Valorization of Water Lettuce (Pistia stratiotes L.) Through Bioconversion for Black Soldier Fly Larvae (Hermetia illucens): Larvae Growth, Survival Rate, and Nutritional Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Obtaining Black Soldier Fly Larvae
2.3. Rearing Substrates
2.4. Experimental Design and Follow-Up
2.5. Larvae Harvest
2.6. Chemical Analysis of the Larvae Samples
2.7. Growth Performance and Survival Rate of the BSLF and the Substrate Utilization Estimates
2.8. Data Analysis
3. Results
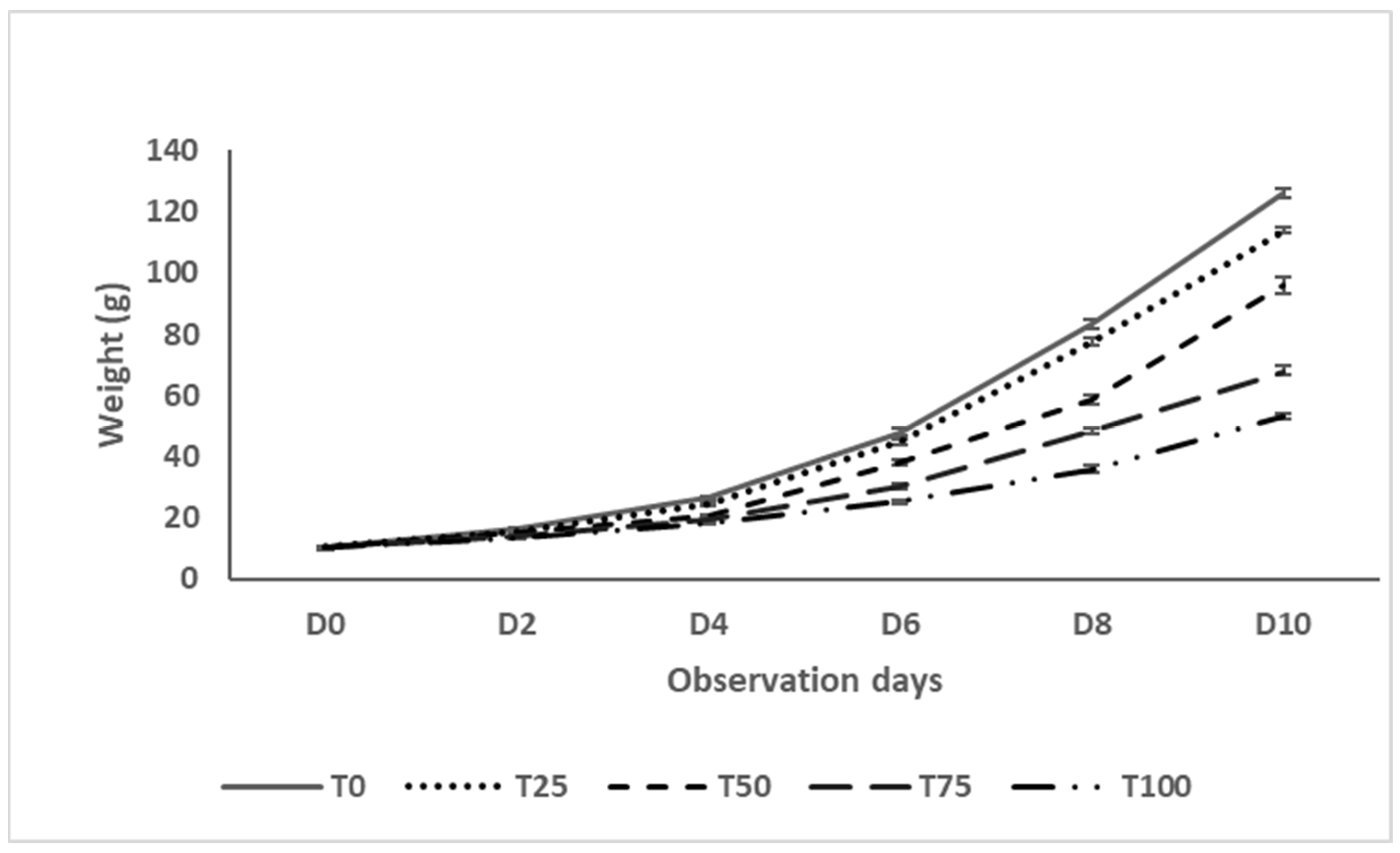
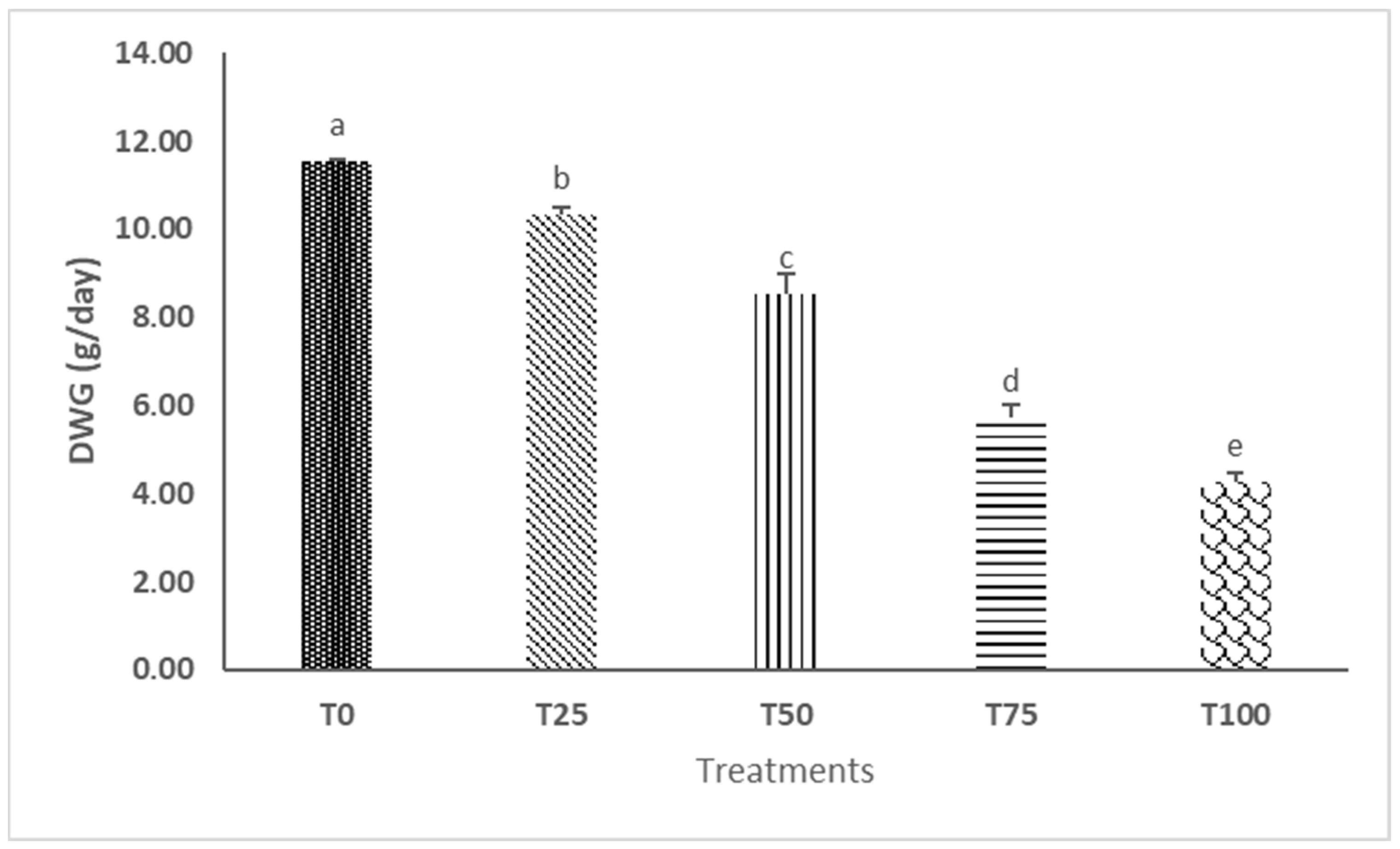
3.1. Growth, Survival Rate, and Substrate Utilization Estimates
3.2. Nutritional Quality of black Soldier Fly Larvae (BSFL) from Different Substrates
4. Discussion
4.1. Growth, Survival Rate, and Substrate Utilization Estimates
4.2. Nutritional Quality of Black Soldier Fly Larvae (BSFL) Produced Using Substrates Made from Water Lettuce Leaves
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odongo, E.E.; Bbosa, W.K.; Kahunde, P.K. Black soldier fly (BSF): A sustainable solution for protein, waste management, and a circular bio-economy. Eur. J. Theor. Appl. Sci. 2024, 2, 822–834. [Google Scholar] [CrossRef]
- Rodrigues, D.P.; Calado, R.; Pinho, M.; Domingues, M.R.; Vázquez, J.A.; Ameixa, O.M. Bioconversion and performance of black soldier fly (Hermetia illucens) in the recovery of nutrients from expired fish feeds. Waste Manag. Res. 2022, 141, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Z.; Zhang, J.; Zhou, S.; Xiong, Q. Conversion of mixtures of soybean curd residue and kitchen waste by black soldier fly larvae (Hermetia illucens L.). Insects 2022, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Pliantiangtam, N.; Chundang, P.; Kovitvadhi, A. Growth performance, waste reduction efficiency and nutritional composition of black soldier fly (Hermetia illucens) larvae and prepupae reared on coconut endosperm and soybean curd residue with or without supplementation. Insects 2021, 12, 682. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture: Influencing Action toward Sustainable Fisheries and Aquaculture; Sustainability in action: Gosnells, WA, Australia, 2020. [Google Scholar] [CrossRef]
- Agbohessou, P.S.; Mandiki, S.N.; Gougbédji, A.; Megido, R.C.; Hossain, M.S.; De Jaeger, P.; Larondelle, Y.; Francis, F.; Lalèyè, P.A.; Kestemont, P. Total replacement of fish meal by enriched-fatty acid Hermetia illucens meal did not substantially affect growth parameters or innate immune status and improved whole body biochemical quality of Nile tilapia juveniles. Aquac. Nutr. 2021, 32, 47–53. [Google Scholar] [CrossRef]
