Simple Summary
The pea aphid, Acyrthosiphon pisum, is a major agricultural pest. It uniquely possesses fungal-derived genes that allow it to produce carotenoid pigments typically found in plants. Our study reveals a previously unknown role for these pigments beyond coloration. Under cold stress (12 °C), high light intensity triggers carotenoids to act like micro solar panels, capturing light to fuel ATP production. This leads to a striking 240% boost in energy, enhancing aphid development and reproduction under cold conditions. In contrast, the system remains inactive at warmer temperatures (22 °C). These findings demonstrate how a single horizontal gene transfer event can provide an organism with a novel energy-capturing ability, markedly increasing its adaptability in variable environments.
Abstract
The pea aphid, Acyrthosiphon pisum, possesses horizontally acquired fungal carotenoid biosynthesis genes, enabling de novo production of carotenoids. Although carotenoids are known to contribute to photo-protection and coloration, their potential role in energy metabolism and population fitness under thermal stress is still unclear. This study investigated the interactive effects of temperature and light intensity on energy homeostasis and life-history traits in A. pisum. Using controlled environmental regimes, we demonstrate that light intensity significantly influenced the ATP content, development, and reproductive output of A. pisum at 12 °C, but not at 22 °C. Under cold stress (12 °C), high light intensity (5000 lux) increased ATP content by 240%, shortened the pre-reproductive period by 46%, extended reproductive duration by 62%, and enhanced the net reproductive rate (R0) and intrinsic rate of increase (rₘ) compared to low light intensity (200 lux). These effects were abolished at the optimal temperature (22 °C), indicating a temperature-gated, light-dependent mechanism. Demographic analyses revealed that carotenoid-associated solar energy harvesting significantly improves fitness under cold conditions, likely compensating for metabolic depression. Our findings reveal a novel ecological adaptation in aphids, where horizontally transferred genes may enable light-driven energy supplementation during thermal stress. This study provides new insights into the physiological mechanisms underlying insect resilience to climate variability and highlights the importance of light as a key environmental factor in shaping life-history strategies in temperate agroecosystems.
1. Introduction
Carotenoids are multifunctional pigments widely distributed in nature [1], serving essential roles in photoprotection, coloration, and energy transfer across organisms [2,3]. While most animals must acquire carotenoids through their diet [4], the pea aphid Acyrthosiphon pisum represents a striking exception. Through horizontal gene transfer (HGT) from fungi, this insect has acquired the genetic machinery to synthesize carotenoids de novo, including lycopene β-cyclase/phytoene synthase (CrtYB) and phytoene desaturase (CrtI) [5,6]. This evolutionary innovation has been linked to aphid pigmentation polymorphism, with green morphs dominating cooler climates [7,8]. However, the potential energetic implications of this HGT event remain poorly understood.
Recent studies suggest that carotenoids in aphids may function beyond passive photoprotection. Observations of light-dependent ATP fluctuations in orange morphs [9] and enhanced ATP production in cold-adapted green morphs [7] hint at a possible role in energy metabolism. Parallel findings in spider mites (Tetranychus urticae)—another arthropod with fungal-derived carotenoid genes—show that these pigments regulate diapause via redox signaling [10,11]. Yet, no study has directly tested whether aphid carotenoids can harness light energy to sustain metabolic demand under thermal stress, nor quantified the fitness consequences of such a mechanism.
Here, we bridge this gap by investigating how HGT-derived carotenoids influence energy homeostasis and life history traits under ecologically relevant light–temperature regimes. We hypothesize that carotenoid-associated light absorption drives ATP synthesis to compensate for cold-induced metabolic depression. By coupling ATP quantification with life table demography across ecologically relevant light intensities (200–5000 lux) and temperatures (12 °C vs 22 °C), we demonstrate that light-dependent ATP synthesis is specifically activated at 12 °C but suppressed at 22 °C, and high light intensity under cold stress accelerates development and increases fecundity by >2-fold. This system represents a novel adaptation where horizontally acquired genes may enable solar energy harvesting in animals.
Our work expands the known functions of HGT in arthropods beyond classical roles in detoxification [12] and reveals a temperature-gated strategy for environmental adaptation. These findings also provide a mechanistic basis for the observed biogeographic distribution of aphid color morphs and open new avenues for studying non-photosynthetic light energy utilization in animals.
