Temporal Dynamics of Bacterial Communities in Ectropis grisescens Following Cryogenic Mortality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Samples Preparation
2.3. DNA Extraction
2.4. PCR Amplification and Sequencing of Microbial 16S rDNA Genes
2.5. Data Analysis
3. Results
3.1. Differential Assembly and Taxonomic Annotation of Symbiotic Bacterial Communities in Ectropis grisescens
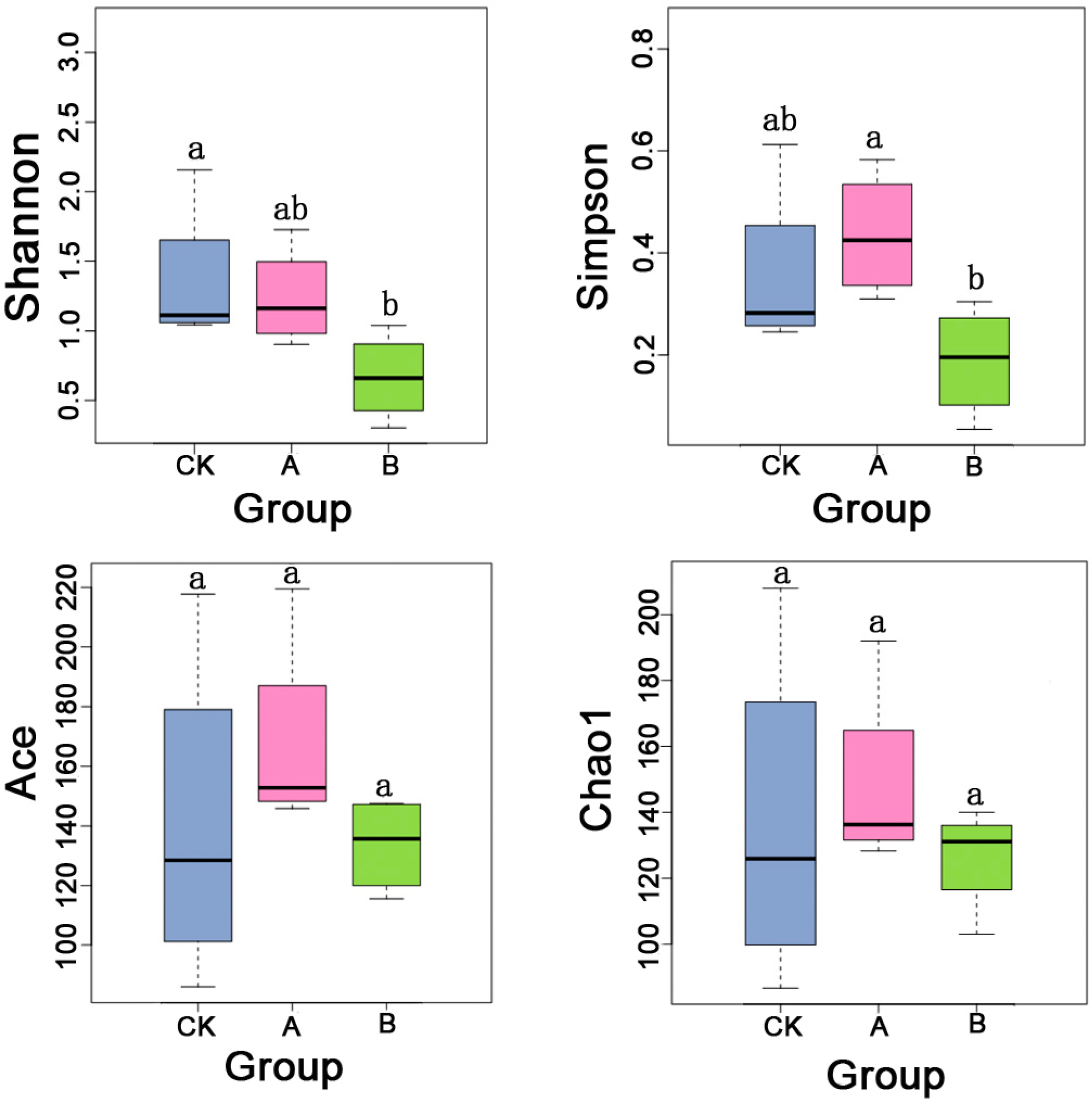
3.2. Differences in Diversity and Richness of Bacterial Communities in Ectropis grisescens Under Different Treatments
3.3. Similarity Analysis of Bacterial Communities in Ectropis grisescens Under Different Treatments
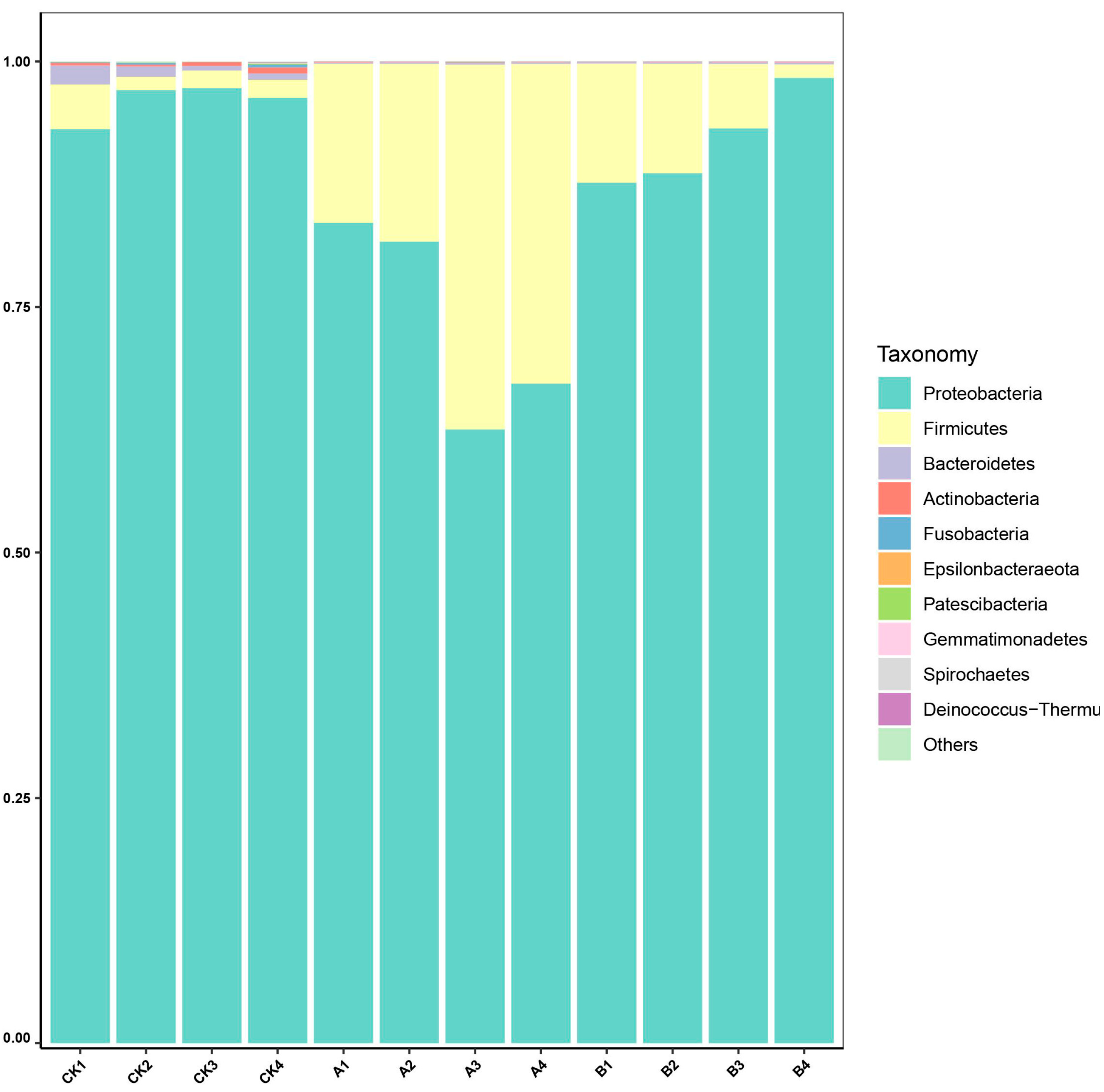
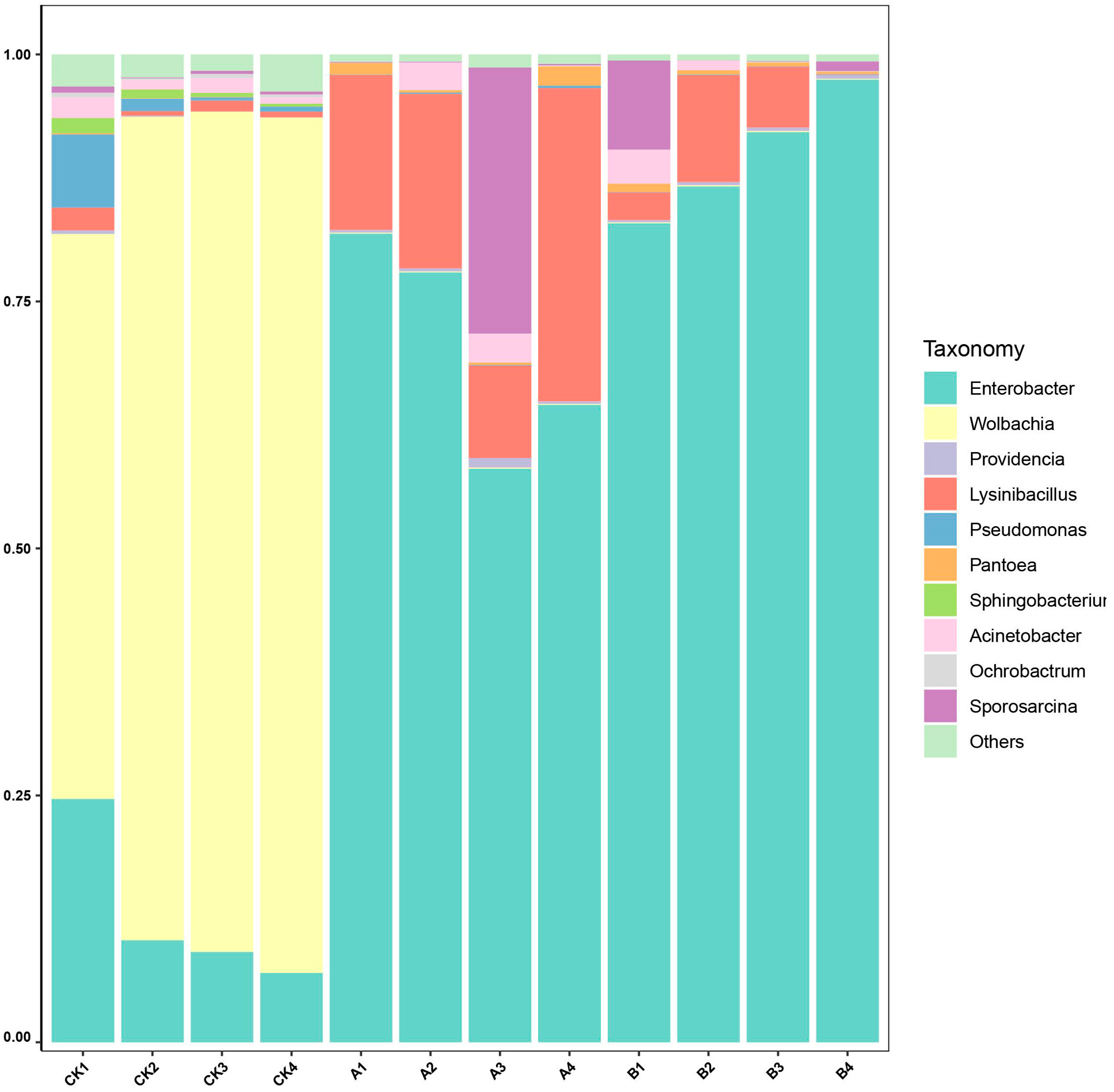
3.4. Analysis of Composition and Abundance of Microbial Communitys in Ectropis grisescens Under Different Treatments
3.5. Temporal Dynamics of Bacteria in Ectropis grisescens Following Liquid Nitrogen-Induced Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Mei, Y.; Li, H.; Tang, M.; He, K.; Xiao, Q. Larval-Transcriptome Dynamics of Ectropis grisescens Reveals Differences in Virulence Mechanism between Two EcobNPV Strains. Insects 2022, 13, 1088. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fang, G.; Wang, Z.; Cao, Y.; Liu, Y.; Li, G.; Liu, X.; Xiao, Q.; Zhan, S. Chromosome-level genome reference and genome editing of the tea geometrid. Mol. Ecol. Resour. 2021, 21, 2034–2049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Liu, S.X.; Xue, D.Y.; Tang, M.J.; Xiao, Q.; Han, H.X. External morphology and molecular identification of two tea geometrid moths from southern China. Chin. J. Appl. Entomol. 2014, 51, 987–1002. [Google Scholar]
- Zhang, G.-H.; Yuan, Z.-J.; Zhang, C.-X.; Yin, K.-S.; Tang, M.-J.; Guo, H.-W.; Fu, J.-Y.; Xiao, Q. Detecting deep divergence in seventeen populations of tea geometrid (Ectropis obliqua Prout) in China by COI mtDNA and cross-breeding. PLoS ONE 2014, 9, e99373. [Google Scholar] [CrossRef]