- Vodounnou, J.V.; Iko, R.; Okou, G.; Kpogue, D.; Montcho, S.A.; Micha, J.-C. Complete substitution of fish meal with black soldier flies Hermetia illucens (L. 1758) larvae meal at varying incorporation rates for feeding Oreochromis niloticus raised in captivity. Aquac. Sci. Manag. 2025, 2, 1. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed. Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.-J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422, 193–201. [Google Scholar] [CrossRef]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. J. Z. Naturforsch C Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Nagpal, R.; Mishra, O.P.; Bhardwaj, N.K.; Mahajan, R. Valorization of Agro-industrial Residue-Rice Straw for Manufacturing Better Quality Paper Using Cleaner, Ultrafiltered Xylano-Pectinolytic Enzymatic Pulping Strategy. Waste Biomass Valorization 2022, 13, 4851–4859. [Google Scholar] [CrossRef]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Black soldier fly-based bioconversion of biosolids creates high-value products with low heavy metal concentrations. Resour. Conserv. Recycl. 2022, 180, 106–149. [Google Scholar] [CrossRef]
- Vodounnou, J.V.; Iko, R.; Sintondji, W.; Tossavi, E.; Kpogue, D.; Micha, J.-C. Rearing of black soldier fly (Hermetia illucens)blarvae as a tool for managing agricultural byproducts. Discov. Agric. 2024, 2, 91. [Google Scholar] [CrossRef]
- Vodounnou, J.V.; Dossa, V.; Djissou, C.; Kpogue, D.; Agadjihouede, H.; Fiogbe, E.D.; Micha, J.-C. Feeding Optimization of Water Hyacinth (Eichhornia crassipes) Leaves as Rearing Substrate for the Production of Black Soldier Fly (Hermetia illucens) Larvae. Waste Biomass Valorization 2024, 15, 3569–3578. [Google Scholar] [CrossRef]
- Ajuonu, O.; Schade, V.; Veltman, B.; Sedjro, K.; Neuenschwander, P. Impact of the weevils Neochetina eichhorniae and N. bruchi (Coleoptera: Curculionidae) on water hyacinth, Eichhornia crassipes (Pontederiaceae), in Benin, West Africa. Afr. Entomol. 2003, 11, 153–171. [Google Scholar]
- Babu, R.M.; Sajeena, A.; Seetharaman, K. Bioassay of the potentiality of Alternaria alternata (Fr.) Keissler as a bioherbicide to control water hyacinth and other aquatic weeds. Crop Prot. 2003, 22, 1005–1013. [Google Scholar] [CrossRef]
