Males of Dalbulus maidis Attract Females Through Volatile Compounds with Potential Pheromone Function: A Tool for Pest Management
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Corn Leafhopper-Dalbulus Maidis
2.2. Maize
2.3. Volatile Collection
2.4. Chemical Analysis
2.5. Chemicals
2.6. Olfactometer Bioassays
2.7. Statistical Analyses
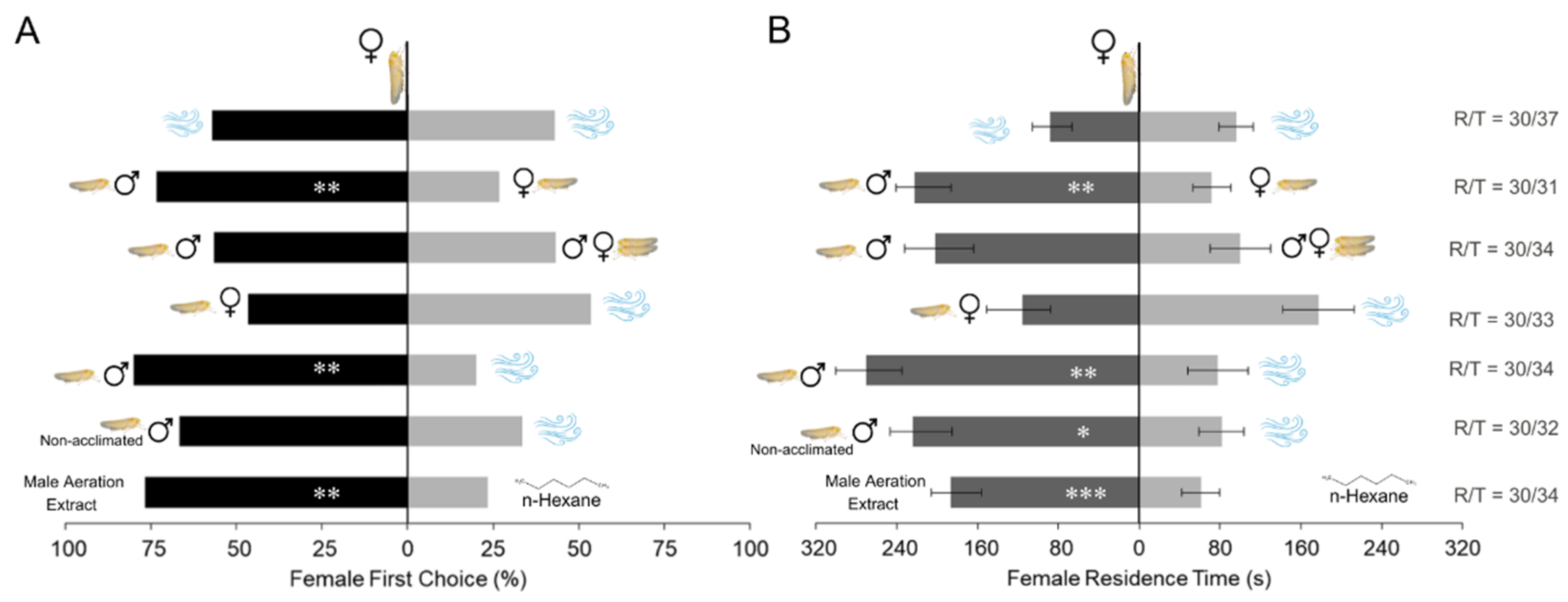
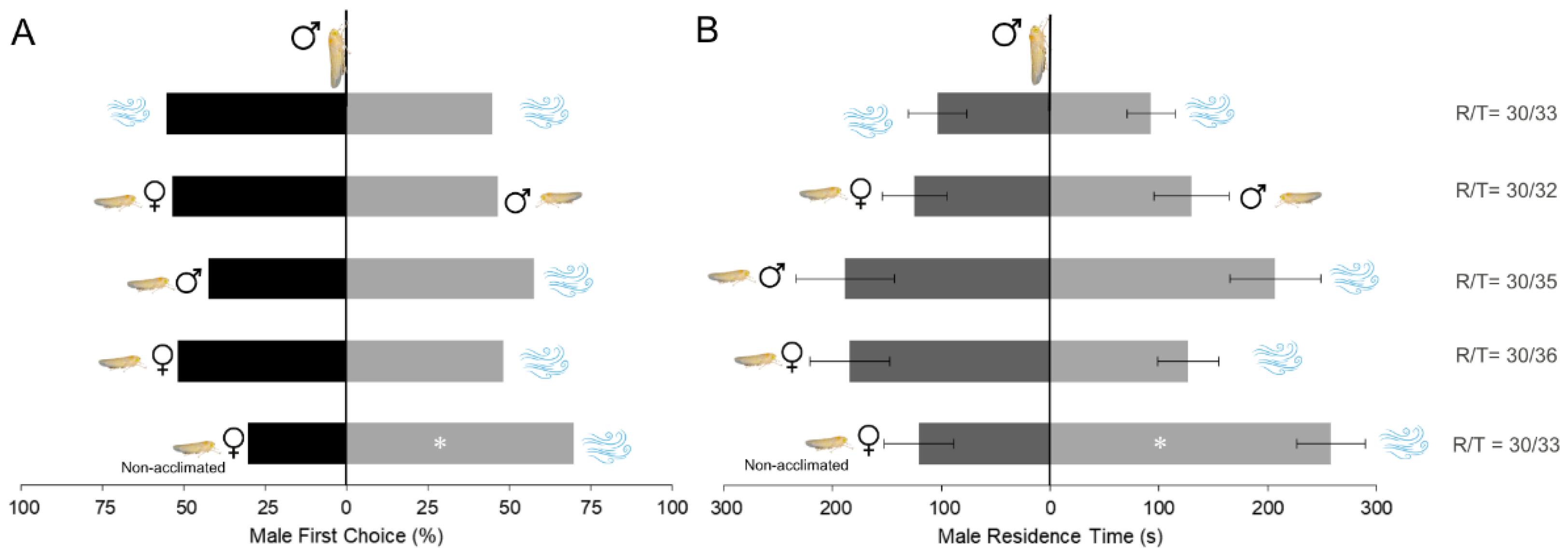
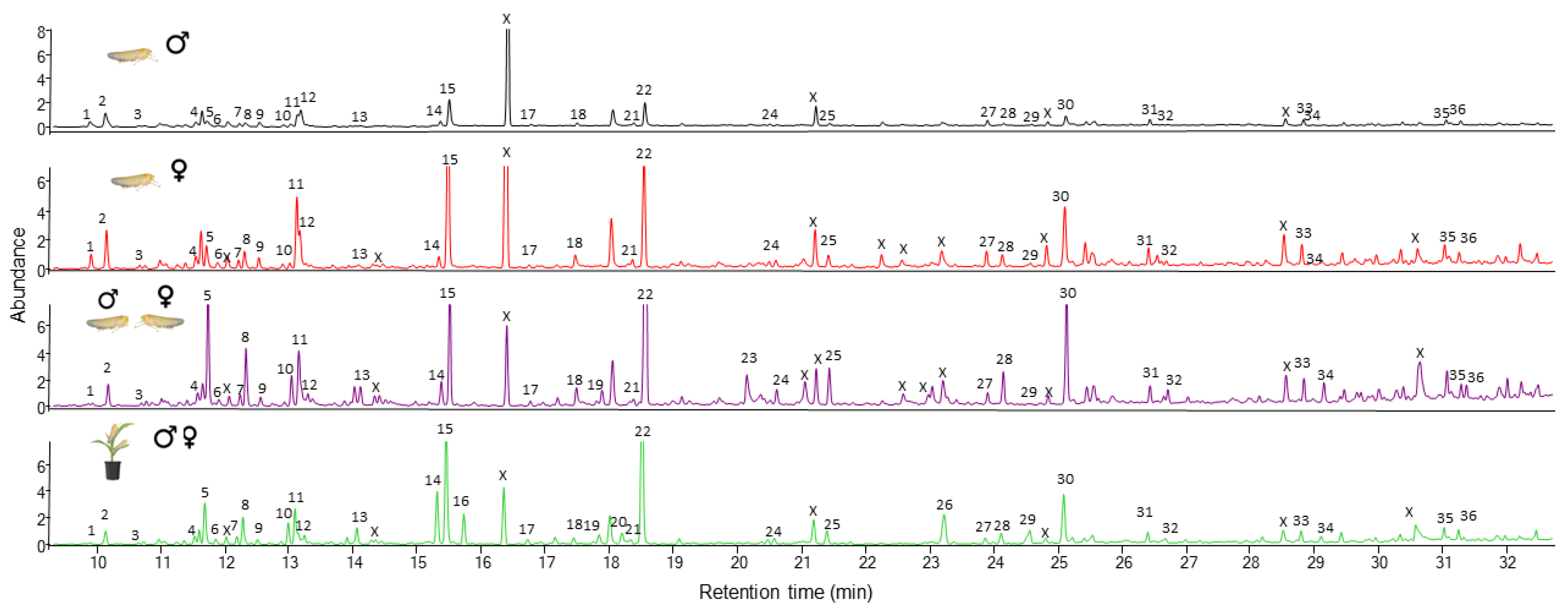
3. Results
3.1. Bioassays
3.2. Volatiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.H.; Feeny, P.P. Allelochemics: Chemical interactions between species: Chemical agents are of major significance in the adaptation of species and organization of communities. Science 1971, 171, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Morgan, E.D. Chemical communication in insect communities: A guide to insect pheromones with special emphasis on social insects. Biol. Rev. 1990, 65, 227–247. [Google Scholar] [CrossRef]
- Yew, J.Y.; Chung, H. Insect pheromones: An overview of function, form, and discovery. Prog. Lipid Res. 2015, 59, 88–105. [Google Scholar] [CrossRef]
- Nault, L.R. Evolution of insect pest maize and leafhopper, a case study. Maydica 1990, 35, 165–175. [Google Scholar]
- Nault, L.R. Maize bushy stunt and corn stunt: A comparison of disease symptoms, pathogen host ranges and vectors. Phytopathology 1980, 70, 659–662. [Google Scholar] [CrossRef]
- Villanova, E.S.; Ramos, A.; de Oliveira, M.C.S.; Esteves, M.B.; Gonçalves, M.C.; Lopes, J.R. First report of a mastrevirus (Geminiviridae) transmitted by the corn leafhopper. Plant Dis. 2022, 106, 1330–1333. [Google Scholar] [CrossRef]