- Zhang, L.H.; Huang, Z.D. Biological control techniques of Ectropis obliqua and Ectropis grisescens. Chin. J. Anhui Agric. Sci. Bull. 2024, 30, 79–83. [Google Scholar]
- Chabanol, E.; Gendrin, M. Insects and microbes: Best friends from the nursery. Curr. Opin. Insect Sci. 2024, 66, 101270. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, B.; Sun, C.; Ishida, K.; Hertweck, C.; Boland, W. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem. Biol. 2017, 24, 66–75. [Google Scholar] [CrossRef]
- Zhang, N.; Qian, Z.; He, J.; Shen, X.; Lei, X.; Sun, C.; Fan, J.; Felton, G.W.; Shao, Y. Gut bacteria of lepidopteran herbivores facilitate digestion of plant toxins. Proc. Natl. Acad. Sci. USA 2024, 121, e2412165121. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, J.; Liu, Y.; Li, H.; Zhan, S.; Xiao, Q. Transcriptomic Analysis Reveals Insect Hormone Biosynthesis Pathway Involved in Desynchronized Development Phenomenon in Hybridized Sibling Species of Tea Geometrids (Ectropis grisescens and Ectropis obliqua). Insects 2019, 10, 381. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Li, Y.; Yin, Y.; Zhang, X.; Zhang, Q.; Kong, X.; Liu, W.; Yao, D.; Zhang, R.; et al. Application of bacteria and bacteriophage cocktails for biological control of houseflies. Parasites Vectors 2024, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; VanLaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef]
- Weatherbee, C.R.; Pechal, J.L.; Benbow, M.E. The Dynamic Maggot Mass Microbiome. Ann. Entomol. Soc. Am. 2017, 110, 45–53. [Google Scholar] [CrossRef]
- Metcalf, J.L.; Wegener Parfrey, L.; Gonzalez, A.; Lauber, C.L.; Knights, D.; Ackermann, G.; Humphrey, G.C.; Gebert, M.J.; Van Treuren, W.; Berg-Lyons, D.; et al. A microbial clock provides an accurate estimate of the postmortem interval in a mouse model system. eLife 2013, 2, e01104. [Google Scholar] [CrossRef]
- Bo-Sheng, C.; Xing-Meng, L.; Yong-Qi, S. Diversity of the gut microbiota in lepidopteran insects and their interaction with hosts. Acta Entomol. Sin. 2017, 60, 710–722. [Google Scholar]
