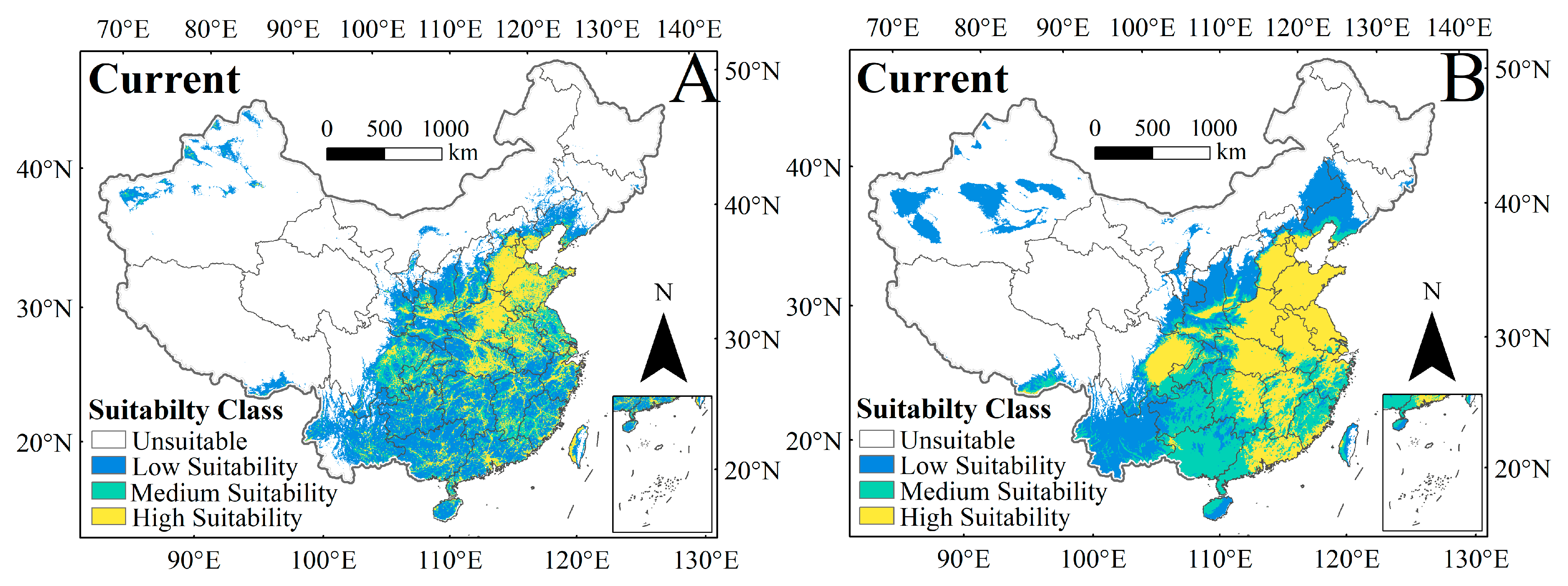



| Human Activity | Highly Suitable Habitat | Moderately Suitable Habitat | Low Suitable Habitat | Unsuitable Habitat |
|---|---|---|---|---|
| Without human activity | 1,222,586 | 1,014,189 | 1,366,816 | 5,996,409 |
| With human activity | 744,908 | 743,224 | 1,686,145 | 6,425,724 |
| Climate Scenario | Decades | Predicted Area (km2) and % of the Corresponding Current Area | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Suitable Region | Contraction | Unchanged | Expansion | Range Change | Contraction Percentage | Expansion Percentage | ||
| 1970–2000 | 3,174,276 | – | – | – | – | – | – | |
| SSP1-2.6 | 2050s | 3,266,075 | 193,198 | 2,980,340 | 278,834 | 2.89% | 6.09% | 8.78% |
| 2070s | 3,330,181 | 148,595 | 3,035,986 | 282,221 | 4.91% | 4.68% | 8.89% | |
| SSP2-4.5 | 2050s | 3,607,075 | 126,993 | 3,046,769 | 552,901 | 13.63% | 4.00% | 17.42% |
| 2070s | 3,805,135 | 80,491 | 3,103,696 | 695,362 | 19.87% | 2.54% | 21.91% | |
| SSP3-7.0 | 2050s | 3,413,899 | 181,632 | 2,991,672 | 410,956 | 7.55% | 5.72% | 12.95% |
| 2070s | 3,872,758 | 153,237 | 3,013,798 | 848,061 | 22.00% | 4.83% | 26.72% | |
| SSP5-8.5 | 2050s | 3,644,669 | 124,532 | 3,049,247 | 592,085 | 14.82% | 3.92% | 18.65% |
| 2070s | 3,897,987 | 122,095 | 3,062,109 | 824,306 | 22.80% | 3.85% | 25.97% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.-F.; Liu, Q.-Z.; Liu, J.; Ma, X.-Y.; He, F.-L. Impacts of Climate Change and Human Activity on the Potential Distribution of Conogethes punctiferalis in China. Insects 2025, 16, 998. https://doi.org/10.3390/insects16100998
Song C-F, Liu Q-Z, Liu J, Ma X-Y, He F-L. Impacts of Climate Change and Human Activity on the Potential Distribution of Conogethes punctiferalis in China. Insects. 2025; 16(10):998. https://doi.org/10.3390/insects16100998
Chicago/Turabian StyleSong, Cheng-Fei, Qing-Zhao Liu, Jiao Liu, Xin-Yao Ma, and Fa-Lin He. 2025. "Impacts of Climate Change and Human Activity on the Potential Distribution of Conogethes punctiferalis in China" Insects 16, no. 10: 998. https://doi.org/10.3390/insects16100998
APA StyleSong, C.-F., Liu, Q.-Z., Liu, J., Ma, X.-Y., & He, F.-L. (2025). Impacts of Climate Change and Human Activity on the Potential Distribution of Conogethes punctiferalis in China. Insects, 16(10), 998. https://doi.org/10.3390/insects16100998






