Behavioral Suppression and Rapid Lethality: Beauveria bassiana B4 Targets Adult Monochamus alternatus for Sustainable Management of Pine Wilt Disease
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Insects and Fungal Strains
2.2. Preparation of Culture Medium and Fungal Suspensions
2.3. Nonwoven Bags and Attractants
2.4. Preparation and Inoculation of Nonwoven Bags
2.5. Determination of the pathogenicity of Beauveria bassiana to Monochamus alternatus
2.6. Forest Test of the B4 Strain Combined with Nonwoven Fabric
2.7. Data Analysis
3. Results
3.1. Screening the Virulence of Beauveria bassiana Strains Following Infection of Monochamus alternatus Adults
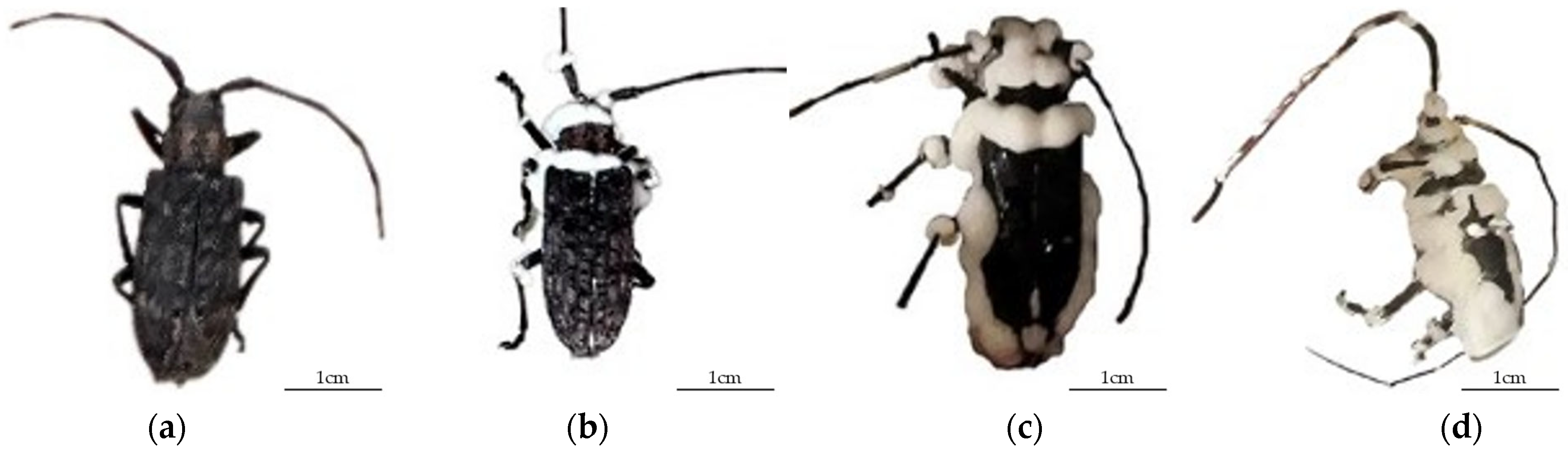
3.2. Symptoms of Beauveria bassiana Infection of Monochamus alternatus Adults
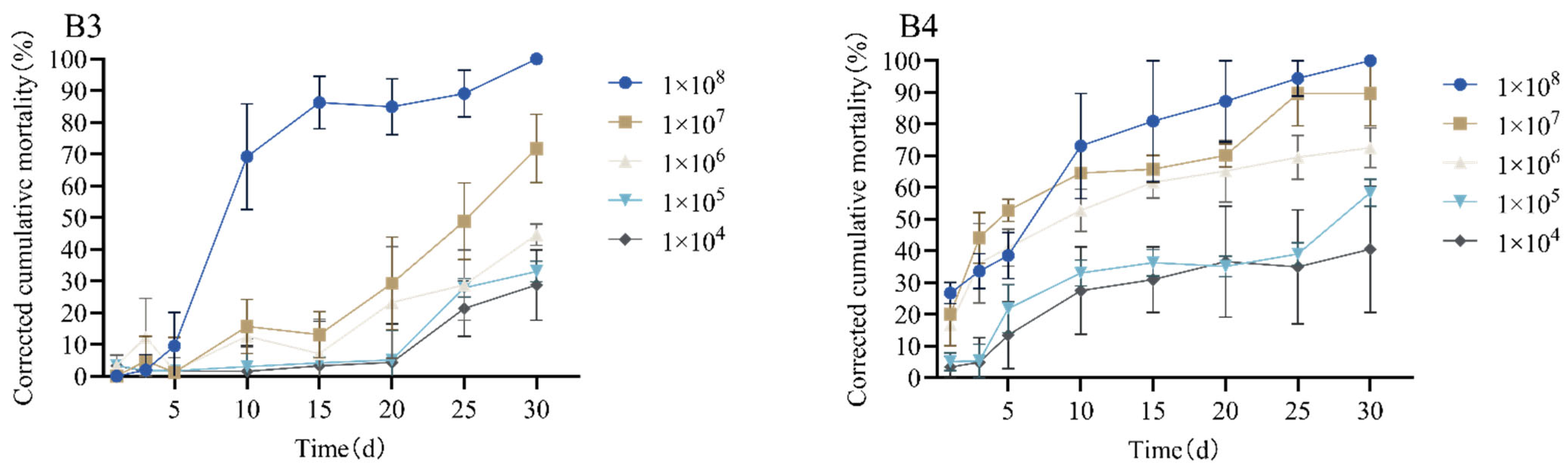
3.3. Determining the Toxicity of Strains B3 and B4 to Monochamus alternatus Adults
3.4. Forest Test of Strain B4 Combined with a Nonwoven Bag
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| PDA | Potato Dextrose Agar |
| RH | Relative Humidity |
| SE | Standard Error |
| DMRT | Duncan’s Multiple Range Test |
References
- Kiyohara, T.; Tokushige, Y. Inoculation Experiments of a Nematode, Bursaphelenchus Sp., onto Pine Trees. J. Jpn. For. Soc. 1971, 53, 210–218. [Google Scholar] [CrossRef]
- Futai, K. Pine Wood Nematode, Bursaphelenchus Xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, H.; Wang, Y.; Chen, Y.; Ma, H.; Yu, Z. The occurrence of major forestry pests in China in 2024 and the trend forecast for 2025. For. Pest Dis. 2025, 44, 52–56. [Google Scholar] [CrossRef]
- Deng, J.; Zhuang, W.; Liu, Y.; Song, L.; Zhang, L. Pathogenicity of white muscardine fungus Beauveria bassiana against Japanese pine sawyer beetle Monochamus alternatus and its compatibility with ectoparasitic beetle Dastarcus helophoroides. J. Plant Prot. 2021, 48, 602–609. [Google Scholar] [CrossRef]
- Luo, L.; Cai, Z.; Lin, T. Research progress on natural enemies against Monochamus alternatus Hope and its bio-control. China Plant Prot. 2015, 35, 21–25. [Google Scholar]
- Dang, Y.; Wang, X.; Yang, Z. Advances in biological control of forest insect pests by using natural enemies in China. J. Environ. Entomol. 2018, 40, 242–255. [Google Scholar]
- Lewis, S.M.; Jusoh, W.F.A.; Walker, A.C.; Fallon, C.E.; Joyce, R.; Yiu, V. Illuminating Firefly Diversity: Trends, Threats and Conservation Strategies. Insects 2024, 15, 71. [Google Scholar] [CrossRef]