- Gómez-Govea, M.A.; de Lourdes Ramírez-Ahuja, M.; Castellanos-López, L.M.; Ruvalcaba-Ortega, I.; Reyes-Cortes, L.M.; de Jesús Trujillo-Rodríguez, G.; Villanueva-Segura, O.K.; Martínez-Fierro, M.; Delgado-Enciso, I.; Ponce-García, G. Bacterial communities associated with Megalopyge opercularis (Smith)(Lepidoptera: Megalopygidae). Fla. Entomol. 2022, 105, 298–306. [Google Scholar]
- Russell, S.L. Transmission mode is associated with environment type and taxa across bacteria-eukaryote symbioses: A systematic review and meta-analysis. FEMS Microbiol. Lett. 2019, 366, fnz013. [Google Scholar] [CrossRef]
- Zhibo, W.; Jiahe, B.; Xiaogui, Z.; Huawei, G.; Qiang, X. Effect of Three Antibiotic Treatments on Bacterial Endosymbiont Community of Ectropis grisescens Warren. J. Tea Sci. 2021, 41, 90–100. [Google Scholar]
- Bai, J.H.; Tang, M.J.; Yin, K.S.; Wang, Z.B.; Xiao, Q. Differential biological characteristics between closely related tea geometrid species, Ectropis obliqua and Ectropis grisescens. Acta Agric. Zhejiangensis 2018, 30, 797–803. [Google Scholar]
- Hong, L.; Meijun, T.; Huawei, G.; Zhibo, W.; Qiang, X. Toxicity difference of EoNPV of two sibling species of tea genometrids. Acta Agric. Zhejiangensis 2020, 32, 1415–1419. [Google Scholar]
- Wang, Z.; Li, H.; Zhou, X.; Tang, M.; Sun, L.; Zhan, S.; Xiao, Q. Comparative characterization of microbiota between the sibling species of tea geometrid moth Ectropis obliqua Prout and E. grisescens Warren. Bull. Entomol. Res. 2020, 110, 684–693. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Jiang, R.; Zhang, C.; Gao, T.; Wang, Y.; Liu, C.; Long, Y.; Zhang, Y.; Yang, Y. Wolbachia strain w Gri from the tea geometrid moth Ectropis grisescens contributes to its host’s fecundity. Front. Microbiol. 2021, 12, 694466. [Google Scholar] [CrossRef]