- Boyette, C.D.; Quimby, P.C.J.R.; Bryson, C.T.; Egley, G.T.; Fulgham, F.E. Biological control of hemp sesbania (Sesbania exaltata) under field conditions with Colletotrichum truncatum formulated in an invert emulsion. Weed Sci. 1993, 41, 497–500. [Google Scholar] [CrossRef]
- Fayad, Y.H.; Ibrahim, A.A.; El-Zoghbyet, A.A.; Shalaby, F.F. Ongoing activities in the biological control of water hyacinth in Egypt. In Biological and Integrated Control of Water Hyacinth, Eichhornia Crassipes, Proceedings of the Second Meeting of the Global Working Group for the Biological and Integrated Control of Water Hyacinth, Beijing, China, 9–12 October 2000; Julien, M.H., Hill, M.P., Center, T.D., Ding, J., Eds.; Australian Centre for International Agricultural Research: Canberra, Australia, 2001. ACIAR Proceeding. Volume 102. [Google Scholar]
- Cilliers, C.J. Biological control of water lettuce, P. stratiotes (Araceae), in South Africa. Agric. Ecosyst. Environ. 1991, 37, 225–229. [Google Scholar] [CrossRef]
- Henry-Silva, G.G.; Camargo, A.F.M.; Pezzato, M.M. Growth of free-floating aquatic macrophytes in different concentrations of nutrients. Hydrobiologia 2008, 610, 153–160. [Google Scholar] [CrossRef]
- Sajna, N.; Haler, M.; Skornik, S.; Kaligaric, M. Survival and expansion of P. stratiotes L. in a thermal stream in Slovenia. Aquat. Bot. 2007, 87, 75–79. [Google Scholar] [CrossRef]
- Gougbedji, A.; Agbohessou, P.; Lalèyè, P.; Francis, R.; Megido, C.R. Technical basis for the small-scale production of black soldierly, Hermetia illucens (L. 1758), meal as Fish feed in Benin. J. Agric. Food Res. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- AOAC. Oicial Methods of Analysis of the Association of Oicial Analytical Chemists, 15th ed.; AOAC: Arlington, TX, USA, 1990. [Google Scholar]
- Bremner, J.M.; Mulvaney, R.G. Nitrogen Total. In Method of Soil Analysis. Part II, Chemical and Microbiological Methods, 2nd ed.; Agronomy Monograph No. 9; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Vodounnou, D.S.J.V.; Kpogue, D.N.S.; Tossavi, C.E.; Mennsah, G.A.; Fiogbe, E.D. Effect of animal waste and vegetable compost on production and growth of earthworm (Eisenia fetida) during vermiculture. Int. J. Recycl. Org. Waste Agric. 2016, 5, 87–92. [Google Scholar] [CrossRef]
