Starvation During the Larval Stage Driving Population Decline in the Butterfly Specialist Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sources
2.2. Larval Rearing and Starvation Treatments
2.3. Adult Mating and Oviposition
2.4. Data Analysis
2.4.1. Life Table Data Analysis
2.4.2. Population Projection
3. Analyses and Results
3.1. Effects of Starvation on Developmental Duration and Survival
3.2. Effects of Starvation on Adult Lifespan and Expectancy
3.3. Effects of Starvation on Butterfly Fecundity
3.4. Effects of Starvation on Butterfly Population Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of Climate Change on the Future of Biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent Responses to Climate Change Reveal the Drivers of Species Extinction and Survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Hung, K.-L.J.; Sandoval, S.S.; Ascher, J.S.; Holway, D.A. Joint Impacts of Drought and Habitat Fragmentation on Native Bee Assemblages in a California Biodiversity Hotspot. Insects 2021, 12, 135. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Further Evidence for a Global Decline of the Entomofauna. Austral Entomol. 2021, 60, 9–26. [Google Scholar] [CrossRef]
- Finn, C.; Grattarola, F.; Pincheira-Donoso, D. More Losers than Winners: Investigating Anthropocene Defaunation through the Diversity of Population Trends. Biol. Rev. 2023, 98, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.M.; Kawahara, A.Y.; Daniels, J.C.; Bateman, C.C.; Scheffers, B.R. Climate Change Effects on Animal Ecology: Butterflies and Moths as a Case Study. Biol. Rev. 2021, 96, 2113–2126. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as Bioindicator: A Hidden Gem for Environmental Monitoring. Front. Environ. Sci. 2023, 11, 1146052. [Google Scholar] [CrossRef]
- Warren, M.S.; Maes, D.; van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The Decline of Butterflies in Europe: Problems, Significance, and Possible Solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Karlsson, B.; Posledovich, D.; Toftegaard, T.; Wiklund, C.; Ehrlén, J.; Gotthard, K. Climate Change, Phenology, and Butterfly Host Plant Utilization. AMBIO 2015, 44, 78–88. [Google Scholar] [CrossRef]
- Herremans, M.; Gielen, K.; Van Kerckhoven, J.; Vanormelingen, P.; Veraghtert, W.; Swinnen, K.R.R.; Maes, D. Abundant Citizen Science Data Reveal That the Peacock Butterfly Aglais io Recently Became Bivoltine in Belgium. Insects 2021, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Bourn, N.; Buiman, C. Landscape-Scale Conservation for Butterflies and Moths: Lessons from the UK; Butterfly Conservation: Wareham, UK, 2012. [Google Scholar]