- Sinclair, L.; Osman, O.A.; Bertilsson, S.; Eiler, A. Microbial community composition and diversity via 16S rRNA gene amplicons: Evaluating the illumina platform. PLoS ONE 2015, 10, e0116955. [Google Scholar] [CrossRef]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.; Park, J.; Chen, Y.-A.; Kawashima, H.; Mizuguchi, K. Impact of quality trimming on the efficiency of reads joining and diversity analysis of Illumina paired-end reads in the context of QIIME1 and QIIME2 microbiome analysis frameworks. BMC Bioinform. 2019, 20, 581. [Google Scholar] [CrossRef]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Weeks, A.; Breeuwer, J. Wolbachia–induced parthenogenesis in a genus of phytophagous mites. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 2245–2251. [Google Scholar] [CrossRef]
- Jiggins, F.M.; Hurst, G.D.; Majerus, M.E. Sex ratio distortion in Acraea encedon (Lepidoptera: Nymphalidae) is caused by a male-killing bacterium. Heredity 1998, 81, 87–91. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zhang, Y.Y.; Wang, X.Y.; Yin, Y.; Du, Y.Z. Wolbachia modify host cell metabolite profiles in response to short-term temperature stress. Environ. Microbiol. Rep. 2024, 16, e70013. [Google Scholar] [CrossRef]
- Rainey, S.M.; Martinez, J.; McFarlane, M.; Juneja, P.; Sarkies, P.; Lulla, A.; Schnettler, E.; Varjak, M.; Merits, A.; Miska, E.A. Wolbachia blocks viral genome replication early in infection without a transcriptional response by the endosymbiont or host small RNA pathways. PLoS Pathog. 2016, 12, e1005536. [Google Scholar] [CrossRef] [PubMed]
- She, L.; Shi, M.; Cao, T.; Yuan, H.; Wang, R.; Wang, W.; She, Y.; Wang, C.; Zeng, Q.; Mao, W. Wolbachia mediates crosstalk between miRNA and Toll pathways to enhance resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2024, 20, e1012296. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef]
- Fenn, K.; Blaxter, M. Wolbachia genomes: Revealing the biology of parasitism and mutualism. Trends Parasitol. 2006, 22, 60–65. [Google Scholar] [CrossRef]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef] [PubMed]