- Wei, Y. Study on the Function of Dopamine Signaling Pathway in the “Tree Top Disease” of Lymantria Dispar. Master’s Thesis, Northwest A & F University, Xianyang, China, 2023. [Google Scholar]
- Rosenheim, J.A.; Kaya, H.K.; Ehler, L.E.; Marois, J.J.; Jaffee, B.A. Intraguild Predation Among Biological-Control Agents: Theory and Evidence. Biol. Control 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Panwar, N.; Szczepaniec, A. Endophytic Entomopathogenic Fungi as Biological Control Agents of Insect Pests. Pest Manag. Sci. 2024, 80, 6033–6040. [Google Scholar] [CrossRef]
- Kim, J.-C.; Lee, M.R.; Yu, J.S.; Park, S.E.; Ha, P.; Kim, J.S. Management of Overwintering Pine Sawyer Beetle, Monochamus alternatus with Colonized Beauveria bassiana ERL836. PLoS ONE 2022, 17, e0274086. [Google Scholar] [CrossRef]
- Wei, Q. The Inhibiting Effect of Beauveria bassiana Induced Solanum lycopersicum Defense on Bemisia tabaci. Ph.D. Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Angel-Ruiz, N.A.; Zavala-Izquierdo, I.; Pérez-Staples, D.; Díaz-Fleisher, F.; Andrade-Torres, A.; Guillén-Navarro, G.K.; Colunga-Salas, P. Bioprospecting of Four Beauveria bassiana Strains and Their Potential as Biological Control Agents for Anastrepha Ludens Loew 1873 (Diptera: Tephritidae). PLoS ONE 2025, 20, e0324441. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Zhang, J.; Lu, Y. Toxic efficiency of biocontrol fungi Beauveria bassiana and Metarhizium anisopliae jointly with four insecticides on controlling cotton aphid Aphis gossypii. J. Plant Prot. 2024, 51, 1457–1465. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Liu, X.; Wang, D. The Control Efficacy of Two Fungal Strains against Locusts of Grassland in Yulin, Shaanxi. Chin. J. Biol. Control 2021, 37, 380–384. [Google Scholar] [CrossRef]
- Wang, Y. Basic Research on the Control of Xylotrechus rusticus L. by Beauveria Spp. and Dastarcus helophoroides. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2021. [Google Scholar]
- Chen, Y.; Cai, S.; Lin, Y.; Zeng, L.; Zhan, F. Isolation and Idetification of a Beauveria bassiana Strain from the Infected Larvae of Monochamus alternatus in the Pine Forest. J. Fujian For. Sci. Technol. 2024, 51, 9–13,32. [Google Scholar] [CrossRef]
- He, X.; Huang, J.; Cai, F.; Yang, X.; Chen, D.; Kang, W. Bioassay of pathogenicity of different Beauveria bassiana isolates to longihorn beetle. In Proceedings of the Research and Application of Entomogenous Fungi in China (Volume V); Fujian Academy of Forestry Sciences: Fuzhou, China, 2003; p. 6. [Google Scholar]