- Marini, L.; Zalucki, M.P. Density-Dependence in the Declining Population of the Monarch Butterfly. Sci. Rep. 2017, 7, 13957. [Google Scholar] [CrossRef]
- Ubach, A.; Páramo, F.; Prohom, M.; Stefanescu, C. Weather and Butterfly Responses: A Framework for Understanding Population Dynamics in Terms of Species’ Life-Cycles and Extreme Climatic Events. Oecologia 2022, 199, 427–439. [Google Scholar] [CrossRef]
- Hunter, A.F.; Lindgren, B.S. Range of Gypsy Moth in British Columbia: A Study of Climatic Suitability. J. Entomol. Soc. Br. Columbia 1995, 92, 45–56. [Google Scholar]
- Varela, L.G.; Bernays, E.A. Behavior of Newly Hatched Potato Tuber Moth Larvae, Phthorimaea operculella Zell. (Lepidoptera: Gelechiidae), in Relation to Their Host Plants. J. Insect Behav. 1988, 1, 261–275. [Google Scholar] [CrossRef]
- Hanspach, J.; Schweiger, O.; Kühn, I.; Plattner, M.; Pearman, P.B.; Zimmermann, N.E.; Settele, J. Host Plant Availability Potentially Limits Butterfly Distributions under Cold Environmental Conditions. Ecography 2014, 37, 301–308. [Google Scholar] [CrossRef]
- Curtis, R.J.; Brereton, T.M.; Dennis, R.L.H.; Carbone, C.; Isaac, N.J.B. Butterfly Abundance Is Determined by Food Availability and Is Mediated by Species Traits. J. Appl. Ecol. 2015, 52, 1676–1684. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Arnold, H.R.; Roy, D.B. Does Diet Breadth Control Herbivorous Insect Distribution Size? Life History and Resource Outlets for Specialist Butterflies. J. Insect Conserv. 2005, 9, 187–200. [Google Scholar] [CrossRef]
- Gotthard, K.; Nylin, S.; Wiklund, C. Adaptive Variation in Growth Rate: Life History Costs and Consequences in the Speckled Wood Butterfly, Pararge aegeria. Oecologia 1994, 99, 281–289. [Google Scholar] [CrossRef]
- Crone, E.E.; Schultz, C.B. Host Plant Limitation of Butterflies in Highly Fragmented Landscapes. Theor. Ecol. 2022, 15, 165–175. [Google Scholar] [CrossRef]
- Jones, L.C. Insects Allocate Eggs Adaptively According to Plant Age, Stress, Disease or Damage. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220831. [Google Scholar] [CrossRef]
- Rausher, M.D.; Papaj, D.R. Demographic Consequences of Descrimination among Conspecific Host Plants by Battus Philenor Butterflies. Ecology 1983, 64, 1402–1410. [Google Scholar] [CrossRef]
- Chen, L. Population Ecology of Luehdorfia chinensis in Taohongling, Jiangxi. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2023. [Google Scholar]