- Pérez-López, E.; Wist, T.; Rodríguez, Y.; Luna-Rodríguez, M.; Olivier, C.Y. Maize bushy stunt in native corn: Implications for Mexican “subsistence farmers”. Environ. Dev. Sustain. 2018, 20, 1797–1805. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Frizzas, M.R. Eight decades of Dalbulus maidis (DeLong and Wolcott) (Hemiptera: Cicadellidae) in Brazil: What we know and what we need to know. Neotrop. Entomol. 2022, 51, 1–17. [Google Scholar] [CrossRef]
- Heady, S.E.; Nault, L.R.; Shambaugh, G.F.; Fairchild, L. Acoustic and mating behavior of Dalbulus leafhoppers (Homoptera: Cicadellidae). Ann. Entomol. Soc. Am. 1986, 79, 727–736. [Google Scholar] [CrossRef]
- Ramirez-Romero, R.; Perez-Ascencio, D.; Garibay-Benítez, D. Courtship behavior of the corn leafhopper Dalbulus maidis (DeLong & Wolcott) (Hemiptera: Cicadellidae). J. Insect Behav. 2014, 27, 804–815. [Google Scholar] [CrossRef]
- Todd, J.L.; Phelan, P.L.; Nault, L.R. Interaction between visual and olfactory stimuli during host-finding by leafhopper, Dalbulus maidis (Homoptera: Cicadellidae). J. Chem. Ecol. 1990, 16, 2121–2133. [Google Scholar] [CrossRef]
- Aráoz, M.C.; Jacobi, V.G.; Fernandez, P.C.; Albarracin, E.L.; Virla, E.G.; Hill, J.G.; Catalan, C.A.N. Volatiles mediate host-selection in the corn hoppers Dalbulus maidis (Hemiptera: Cicadellidae) and Peregrinus maidis (Hemiptera: Delphacidae). Bull. Entomol. Res. 2019, 109, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, A.P. Identification