- Kremer, N.; Voronin, D.; Charif, D.; Mavingui, P.; Mollereau, B.; Vavre, F. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 2009, 5, e1000630. [Google Scholar] [CrossRef]
- Yadav, K.K.; Bora, A.; Datta, S.; Chandel, K.; Gogoi, H.K.; Prasad, G.B.; Veer, V. Molecular characterization of midgut microbiota of Aedes albopictus and Aedes aegypti from Arunachal Pradesh, India. Parasites Vectors 2015, 8, 641. [Google Scholar] [CrossRef]
- Liu, H.; Yin, J.; Huang, X.; Zang, C.; Zhang, Y.; Cao, J.; Gong, M. Mosquito Gut Microbiota: A Review. Pathogens 2024, 13, 691. [Google Scholar] [CrossRef]
- Azis, K.; Zerva, I.; Melidis, P.; Caceres, C.; Bourtzis, K.; Ntougias, S. Biochemical and nutritional characterization of the medfly gut symbiont Enterobacter sp. AA26 for its use as probiotics in sterile insect technique applications. BMC Biotechnol. 2019, 19, 90. [Google Scholar] [CrossRef]
- Kyritsis, G.A.; Augustinos, A.A.; Ntougias, S.; Papadopoulos, N.T.; Bourtzis, K.; Cáceres, C. Enterobacter sp. AA26 gut symbiont as a protein source for Mediterranean fruit fly mass-rearing and sterile insect technique applications. BMC Microbiol. 2019, 19, 288. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Zhang, X.; Zhang, K.; Liu, W.; Zhang, R.; Zhang, Z. Enterobacter hormaechei in the intestines of housefly larvae promotes host growth by inhibiting harmful intestinal bacteria. Parasites Vectors 2021, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Djihinto, O.; Medjigbodo, A.; Gangbadja, A.; Saizonou, H.; Lagnika, H.; Nanmede, D.; Djossou, L.; Bohounton, R.; Sovegnon, P.; Fanou, M. Malaria-transmitting vectors microbiota: Overview and interactions with Anopheles mosquito biology. Front. Microbiol. 2022, 13, 891573. [Google Scholar] [CrossRef]
- Willimsky, G.; Bang, H.; Fischer, G.; Marahiel, M. Characterization of cspB, a Bacillus subtilis inducible cold shock gene affecting cell viability at low temperatures. J. Bacteriol. 1992, 174, 6326–6335. [Google Scholar] [CrossRef][Green Version]