- Xu, X.L. A Discussion on the Distribution Boundaries of Luehdorfia chinensis. Agric. Technol. 2014, 34, 254. [Google Scholar]
- Yuan, D.C.; Mai, G.Q.; Xue, D.Y.; Hu, C.; Ye, G.Y. The Habitat Biology and Conservation Status of Luehdorfia chinensis (Lepidoptera: Papilionidae). Chin. Biodivers. 1998, 6, 26–36. [Google Scholar] [CrossRef]
- Stetten, J.; Bozano, G. Tribes Zerynthiini, Luehdorfini. Subfamily Parnasiinae. In Guide to the Butterflies of the Palearctic Region. Papilionidae, Part III; Bozano, G.M., Ed.; Omnes Artes: Milan, Italy, 2021; p. 96. [Google Scholar]
- Hu, C.; Wu, X.J.; Wang, X.M. The Biology of Luehdorfia chinensis Leech, A Rare and Endangered Butterfly. Acta Entomol. Sin. 1992, 35, 195–199. [Google Scholar] [CrossRef]
- Li, C.L. The Early Stages of Chinese Rhopalocera—Luehdorfia chinensis Leech (Parnassiidae: Zerynthiinae). Acta Entomol. Sin. 1978, 21, 161–163, 239. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, C. A Synopsis of the Chinese Species of Asarum (Aristolochiaceae). J. Arnold Arbor. 1983, 64, 565–597. [Google Scholar] [CrossRef]
- Liu, S.; Xian, Z.; Zhao, Y.; Wang, L.; Tian, J.; Pan, C.; Han, J.; Zhang, Y.; Li, C.; Yi, Y.; et al. Quantitative Determination and Toxicity Evaluation of Aristolochic Acid Analogues in Asarum heterotropoides F. Schmidt (Xixin) and Traditional Chinese Patent Medicines. Front. Pharmacol. 2021, 12, 761593. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Yang, W.J.; Wu, W.G.; Liu, X.H.; Zhang, Y.; Zeng, J.P. Traits Variability of Asarum forbesii and Conservation Implications to the Rare Butterfly of Luehdorfia chinensis in Taohongling, South China. Acta Agric. Univ. Jiangxiensis 2022, 44, 1122–1134. [Google Scholar] [CrossRef]
- Zou, M.H. Limitation Effect and Growth Conditions of the Hostplant Asarum forbesii on the Butterfly Population of Luehdorfia chinensis in Taohongling. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2025. [Google Scholar]
- Zalucki, M.P.; Lammers, J.H. Dispersal and Egg Shortfall in Monarch Butterflies: What Happens When the Matrix Is Cleaned Up? Ecol. Entomol. 2010, 35, 84–91. [Google Scholar] [CrossRef]
- He, G.Q.; Jia, F.H.; Zhu, H.B. The Species Distribution and Quantity Surveying of Luehdorfia chinese (Leech) in JiangXi. J. Jiangxi Univ. TCM 2011, 23, 75–76. [Google Scholar]
- Su, J. Geographical Distributions, Environmental Niches and Conservation in the Rare Butterflies of Luehdorfia spp. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2020. [Google Scholar]