- Jayanegara, A.; Novandri, B.; Yantina, N.; Ridla, M. Use of BSF larvae (Hermetia illucens) to substitute soybean meal in ruminant diet: An in vitro rumen fermentation study. Vet. World 2017, 10, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Hervás, G.; González-Rosales, M.G.; Mendoza, A.G.; Robles-Jiménez, L.E.; Frutos, P. Insects as alternative feed for ruminants: Comparison of protein evaluation methods. J. Anim. Sci. Biotechnol. 2022, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Falabella, P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustainability 2021, 13, 10198. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Hopkins, I.; Newman, L.P.; Gill, H.; Danaher, J. The influence of food waste rearing substrates on black soldier fly larvae protein composition: A systematic review. Insects 2021, 12, 608. [Google Scholar] [CrossRef]
- Nugroho, R.A.; Aryani, R.; Hardi, E.H.; Manurung, H.; Rudianto, R.; Wirawan, N.A.; Syalsabillah, N.; Jati, W.N. Nutritive value, material reduction, biomass conversion rate, and survival of black solider fly larvae reared on palm kernel meal supplemented with fish pellets and fructose. Int. J. Trop. Insect Sci. 2023, 43, 1243–1254. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Sumner, S.M. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Ann. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Da-Silva, W.C.; da Silva, É.B.R.; da Silva, J.A.R.; Martorano, L.G.; Belo, T.S.; Sousa, C.E.L.; Camargo-Júnior, R.N.C.; Andrade, R.L.; Santos, A.G.d.S.; de Carvalho, K.C.; et al. Nutritional Value of the Larvae of the Black Soldier Fly (Hermetia illucens) and the House Fly (Musca domestica) as a Food Alternative for Farm Animals—A Systematic Review. Insects 2024, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Tomberlin, T.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Sharanabasappa, S.D.; Srikanth, B.H.; Manju, G.U.; Nandhini, D.; Revannavar, R.; Pavana, J.K.; Pradeep, S.; Sridhara, S. Nutritional composition of black soldier fly, Hermetia illucens (L.) during various life stages reared on vegetable and fruit waste. Entomol. News 2024, 131, 121–128. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, 0182601. [Google Scholar] [CrossRef]
- Adebayo, H.A.; Kemabonta, K.A.; Ogbogu, S.S.; Elechi, M.C.; Obe, M.T. Comparative assessment of developmental parameters, proximate analysis and mineral compositions of black soldier fly (Hermetia illucens) prepupae reared on organic waste substrates. Int. J. Trop. Insect Sci. 2021, 2, 1953–1959. [Google Scholar] [CrossRef]
- Eggink, K.M.; Donoso, I.G.; Dalsgaard, J. Optimal dietary protein to carbohydrate ratio for black soldier fly (Hermetia illucens) larvae. J. Insects Food Feed. 2023, 9, 789–798. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed. 2015, 1, 249–259. [Google Scholar] [CrossRef]
| Parameters | Plant Part | |
|---|---|---|