of a self-regulatory pheromone system that controls nymph aggregation behavior of rice spittlebug Callitettix versicolor. Front. Zool. 2015, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sevarika, M.; Rondoni, G.; Ganassi, S.; Pistillo, O.M.; Germinara, G.S.; De Cristofaro, A.; Romani, R.; Conti, E. Behavioural and electrophysiological responses of Philaenus spumarius to odours from conspecifics. Sci. Rep. 2022, 12, 8402. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Rizvi, S.A.H.; George, J.; Reddy, G.V.; Zeng, X.; Guerrero, A. Latest developments in insect sex pheromone research and its application in agricultural pest management. Insects 2021, 12, 484. [Google Scholar] [CrossRef]
- Massola Júnior, N.S.; Bedendo, I.P.; Amorim, L.; Lopes, J.R.S. Effects of the inoculation time on corn with Spiroplasma kunkelii on yield components. Fitopal. Bras. 1999, 24, 4, 570–573. [Google Scholar]
- Oliveira, C.M.; Sabato, E.O. Doenças em milho: Insetos-vetores, molicutes e vírus, 2nd ed.; Embrapa: Brasília, Brazil, 2017; pp. 153–184. [Google Scholar]
- Sanches, M.S.; Michereff, M.F.F.; Borges, M.; Laumann, R.A.; Oliveira, C.M.; Frizzas, M.R.; Blassioli-Moraes, M.C. How much, how long and when: Density, duration and plant stage affect herbivore-induced plant volatiles in maize by the corn leafhopper. J. Chem. Ecol. 2025, 51, 53. [Google Scholar] [CrossRef]
- NIST. National Institute of Standards and Technology. Mass Spectrometry Data Center. 2020. Available online: https://chemdata.nist.gov/ (accessed on 14 April 2025).