- Wang, D.Q.; Huang, S.H. Medicinal Plants of Asarumin Anhui Province. China J. Chin. Mater. Med. 1989, 14, 198–200. [Google Scholar]
- Nie, A.Z.; Bian, M.; Zhu, C.S.; Gao, M.M. Mechanism of Asari Radix et Rhizoma water extract induced liver injury based on proteomics. Chin. Tradit. Herb. Drugs 2024, 55, 5145–5153. [Google Scholar]
- Jeong, H.J.; Kim, J.G. Small-Scale Spatial Genetic Structure of Asarum sieboldii Metapopulation in a Valley. J. Ecol. Environ. 2021, 45, 11. [Google Scholar] [CrossRef]
- Yang, Z.L. The Geographical Distribution of Asarum (Aristolochiaceae) from Sichuan Province in China. Guihaia 1988, 81, 83–88. [Google Scholar]
- Jonathan, B.; Craig, H.-T. Red List of Threatened Species: A Global Species Assessment; IUCN-The World Conservation Union: Gland, Switzerland, 2004. [Google Scholar]
- Taohongling Sika Deer Reserve. Jiangxi Taohongling Meihualu Baohuqu; China Forestry Publishing House: Beijing, China, 2000. [Google Scholar]
- Guo, H.; Jia, N.; Chen, H.; Xie, D.; Chi, D. Preliminary Analysis of Transcriptome Response of Dioryctria sylvestrella (Lepidoptera: Pyralidae) Larvae Infected with Beauveria bassiana under Short-Term Starvation. Insects 2023, 14, 409. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two New Methods for the Study of Insect Population Ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Iranipour, S.; Mahmoodi Arabi, S.; Michaud, J.P. Does the Two-Sex Life Table for Sexual Populations Invalidate Those Based Solely on Female Cohorts? Ann. Entomol. Soc. Am. 2025, 118, 189–205. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, F.; Tan, X.; Zhang, T.; Teng, Z.; Fan, Y.; Wan, F.; Zhou, H. Use of Age-Stage, Two-Sex Life Table to Compare the Fitness of Bactrocera dorsalis (Diptera: Tephritidae) on Northern and Southern Host Fruits in China. Insects 2022, 13, 258. [Google Scholar] [CrossRef]
- Abbes, K.; Harbi, A.; Guerrieri, E.; Chermiti, B. Using Age-Stage Two-Sex Life Tables to Assess the Suitability of Three Solanaceous Host Plants for the Invasive Cotton Mealybug Phenacoccus solenopsis Tinsley. Plants 2024, 13, 1381. [Google Scholar] [CrossRef]
- Rismayani Ullah, M.S.; Chi, H.; Gotoh, T. Impact of Constant and Fluctuating Temperatures on Population Characteristics of Tetranychus pacificus (Acari: Tetranychidae). J. Econ. Entomol. 2021, 114, 638–651. [Google Scholar] [CrossRef]