| Leaves | Roots | |
| Organic matter (%) | 63.25 ± 0.22 | 54.85 ± 0.19 |
| Ash (%) | 36.74 ± 0.14 | 45.15 ± 0.16 |
| Crude protein (%) | 7.12 ± 0.10 | 3.08 ± 0.12 |
| Crude lipids (%) | 2.8 ± 0.08 | 1.74 ± 0.05 |
| Crude fiber (%) | 18.11 ± 0.11 | 21.6 ± 0.09 |
| Ingredients | T0 | T25 | T50 | T75 | T100 | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Farm Feed for BSFL | 100 | 75 | 50 | 25 | 0 | - | - |
| Water Lettuce LeaF | 0 | 25 | 50 | 75 | 100 | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | - | - |
| Parameters for the Physio-chemical and Biochemical Compositions of the Substrates | |||||||
| pH | 7.56 ± 0.12 a | 7.03 ± 0.39 a | 7.06 ± 0.21 a | 7.13 ± 0.43 a | 7.60 ± 0.15 a | 0.91 | 0.49 |
| Dry Matter (%) | 92.30 ± 0.12 a | 91.70 ± 0.36 a | 91.46 ± 0.17 ab | 90.70 ± 0.40 b | 89.66 ± 0.20 c | 13.37 | 0.0005 |
| Organic Matter (%) | 70.46 ± 0.17 a | 68.87 ± 0.01 b | 66.95 ± 0.01 c | 65.02 ± 0.01 d | 63.19 ± 0.04 e | 1284.14 | <0.0001 |
| Ash (%) | 29.43 ± 0.17 a | 31.05 ± 0.01 b | 33.00 ± 0.01 c | 34.96 ± 0.01 d | 36.80 ± 0.04 e | 1321.52 | <0.0001 |
| Crude Protein (%) | 21.30 ± 0.11 a | 17.77 ± 0.01 b | 14.22 ± 0.01 c | 10.70 ± 0.01 d | 7.15 ± 0.02 e | 11,179.52 | <0.0001 |
| Crude Lipids (%) | 7.18 ± 0.16 a | 5.89 ± 0.01 b | 4.76 ± 0.01 c | 3.70 ± 0.01 d | 2.76 ± 0.08 e | 451.89 | <0.0001 |
| Carbohydrates (%) | 44.63 ± 0.31 a | 43.78 ± 0.01 b | 42.34 ± 0.01 c | 40.94 ± 0.01 d | 39.53 ± 0.37 e | 88.78 | <0.0001 |
| Crude Fiber (%) | 12.81 ± 0.03 a | 14.20 ± 0.01 b | 15.51 ± 0.01 c | 16.83 ± 0.01 d | 18.15 ± 0.03 e | 8185.28 | <0.0001 |
| Protein/Carbohydrate Ratio | 0.47 ± 0.18 a (≃1:2) | 0.40 ± 0.01 b (≃1:2.5) | 0.33 ± 0.04 c (≃1:3) | 0.26 ± 0.03 d (≃1:4) | 0.18 ± 0.05 e (≃1:5) | 869.66 | <0.0001 |
| Energy (kcal/kg) | 3539.90 ± 12.61 a | 3276.36 ± 1.10 b | 3001.73 ± 1.51 c | 2735.46 ± 1.12 d | 2479.39 ± 20.94 e | 1468.01 | <0.0001 |
| Parameters | T0 | T25 | T50 | T75 | T100 | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Initial biomass weight (g) | 10.55 ± 0.01 a | 10.56 ± 0.01 a | 10.56 ± 0.01 a | 10.57 ± 0.01 a | 10.57 ± 0.01 a | 0.61 | 0.66 |
| Final biomass weight (g) | 126.00 ± 0.26 a | 113.83 ± 0.88 b | 95.73 ± 2.66 c | 67.83 ± 1.72 d | 53.24 ± 1.08 e | 381.35 | <0.0001 |
| Survival rate (%) | 92.66 ± 1.76 a | 93.00 ± 1.52 a | 93.33 ± 1.33 a | 94.73 ± 0.26 a | 90.13 ± 2.82 a | 1.061 | 0.424 |
| Production (g/kg substrate) | 92.35 ± 0.22 a | 82.62 ± 0.71 b | 68.13 ± 2.14 c | 45.80 ± 1.38 d | 34.14 ± 0.87 e | 380.39 | <0.0001 |