- Leopold, E. Selective hydroboration of a 1, 3, 7-trene: Homogeraniol. J. Org. Synth. 1986, 64, 164–174. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2016. Available online: https://www.r-project.org (accessed on 20 June 2025).
- Faal, H.; Meier, L.R.; Canlas, I.J.; Murman, K.; Wallace, M.; Carrillo, D.; Cooperband, M.F. Volatiles from male honeydew excretions attract conspecific male spotted lanternflies, Lycorma delicatula (Hemiptera: Fulgoridae). Front. Insect Sci. 2022, 2, 982965. [Google Scholar] [CrossRef]
- Moraes, M.C.; Borges, M.; Pareja, M.; Vieira, H.G.; Sereno, F.T.S.; Laumann, R.A. Food and humidity affect sex pheromone ratios in the stink bug, Euschistus heros. Physiol. Entomol. 2008, 33, 43–50. [Google Scholar] [CrossRef]
- Claridge, M.F. Acoustic signals in the Homoptera: Behavior, taxonomy, and evolution. Annu Rev Entomol 1985, 30, 297–317. [Google Scholar] [CrossRef]
- Virant-Doberlet, M.; Cokl, A. Vibrational communication in insects. Neotrop. Entomol. 2004, 33, 121–134. [Google Scholar] [CrossRef]
- Strübing, H. Sound Production: The Crucial Factor for Mate Finding in Planthoppers (Homoptera: Auchenorrhyncha); Studying Vibrational Communication; Springer: Cham, Switzerland, 2014; pp. 53–61. [Google Scholar]
- Ranger, C.M.; Winter, R.E.; Backus, E.A.; Rottinghaus, G.E.; Ellersieck, M.R.; Johnson, D.W. Discrimination by the potato leafhopper (Hemiptera: Cicadellidae) of host volatiles from resistant and susceptible alfalfa, Medicago sativa L. Environ. Entomol. 2005, 34, 271–280. [Google Scholar] [CrossRef]
- Rodrigues, I.; Benhadi-Marín, J.; Rodrigues, N.; Baptista, P.; Pereira, J.A. Olfactory responses to volatile organic compounds and movement parameters of Philaenus spumarius and Cicadella viridis. J. Appl. Entomol. 2022, 146, 486–497. [Google Scholar] [CrossRef]
- Faal, H.; Cooperband, M.F.; Canlas, I.; Carrillo, D. Evidence of pheromone use in a fulgorid, spotted lanternfly. Forests 2022, 13, 1639. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Lopes, J.R.; Nault, L.R. Survival Strategies of Dalbulus maidis During Maize Off-Season in Brazil. Entomol. Exp. Appl. 2013 147, 141–153. [CrossRef]
- Canale, M.C.; Lopes, J.R.S.; Nesi, C.N.; Prado, S.d.S. Role of Dalbulus maidis (Hemiptera: Cicadellidae) gender on maizebushy stunt phytoplasma transmission. Phytopathogenic Mollicutes 2018, 8, 32–39. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Querino, R.B.; Jesus, E.A.G.; Sanches, M.S.; Aquino, M.F.S.; Souza, H.R.; Brito, M.L.; Malaquias, J.V.; Conceição, G.V.; Frizzas, M.R. Escape Behaviour of Dalbulus maidis (DeLong and Wolcott): Subsidies for Insecticide Application and Field Sampling. J. Appl. Entomol. 2025. [CrossRef]
- Verheggen, F.J.; Haubruge, E.; Mescher, M.C. Alarm pheromones—Chemical signaling in response to danger. Vitam. Horm. 2010, 83, 215–239. [Google Scholar]
- Boullis, A.; Verheggen, F.J. Chemical Ecology of Aphids (Hemiptera: Aphididae); Biology and Ecology of Aphids. In Biology and Ecology of Aphids; CRC Press: Boca Raton, FL, USA, 2016; p. 171. [Google Scholar]
- Nault, L.R.; Wood, T.K.; Goff, A.M. Treehopper (Membracidae) alarm pheromones. Nature 1974, 249, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Blassioli-Moraes, M.C.; Laumann, R.A.; Michereff, M.F.; Borges, M. Semiochemicals for Integrated Pest Management. In Sustainable Agrochemistry: A Compendium of Technologies; Peres, F., Lichtenstein, G., Eds.; Springer: Cham, Switzerland, 2019; pp. 85–112. [Google Scholar]