- Li, W.; Bashir, N.H.; Naeem, M.; Tian, R.; Tian, X.; Chen, H. Age-Stage, Two-Sex Life Table of Atractomorpha lata (Orthoptera: Pyrgomorphidae) at Different Temperatures. Insects 2024, 15, 493. [Google Scholar] [CrossRef]
- El Aalaoui, M.; Sbaghi, M.; Mokrini, F. Effect of Temperature on the Development and Reproduction of Olive Psyllid Euphyllura olivina Costa (Hemiptera: Psyllidae). Crop Prot. 2025, 190, 107131. [Google Scholar] [CrossRef]
- Bankar, D.R.; Bhamare, V.K. Comparative Biology, Life Tables and Intrinsic Rate of Increase of Spodoptera frugiperda (J.E. Smith) Reared on Pearl Millet and Sugarcane. J. Entomol. Res. 2023, 47, 866–870. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MSChart: A Computer Program for the Population Projection Based on Age-Stage, Two-Sex Life Table. Available online: https://zenodo.org/records/7484085 (accessed on 20 January 2020).
- Chi, H. TWOSEX-MSChart: A Computer Program for Age Stage, Two-Sex Life Table Analysis; National Chung Hsing University: Taichung, Taiwan, 2018. [Google Scholar]
- Lehmann, P.; Ammunét, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemelä, P.; Terblanche, J.S.; et al. Complex Responses of Global Insect Pests to Climate Warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Chen, A.; Liu, B.; Zhou, R.; Zhang, H.; Zhou, L.; Xie, X.; Zhuo, Z.; Xu, D. Habitat Suitability Analysis for Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae) in the Middle and Lower Yangtze River: A Study Based on the MaxEnt Model. Insects 2025, 16, 396. [Google Scholar] [CrossRef]
- Wang, R.P.; Li, L. The Extinction Vortex of Small Population. J. Biol. 2008, 25, 14–16. [Google Scholar]
- Bin, W.; Wang, W.; Wang, H.; He, G. A Retrospective Analysis on the Population Viability of the Yangtze River Dolphin or Baiji (Lipotes vexillifer). Indian J. Anim. Res. 2020, 56, 775–779. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Towards a Functional Resource-Based Concept for Habitat: A Butterfly Biology Viewpoint. Oikos 2003, 102, 417–426. [Google Scholar] [CrossRef]
- Iutzi, F.; Crews, T. Perennializing Grain Crop Agriculture: A Pathway; The Land Institute: Salina, KS, USA, 2020; pp. 823–5376. [Google Scholar]
- McCue, M.D. Starvation Physiology: Reviewing the Different Strategies Animals Use to Survive a Common Challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef]