| Degradation rate (%) | 67.38 ± 0.69 a | 62.18 ± 1.17 b | 60.16 ± 0.79 b | 53.20 ± 1.00 c | 46.40 ± 1.22 d | 67.21 | <0.0001 |
| Parameters | T0 | T25 | T50 | T75 | T100 | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Dry Matter (%) | 39.75 ± 0.80 a | 39.67 ± 0.64 a | 39.83 ± 0.33 a | 40.44 ± 0.39 a | 39.57 ± 0.82 a | 0.28 | 0.87 |
| Organic Matter (%) | 90.16 ± 0.44 a | 89.50 ± 0.88 a | 89.00 ± 0.57 a | 88.00 ± 0.57 b | 87.33 ± 0.88 b | 3.76 | 0.04 |
| Ash (%) | 9.83 ± 0.44 a | 10.50 ± 0.29 a | 11.00 ± 0.57 ab | 12.00 ± 0.57 b | 12.66 ± 0.88 b | 3.76 | 0.04 |
| Crude Protein (%) | 42.96 ± 0.31 a | 42.16 ± 0.72 ab | 40.16 ± 0.44 b | 38.16 ± 0.44 c | 33.00 ± 1.15 d | 33.70 | <0.0001 |
| Crude Lipids (%) | 31.40 ± 0.30 a | 31.00 ± 0.57 a | 27.00 ± 2.08 b | 21.00 ± 1.00 c | 15.00 ± 1.15 d | 38.30 | <0.0001 |
| Crude Fiber (%) | 20.41 ± 0.30 a | 19.86 ± 0.46 a | 19.16 ± 0.93 a | 18.33 ± 0.60 b | 17.11 ± 0.19 b | 5.40 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vodounnou, J.V.; Iko, R.; Luthada-Raswiswi, R.; Sintondji, S.W.; Alofa, C.S.; Djidohokpin, G.; Niass, F.; Micha, J.-C. Valorization of Water Lettuce (Pistia stratiotes L.) Through Bioconversion for Black Soldier Fly Larvae (Hermetia illucens): Larvae Growth, Survival Rate, and Nutritional Quality. Insects 2025, 16, 1068. https://doi.org/10.3390/insects16101068
Vodounnou JV, Iko R, Luthada-Raswiswi R, Sintondji SW, Alofa CS, Djidohokpin G, Niass F, Micha J-C. Valorization of Water Lettuce (Pistia stratiotes L.) Through Bioconversion for Black Soldier Fly Larvae (Hermetia illucens): Larvae Growth, Survival Rate, and Nutritional Quality. Insects. 2025; 16(10):1068. https://doi.org/10.3390/insects16101068
Chicago/Turabian StyleVodounnou, Juste Vital, Romaric Iko, Rendani Luthada-Raswiswi, Sèlomè Wilfried Sintondji, Cayen Sédro Alofa, Gildas Djidohokpin, Farokh Niass, and Jean-Claude Micha. 2025. "Valorization of Water Lettuce (Pistia stratiotes L.) Through Bioconversion for Black Soldier Fly Larvae (Hermetia illucens): Larvae Growth, Survival Rate, and Nutritional Quality" Insects 16, no. 10: 1068. https://doi.org/10.3390/insects16101068
APA StyleVodounnou, J. V., Iko, R., Luthada-Raswiswi, R., Sintondji, S. W., Alofa, C. S., Djidohokpin, G., Niass, F., & Micha, J.-C. (2025). Valorization of Water Lettuce (Pistia stratiotes L.) Through Bioconversion for Black Soldier Fly Larvae (Hermetia illucens): Larvae Growth, Survival Rate, and Nutritional Quality. Insects, 16(10), 1068. https://doi.org/10.3390/insects16101068