2. Materials and Methods
2.1. Aphid Rearing and Maintenance
The green morph of A. pisum was collected from broad bean (Vicia faba, cv. ‘Lincan-5’) fields in Xinxiang, Henan Province, China (35°18′ N, 113°52′ E). Colonies were maintained in controlled chambers (22 ± 1 °C, 70 ± 5% RH, 16:8 h L:D) on 4-week-old bean plants for more than 10 generations prior to experiments. To initiate experimental cohorts, ten adult aphids were placed on host plants and allowed to produce offspring for a 12-h period. All neonates produced within this period were collected and pooled from multiple mothers (>50 individuals) to minimize maternal and genetic bias. From this pooled mixture, 30 neonates were randomly selected to establish the initial experimental cohort.
2.2. ATP Content Determination
To investigate light intensity effects on ATP content in A. pisum, aphids were subjected to six environmental regimes comprising two temperatures (12 °C and 22 °C, ±1 °C) and three light intensities (5000 lux, 1000 lux, and 200 lux) in controlled climate chambers equipped with cool white LED lamps (spectral range: 400–780 nm, T8, Tuanpu Lighting Co., Ltd., Zhongshan, China). For each treatment, fifteen 2-day-old wingless adults were acclimated for 12 h on host plants under the respective conditions. Then, ten aphids were randomly selected and pooled for ATP quantification, with three independent biological replicates per treatment; each replicate came from a different batch and a separate plan.
ATP content was determined using the ATP Assay Kit (Fluorometric) (Beyotime, Shanghai, China, #S0026) according to the manufacturer’s instructions. Aphid samples were homogenized in lysis buffer on ice and centrifuged at 12,000× g for 5 min at 4 °C to collect the supernatant. Following reaction initiation, samples were incubated at 37 °C for 20 min to ensure complete color development. Fluorescence was measured using a BioTek Synergy HT microplate reader (Synergy HTX, BioTek Instruments Incorporated, Winooski, VT, USA) with an excitation/emission wavelength set of 535/700 nm and an integration time of 10 s. A standard curve generated with known ATP concentrations (R2 > 0.99) was used for quantification. ATP levels were normalized to sample fresh mass and expressed as μmol ATP per gram of fresh weight (μmol/g FW). No data points were excluded from the analyses.
2.3. Demographic Effects of Temperature and Light Intensity
Age-specific life tables for A. pisum on broad bean (Vicia faba) were constructed under controlled conditions in constant-temperature environmental chambers (GXM-508, Jiangnan Instrument Factory, Ningbo, China) at 12 °C (cold) and 22 °C (optimum). The experiment was initiated by placing 10 adult aphids on host plants. After production of >30 offspring, adults were removed and 30 first-instar nymphs were retained to establish experimental cohorts. Each cohort was maintained individually on a separate plant under one of three light intensities (5000, 1000, or 200 lux) with a 16:8 h light: dark cycle and 70 ± 5% relative humidity. Three independent plant-based replicates were included per treatment (n = 3).
To facilitate observation, black paper was placed beneath host plants to enhance visibility of aphid exuviae. Daily monitoring was conducted 3 h into the light phase to record developmental progression and reproductive output until all adults expired. During the reproductive period, newborn nymphs were counted and removed daily. The life table parameters included [13]:
- Net reproductive rate (R0), calculated as R0 = Σlxmx.
- The intrinsic rate of increase (rₘ), determined by numerically solving the Euler–Lotka equation: Σe(−rₘx)lxmx = 1, using an iterative algorithm with a convergence threshold of 10−6.
- Generation time (T), computed as T = Σlxmxx/R0.
- Doubling time (DT), defined as DT = ln(2)/rₘ.
In these expressions, x denotes age or developmental stage (in days), lx represents the age-specific survival rate (probability of survival from birth to age x, where 0 ≤ lx ≤ 1), and mₓ refers to the age-specific fecundity (mean number of female offspring produced per female at age x).