- McKay, A.F.; Ezenwa, V.O.; Altizer, S. Consequences of Food Restriction for Immune Defense, Parasite Infection, and Fitness in Monarch Butterflies. Physiol. Biochem. Zool. 2016, 89, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Bauerfeind, S.S.; Fischer, K. Effects of Food Stress and Density in Different Life Stages on Reproduction in a Butterfly. Oikos 2005, 111, 514–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zheng, J.; Li, T.; Zhao, L. Effects of Larval Starvation Stress on the Life History and Adult Fitness of Fall Webworm, Hyphantria cunea. Insects 2025, 16, 410. [Google Scholar] [CrossRef]
- Brueggemann, L.; Singh, P.; Müller, C. Life Stage- and Sex-Specific Sensitivity to Nutritional Stress in a Holometabolous Insect. Ecol. Evol. 2025, 15, e70764. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Fiedler, K. Effects of Larval Starvation on Adult Life-History Traits in the Butterfly Species Lycaena tityrus (Lepidoptera: Lycaenidae). Entomol. Gen. 2001, 25, 249–254. [Google Scholar] [CrossRef]
- Lee, K.P.; Kwon, S.-T.; Roh, C. Caterpillars Use Developmental Plasticity and Diet Choice to Overcome the Early Life Experience of Nutritional Imbalance. Anim. Behav. 2012, 84, 785–793. [Google Scholar] [CrossRef]
- Bauerfeind, S.S.; Fischer, K. Effects of Larval Starvation and Adult Diet-Derived Amino Acids on Reproduction in a Fruit-Feeding Butterfly. Entomol. Exp. Appl. 2009, 130, 229–237. [Google Scholar] [CrossRef]
- Elkin, C.M.; Reid, M.L. Low Energy Reserves and Energy Allocation Decisions Affect Reproduction by Mountain Pine Beetles, Dendroctonus ponderosae. Funct. Ecol. 2005, 19, 102–109. [Google Scholar] [CrossRef]
- García-Roger, E.M.; Martínez, A.; Serra, M. Starvation Tolerance of Rotifers Produced from Parthenogenetic Eggs and from Diapausing Eggs: A Life Table Approach. J. Plankton Res. 2006, 28, 257–265. [Google Scholar] [CrossRef]
- Billings, A.C.; Schultz, K.E.; Hernandez, E.A.; Jones, W.E.; Price, D.K. Male Courtship Behaviors and Female Choice Reduced during Experimental Starvation Stress. Behav. Ecol. 2019, 30, 231–239. [Google Scholar] [CrossRef]
- Gols, R.; Croijmans, L.; Dicke, M.; van Loon, J.J.A.; Harvey, J.A. Plant Quantity Affects Development and Reproduction of a Gregarious Butterfly More than Plant Quality. Entomol. Exp. Appl. 2022, 170, 646–655. [Google Scholar] [CrossRef]
- Zhao, X.; Geng, Y.; Hu, T.; Xie, C.; Xu, W.; Zuo, Z.; Xue, M.; Hao, D. Ecological Strategies of Hyphantria cunea (Lepidoptera: Arctiidae) Response to Different Larval Densities. Front. Ecol. Evol. 2023, 11, 1177029. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Zhang, S.-S.; Niu, B.-L.; Ji, D.-F.; Liu, X.-J.; Li, M.-W.; Bai, H.; Palli, S.R.; Wang, C.-Z.; Tan, A.-J. A Determining Factor for Insect Feeding Preference in the Silkworm, Bombyx mori. PLoS Biol. 2019, 17, e3000162. [Google Scholar] [CrossRef]