- Adebo, O.A.; Njobeh, P.B.; Mavumengwana, V. Degradation and detoxification of AFB1 by Staphylocococcus warneri, Sporosarcina sp. and Lysinibacillus fusiformis. Food Control 2016, 68, 92–96. [Google Scholar] [CrossRef]
- Pechal, J.L.; Crippen, T.L.; Benbow, M.E.; Tarone, A.M.; Dowd, S.; Tomberlin, J.K. The potential use of bacterial community succession in forensics as described by high throughput metagenomic sequencing. Int. J. Leg. Med. 2014, 128, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.L.; Xu, Z.Z.; Weiss, S.; Lax, S.; Van Treuren, W.; Hyde, E.R.; Song, S.J.; Amir, A.; Larsen, P.; Sangwan, N. Microbial community assembly and metabolic function during mammalian corpse decomposition. Science 2016, 351, 158–162. [Google Scholar] [CrossRef] [PubMed]



| Group | Raw_PE | Effective Tags | OTUs | Q20/% | Goods_Coverage |
|---|---|---|---|---|---|
| CK | 209,019 | 193,753 | 250 | 99.05 | 0.999 |
| A | 181,515 | 152,690 | 171 | 98.87 | 0.999 |
| B | 191,956 | 162,373 | 137 | 98.85 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, Z.; Feng, G.; Xiao, Q.; Tang, M. Temporal Dynamics of Bacterial Communities in Ectropis grisescens Following Cryogenic Mortality. Insects 2025, 16, 1040. https://doi.org/10.3390/insects16101040
Zhang X, Wang Z, Feng G, Xiao Q, Tang M. Temporal Dynamics of Bacterial Communities in Ectropis grisescens Following Cryogenic Mortality. Insects. 2025; 16(10):1040. https://doi.org/10.3390/insects16101040
Chicago/Turabian StyleZhang, Xinxin, Zhibo Wang, Guozhong Feng, Qiang Xiao, and Meijun Tang. 2025. "Temporal Dynamics of Bacterial Communities in Ectropis grisescens Following Cryogenic Mortality" Insects 16, no. 10: 1040. https://doi.org/10.3390/insects16101040
APA StyleZhang, X., Wang, Z., Feng, G., Xiao, Q., & Tang, M. (2025). Temporal Dynamics of Bacterial Communities in Ectropis grisescens Following Cryogenic Mortality. Insects, 16(10), 1040. https://doi.org/10.3390/insects16101040