- He, X.; Chen, S.; Huang, J. Preliminary screening of virulent strains of Metarhizium anisopliae against Monochamus alternatus. Acta Entomol. Sin. 2005, 975–981. [Google Scholar] [CrossRef]
- Liu, H.; Piao, C.; Wang, L.; Shen, X.; Zheng, R.; Shu, Q. Biocontrol of Monochamus alternatus by Beauveria bassiana and Scleroderma guani. Sci. Silvae Sin. 2007, 43, 64–68. [Google Scholar]
- Zhang, Y.; Wang, X.; Yang, Z.; Wei, K.; Cao, L. Research Progress on Natural Enemies and Their Application of the Vector Insects of Bursaphelenchus Xylophilus. For. Pest Dis. 2022, 41, 21–29. [Google Scholar] [CrossRef]
- Zhang, Z. Study on Screening and Cultural Condition Optimization for Pathogenic Fungi Against Longhorn Beetle. Master’s Thesis, Northwest A & F University, Xianyang, China, 2023. [Google Scholar]
- Cai, S.; Liu, J.; He, X.; Li, Z.; Wu, L. Bioassay of different strains of Beauveria bassiana and Metarhizium anisopliae to Anoplophora chinensis adults. For. Pest Dis. 2008, 27, 1–3. [Google Scholar]
- Guo, H.; Liu, Z.; Sun, J. Effects of spore suspension concentration and host body size on the pathogenicity of Beauveria bassiana against Monochamus alternatus (Coleoptera: Cerambycidae) larvae. Acta Entomol. Sin. 2020, 63, 835–842. [Google Scholar] [CrossRef]
- Kovač, M.; Lacković, N.; Pernek, M. Effect of Beauveria bassiana Fungal Infection on Survival and Feeding Behavior of Pine-Tree Lappet Moth (Dendrolimus Pini L.). Forests 2020, 11, 974. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Shu, Q.; Meng, Z.; Dong, G.; Fang, J. Influence of Beauveria bassiana on the life habits of Monochamus alternatus larvae. J. Anhui Agric. Univ. 2010, 37, 196–199. [Google Scholar] [CrossRef]
- Cappa, F.; De Fazi, L.; Baracchi, D.; Cervo, R. Adverse Effects of the Fungal Biopesticide Beauveria bassiana on a Predatory Social Wasp. Sci. Total Environ. 2024, 908, 168202. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Control Trail of Monochamus alternatus (Hope) in the Forest. Biol. Disaster Sci. 2013, 36, 202–205. [Google Scholar]
- Wang, B.; Fan, M.; Li, Z. Control Forest Beetles with Beauveria bassiana Nonwoven Fabric Sheet in Combination with Beetle Attractants. Chin. J. Biol. Control 2003, 91–92. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Wu, H. The Screening Virulent Strain of Beauveria bassiana to Monochamus alternatus. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2000, 33–37. [Google Scholar]