- Boggs, C.L. Understanding Insect Life Histories and Senescence through a Resource Allocation Lens. Funct. Ecol. 2009, 23, 27–37. [Google Scholar] [CrossRef]
- Fagan, W.F.; Holmes, E.E. Quantifying the Extinction Vortex. Ecol. Lett. 2006, 9, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, M.E.; Soule, M.E. Minimum Viable Populations: Processes of Extinction. In Conservation Biology: The Science of Scarcity and Diversity; Soule’, M.E., Ed.; Sinauer Associates: Sunderland, MA, USA, 1986; pp. 19–34. [Google Scholar]
- Nordstrom, S.W.; Hufbauer, R.A.; Olazcuaga, L.; Durkee, L.F.; Melbourne, B.A. How Density Dependence, Genetic Erosion and the Extinction Vortex Impact Evolutionary Rescue. Proc. R. Soc. B 2023, 290, 20231228. [Google Scholar] [CrossRef] [PubMed]
- Brunbjerg, A.K.; Høye, T.T.; Eskildsen, A.; Nygaard, B.; Damgaard, C.F.; Ejrnæs, R. The Collapse of Marsh Fritillary (Euphydryas aurinia) Populations Associated with Declining Host Plant Abundance. Biol. Conserv. 2017, 211, 117–124. [Google Scholar] [CrossRef]
- James, D.G. Monarch Butterflies in Western North America: A Holistic Review of Population Trends, Ecology, Stressors, Resilience and Adaptation. Insects 2024, 15, 40. [Google Scholar] [CrossRef]
- Lukens, L.; Thieme, J.; Thogmartin, W.E. Milkweed and Floral Resource Availability for Monarch Butterflies (Danaus plexippus) in the United States. Front. Ecol. Evol. 2024, 12, 1330583. [Google Scholar] [CrossRef]
- Kukkonen, J.M.; von Numers, M.; Brommer, J.E. Conserving Apollo Butterflies: Habitat Characteristics and Conservation Implications in Southwest Finland. J. Insect Conserv. 2024, 28, 1199–1210. [Google Scholar] [CrossRef]
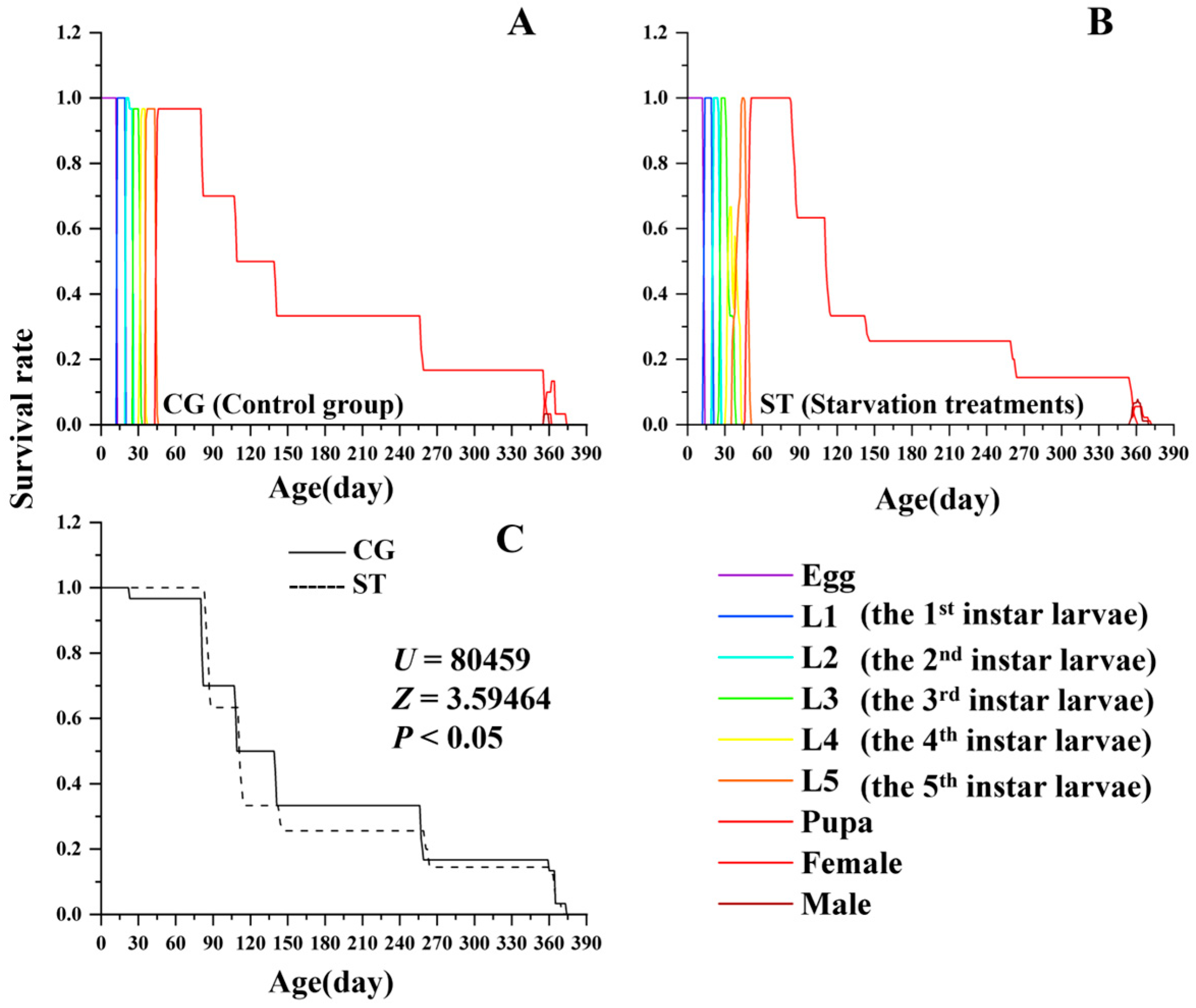

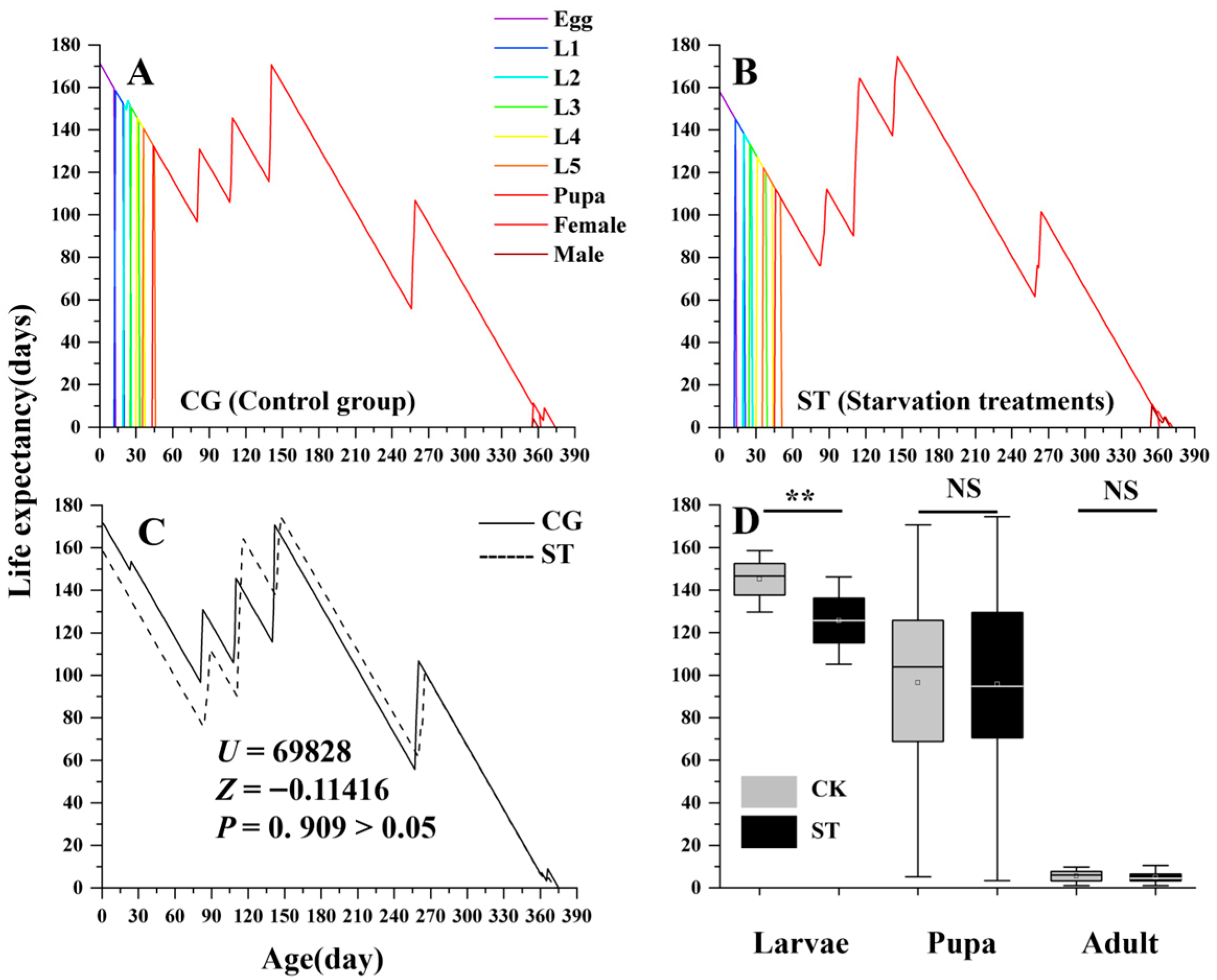
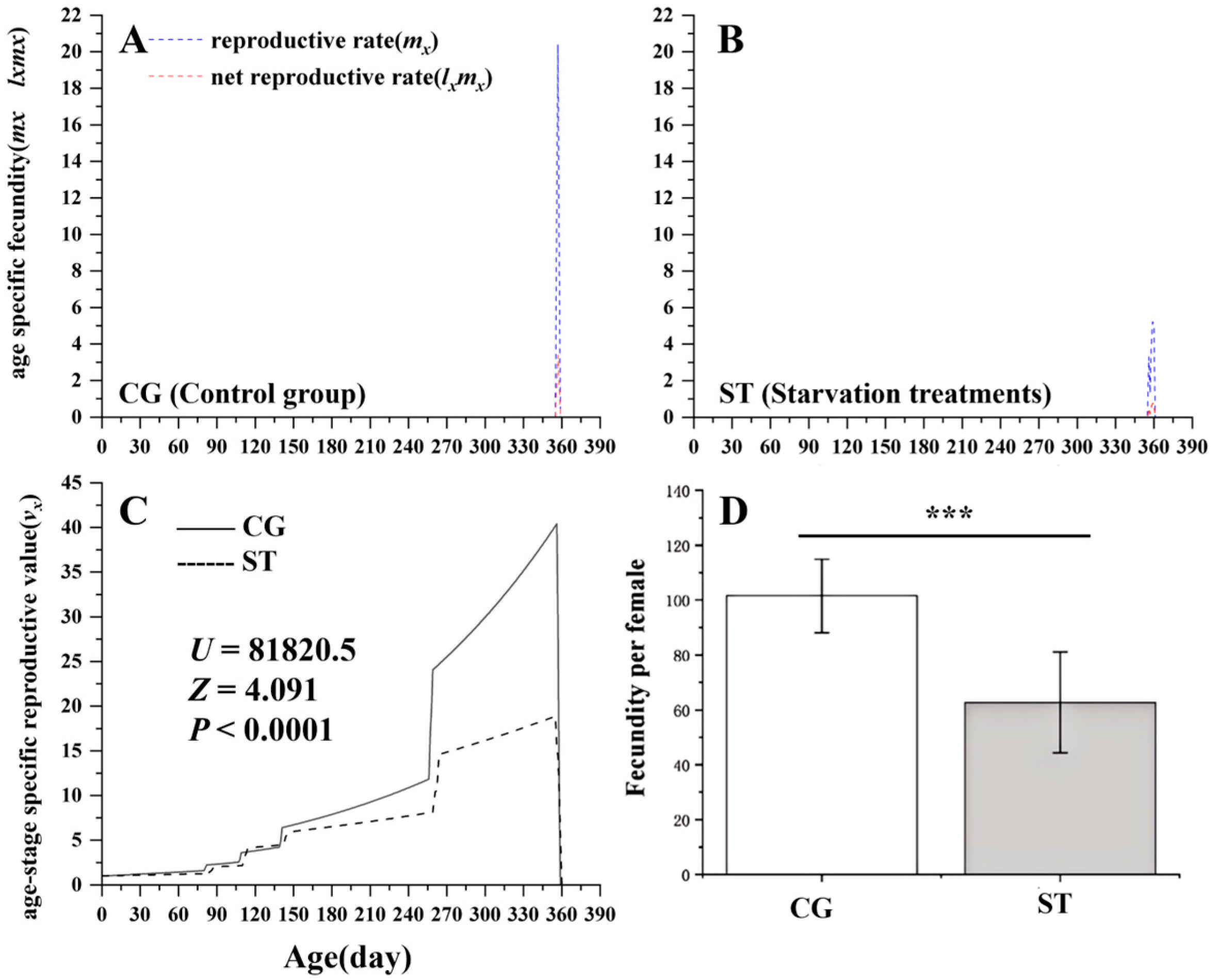
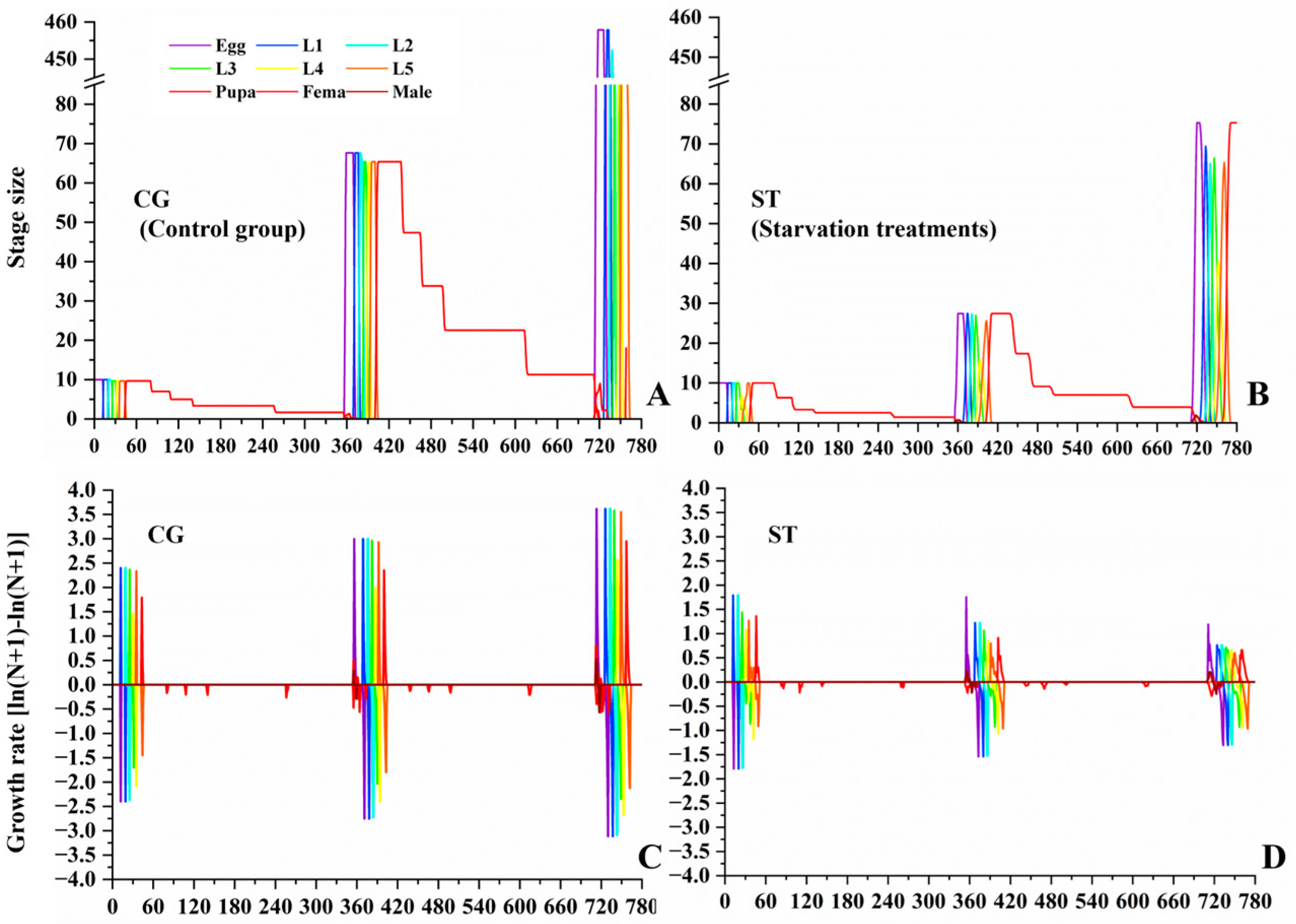
| Stages | CG (n) | Starvation Treatments (ST) | Kruskal–Wallis Test | ||
|---|---|---|---|---|---|
| 3rd (n) | 4th (n) | 5th (n) | |||
| egg | 13.00 ± 0.00 a (30) | 13.97 ± 0.18 a (30) | 13.33 ± 0.48 a (30) | 13.20 ± 0.41 a (30) | χ2 = 6.86538, df = 3, p = 0.07631 |
| 1st instar larvae | 7.00 ± 0.00 a (30) | 7.00 ± 0.00 a (30) | 7.00 ± 0.00 a (30) | 7.00 ± 0.00 a (30) | χ2 = 0, df = 3, p = 1 |
| 2nd instar larvae | 6.00 ± 0.00 a (30) | 6.00 ± 0.00 a (30) | 5.83 ± 0.38 a (30) | 6.00 ± 0.00 a (30) | χ2 = 6.40, df = 3, p = 0.0938 |