- Cocco, A.; Deliperi, S.; Delrio, G. Potential of mass trapping for Tuta absoluta management in greenhouse tomato crops using light and pheromone traps. IOBC-WPRS Bull. 2012, 80, 319–324. [Google Scholar]
- Hasan, M.M.; Mahroof, R.M.; Aikins, M.J.; Athanassiou, C.G.; Phillips, T.W. Pheromone-based auto-confusion for mating disruption of Plodia interpunctella (Lepidoptera: Pyralidae) in structures with raw and processed grain products. J. Stored. Prod. Res. 2023, 104, 102201. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, S.; Kim, Y.; Lee, Y. Real-time monitoring of oriental fruit moth, Grapholita molesta, populations using a remote sensing pheromone trap in apple orchards. J. Asia Pac. Entomol. 2011, 14, 259–262. [Google Scholar] [CrossRef]
- Laumann, R.A.; Moraes, M.C.B.; Khrimian, A.; Borges, M. Field capture of Thyanta perditor with pheromone-baited traps. Pesq. Agropec. Bras. 2011, 46, 113–119. [Google Scholar] [CrossRef]
- Silva, V.P.D.; Pereira, M.J.B.; Vivan, L.M.; Blassioli-Moraes, M.C.; Laumann, R.A.; Borges, M. Monitoring of the brown stink bug Euschistus heros (Hemiptera: Pentatomidae) with sex pheromone in soybean fields. Pesq. Agropec. Bras. 2014, 49, 844–852. [Google Scholar] [CrossRef]
- Krupke, C.H.; Roitberg, B.D.; Judd, G.J.R. Field and laboratory responses of male codling moth (Lepidoptera: Tortricidae) to a pheromone-based attract-and-kill strategy. Environ. Entomol. 2002, 31, 189–197. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Gao, Y.; Bian, L.; Sun, X.; Chen, Z. Volatiles from non-host aromatic plants repel tea green leafhopper Empoasca vitis. Entomol. Exp. Appl. 2014, 153, 156–169. [Google Scholar] [CrossRef]
- Niu, Y.; Han, S.; Wu, Z.; Pan, C.; Wang, M.; Tang, Y.; Zhang, Q.H.; Tan, G.; Han, B. A push–pull strategy for controlling the tea green leafhopper (Empoasca flavescens F.) using semiochemicals from Tagetes erecta and Flemingia macrophylla. Pest Manag. Sci. 2022, 78, 2161–2172. [Google Scholar] [CrossRef]
- Cooperband, M.F.; Murman, K.M. Spotted lanternflies respond to natural pheromone lures for mate-finding and oviposition. Insects 2024, 15, 447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanches, M.S.; Borges, M.; Laumann, R.A.; Oliveira, C.M.; Frizzas, M.R.; Blassioli-Moraes, M.C. Males of Dalbulus maidis Attract Females Through Volatile Compounds with Potential Pheromone Function: A Tool for Pest Management. Insects 2025, 16, 1021. https://doi.org/10.3390/insects16101021
Sanches MS, Borges M, Laumann RA, Oliveira CM, Frizzas MR, Blassioli-Moraes MC. Males of Dalbulus maidis Attract Females Through Volatile Compounds with Potential Pheromone Function: A Tool for Pest Management. Insects. 2025; 16(10):1021. https://doi.org/10.3390/insects16101021
Chicago/Turabian StyleSanches, Mateus Souza, Miguel Borges, Raul Alberto Laumann, Charles Martins Oliveira, Marina Regina Frizzas, and Maria Carolina Blassioli-Moraes. 2025. "Males of Dalbulus maidis Attract Females Through Volatile Compounds with Potential Pheromone Function: A Tool for Pest Management" Insects 16, no. 10: 1021. https://doi.org/10.3390/insects16101021
APA StyleSanches, M. S., Borges, M., Laumann, R. A., Oliveira, C. M., Frizzas, M. R., & Blassioli-Moraes, M. C. (2025). Males of Dalbulus maidis Attract Females Through Volatile Compounds with Potential Pheromone Function: A Tool for Pest Management. Insects, 16(10), 1021. https://doi.org/10.3390/insects16101021