- Bai, Y. Effecfs of Humidity Temperature and Initial Infection Ratio on Fungal Disease of B. bassiana Against Stephanitis nashi and Locusta Migratoria Manilensis and Tts Model Construction. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2017. [Google Scholar]
- Lu, L. Scleroderma sichuanensis Carrying B. bassiana Microcapsules to Control M. alternatus. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2023. [Google Scholar]
- Wu, S.; Gao, Y.; Smagghe, G.; Xu, X.; Lei, Z. Interactions between the Entomopathogenic Fungus Beauveria bassiana and the Predatory Mite Neoseiulus Barkeri and Biological Control of Their Shared Prey/Host Frankliniella Occidentalis. Biol. Control 2016, 98, 43–51. [Google Scholar] [CrossRef]
- Fattorini, S. Upward and Poleward (but Not Phenological) Shifts in a Forest Tenebrionid Beetle in Response to Global Change in a Mediterranean Area. Insects 2024, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S.; Vitozzi, A.; Di Biase, L.; Bergamaschi, D. Macroecology of Dung Beetles in Italy. Insects 2024, 15, 39. [Google Scholar] [CrossRef] [PubMed]

| Days After Inoculation | Fungal Strains | |||
|---|---|---|---|---|
| B1 | B2 | B3 | B4 | |
| 1 d | 0.00 ± 0.00 b | 6.67 ± 11.55 b | 0.00 ± 0.00 b | 26.67 ± 5.77 a |
| 3 d | 32.85 ± 15.00 a | 1.95 ± 8.36 b | 1.95 ± 8.36 b | 33.59 ± 18.70 a |
| 5 d | 34.12 ± 12.23 ab | 1.18 ± 25.45 b | 9.54 ± 18.32 ab | 38.56 ± 12.61 a |
| 10 d | 40.90 ± 12.19 ab | −1.63 ± 29.30 b | 69.19 ± 28.83 a | 73.11 ± 28.17 a |
| 15 d | 50.00 ± 7.14 ab | 7.74 ± 26.75 b | 86.31 ± 14.32 a | 80.95 ± 32.99 a |
| 20 d | 60.07 ± 23.22 a | 17.21 ± 10.56 b | 84.98 ± 15.40 a | 87.18 ± 22.20 a |
| 25 d | 66.00 ± 19.15 b | 19.56 ± 9.95 c | 89.10 ± 12.80 ab | 94.44 ± 9.62 a |
| 30 d | 71.13 ± 22.25 b | 19.56 ± 9.95 c | 100 ± 0.00 a | 100 ± 0.00 a |
| Strain | LC50 (spores/mL) | 95% FL 1 (spores/mL) | Regression Equation | (χ2) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| B3 | 4.93 × 107 | 7.01 × 106 | 100.323 | y = 0.728x − 5.597 | 11.574 |
| B4 | 9.63 × 105 | 3.18 ×105 | 2.89 × 106 | y = 0.326x − 1.949 | 0.847 |
| Strain | Spore Concentration (spores/mL) | LT50 (d) | LT90 (d) | Regression Equation | Correlation Coefficient |
|---|---|---|---|---|---|
| B3 | 1 × 108 | 11.36 | 19.93 | y = 0.150x − 1.699 | 0.804 |
| 1 × 107 | 24.92 | 39.15 | y = 0.900x − 2.243 | 0.896 | |
| 1 × 106 | 35.23 | 60.86 | y = 0.050x − 1.762 | 0.724 | |