| 3rd instar larvae | 5.69 ± 0.10 b (30) | 11.60 ± 0.40 a (30) | 5.47 ± 0.51 b (30) | 5.73 ± 0.57 b (30) | χ2 = 86.39, df = 3, p < 0.0001 |
| 4th instar larvae | 4.34 ± 0.09 b (30) | 4.77 ± 0.43 b (30) | 7.03 ± 0.18 a (30) | 4.33 ± 0.48 b (30) | χ2 = 83.23, df = 3, p < 0.0001 |
| 5th instar larvae | 8.48 ± 0.09 b (30) | 7.20 ± 0.41 c (30) | 8.37 ± 0.49 b (30) | 11.23 ± 0.41 a (30) | χ2 = 100.83, df = 3, p < 0.0001 |
| Larval duration | 31.37 ± 0.76 c (30) | 35.93 ± 0.64 a (30) | 33.77 ± 0.90 b (30) | 33.97 ± 0.72 b (30) | χ2 = 99.97, df = 3, p < 0.0001 |
| Pupa | 313.2 ± 0.97 a (14) | 308.14 ± 0.71 b (6) | 309.00 ± 0.00 b (2) | 308.75 ± 0.85 b (5) | χ2 = 9.83, df = 3, p < 0.05 |
| Pre-adult emergence (Female) | 365.20 + 3.56 a (8) | 359.5 ± 0.5 b (2) | 358 (1) | 356.33 + 2.33 b (3) | χ2 = 8.79, df = 2, p < 0.05 |
| Pre-adult emergence (Male) | 365.75 + 3.41 a (6) | 357.4 + 4.8 b (4) | 358 (1) | 356.50 + 1.36 b (2) | χ2 = 7.02, df = 2, p < 0.05 |
| Population Parameters | CG (n = 30) | ST (n = 90) |
|---|---|---|
| Intrinsic rate of increase (r) | 0.0064 ± 0.0026 a | 0.0033 ± 0.0016 b |
| Net reproductive rate (R0) | 6.77 ± 4.72 a | 2.74 ± 1.4 b |
| Finite rate of increase (λ) | 1.0064 ± 0.0027 a | 1.0033 ± 0.0017 a |
| Total fecundity (F) | 40.6 ± 27.53 a | 19 ± 8.92 b |
| Mean generation time (T) | 357.87 ± 0.69 a | 358.66 ± 0.84 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Zhu, Q.; Zou, Y.; Yang, C.; Wu, W.; Zou, Q.; Zeng, J. Starvation During the Larval Stage Driving Population Decline in the Butterfly Specialist Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae). Insects 2025, 16, 995. https://doi.org/10.3390/insects16100995
Yang W, Zhu Q, Zou Y, Yang C, Wu W, Zou Q, Zeng J. Starvation During the Larval Stage Driving Population Decline in the Butterfly Specialist Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae). Insects. 2025; 16(10):995. https://doi.org/10.3390/insects16100995
Chicago/Turabian StyleYang, Wenjing, Qi Zhu, Yunhao Zou, Chao Yang, Wenguo Wu, Qin Zou, and Juping Zeng. 2025. "Starvation During the Larval Stage Driving Population Decline in the Butterfly Specialist Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae)" Insects 16, no. 10: 995. https://doi.org/10.3390/insects16100995
APA StyleYang, W., Zhu, Q., Zou, Y., Yang, C., Wu, W., Zou, Q., & Zeng, J. (2025). Starvation During the Larval Stage Driving Population Decline in the Butterfly Specialist Luehdorfia chinensis Leech, 1893 (Lepidoptera: Papilionidae). Insects, 16(10), 995. https://doi.org/10.3390/insects16100995