| 1 × 105 | 37.76 | 57.91 | y = 0.064x − 2.401 | 0.799 | |
| 1 × 104 | 40.95 | 62.41 | y = 0.060x − 2.444 | 0.750 | |
| B4 | 1 × 108 | 6.61 | 19.67 | y = 0.980x − 0.649 | 0.965 |
| 1 × 107 | 7.47 | 28.64 | y = 0.061x − 0.452 | 0.879 | |
| 1 × 106 | 12.40 | 41.32 | y = 0.044x − 0.549 | 0.833 | |
| 1 × 105 | 26.28 | 52.98 | y = 0.048x − 1.262 | 0.786 | |
| 1 × 104 | 31.33 | 60.64 | y = 0.044x − 1.370 | 0.785 |
| Study Year | Time Point | Treatment Group | Mean ± SD | 95% FL 1 (mg/mL) | p-Value | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 2023 | 30 d | T1 | 9.5 ± 1.9 | 6.5 | 12.5 | p < 0.001 |
| - | CK | 28.3 ± 4.1 | 21.7 | 34.8 | - | |
| 60 d | T1 | 5.0 ± 2.4 | 1.1 | 8.9 | p < 0.001 | |
| - | CK | 18.8 ± 3.2 | 13.7 | 23.8 | - | |
| One year | T1 | 14.5 ± 2.4 | 10.7 | 18.3 | p < 0.001 | |
| - | CK | 47.0 ± 2.8 | 42.5 | 51.5 | - | |
| 2024 | 30 d | T1 | 3.3 ± 2.8 | −1.1 | 7.6 | 0.002 |
| - | CK | 22.5 ± 7.0 | 11.3 | 33.7 | - | |
| 60 d | T1 | 2.5 ± 0.6 | 1.6 | 3.4 | 0.002 | |
| - | CK | 14.8 ± 4.6 | 7.5 | 22.0 | - | |
| One year | T1 | 5.75 ± 2.75 | 1.37 | 10.13 | p < 0.001 | |
| - | CK | 37.3 ± 10.1 | 21.21 | 53.29 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, X.; An, L.; Gong, D.; Wang, J.; Bi, H.; Zheng, Y.; Cao, L.; Lu, S. Behavioral Suppression and Rapid Lethality: Beauveria bassiana B4 Targets Adult Monochamus alternatus for Sustainable Management of Pine Wilt Disease. Insects 2025, 16, 1045. https://doi.org/10.3390/insects16101045
Zhang Y, Zhang X, An L, Gong D, Wang J, Bi H, Zheng Y, Cao L, Lu S. Behavioral Suppression and Rapid Lethality: Beauveria bassiana B4 Targets Adult Monochamus alternatus for Sustainable Management of Pine Wilt Disease. Insects. 2025; 16(10):1045. https://doi.org/10.3390/insects16101045
Chicago/Turabian StyleZhang, Yaqi, Xuejie Zhang, Liudi An, Dongfeng Gong, Jinsheng Wang, Huitao Bi, Yi Zheng, Lei Cao, and Shaohui Lu. 2025. "Behavioral Suppression and Rapid Lethality: Beauveria bassiana B4 Targets Adult Monochamus alternatus for Sustainable Management of Pine Wilt Disease" Insects 16, no. 10: 1045. https://doi.org/10.3390/insects16101045
APA StyleZhang, Y., Zhang, X., An, L., Gong, D., Wang, J., Bi, H., Zheng, Y., Cao, L., & Lu, S. (2025). Behavioral Suppression and Rapid Lethality: Beauveria bassiana B4 Targets Adult Monochamus alternatus for Sustainable Management of Pine Wilt Disease. Insects, 16(10), 1045. https://doi.org/10.3390/insects16101045