2.4. Data Analyses
Life table parameters of A. pisum under different light intensities at 12 °C and 22 °C were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) post hoc test to assess differences among light intensity treatments within each temperature. For all other data (including ATP content, developmental timing, and fecundity), two-way ANOVA was applied with temperature and light intensity as fixed factors, followed by Tukey’s honestly significant difference (HSD) post hoc tests when significant interactions or main effects were detected (p < 0.05). All data are presented as mean ± standard error (SEM). Statistical analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Light-Dependent ATP Synthesis Is Temperature-Sensitive
We discovered that light-dependent ATP synthesis in A. pisum is bidirectionally regulated by temperature, as evidenced by a highly significant temperature × light intensity interaction (Two-way ANOVA: F2,12 = 75.472, p < 0.0001) (Figure 1). Under cold stress (12 °C), ATP content increased progressively with light intensity, reaching a 2.4-fold higher level under high light (5000 lux: 12.165 ± 0.42 μmol/g) compared to low light (200 lux: 3.885 ± 0.21 μmol/g; p < 0.001), demonstrating active solar energy harvesting (Figure 1A). Conversely, at the optimal temperature (22 °C), ATP content significantly decreased with increasing light intensity, showing a 43% reduction under high light (5000 lux: 6.304 ± 0.33 μmol/g) relative to low light (200 lux: 11.118 ± 0.45 μmol/g; p < 0.001) (Figure 1B). This temperature-gated reversal of the light response suggests carotenoid-associated light-driven ATP synthesis is specifically activated during cold stress.
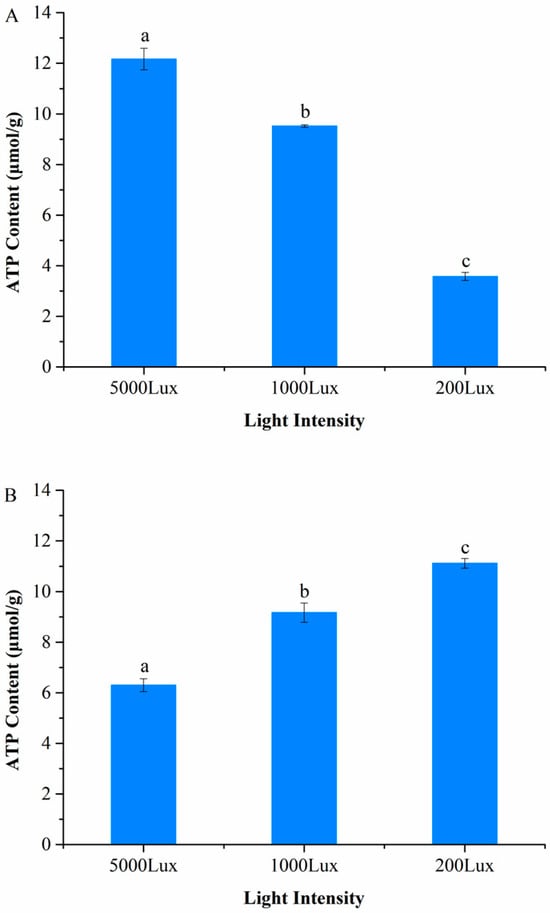
Figure 1.
Temperature-dependent effects of light intensity on ATP content in A. pisum. (A) At 12 °C. (B) At 22 °C. Data represent mean ± SEM (n = 3 independent biological replicates, each with 10 pooled aphids). Different lowercase letters indicate significant differences among light intensities within the same temperature (Tukey’s HSD test, p < 0.05).
3.2. Light-Accelerated Life History Under Cold Stress
A two-way ANOVA revealed that both temperature and light intensity significantly influenced the pre-reproductive period of A. pisum, with a highly significant interaction between the two factors (Temperature: F1,12 = 150.25, p < 0.0001; Light: F2,12 = 12.58, p = 0.0015; T×L: F2,12 = 28.36, p < 0.0001) (Figure 2). At 12 °C, the pre-reproductive period was significantly shortened under high light intensity (5000 lux: 13 ± 0.5 days) compared to low light (200 lux: 24 ± 1.2 days; p < 0.001). Conversely, at 22 °C, light intensity had no significant effect on the pre-reproductive duration (p > 0.05). A similar interaction pattern was observed for reproductive duration (T×L: F2,12 = 22.15, p < 0.0001), which was extended by 62% under high light at 12 °C but remained unaffected at 22 °C. These results suggest a temperature-gated, light-responsive plasticity in the life history strategies of A. pisum.
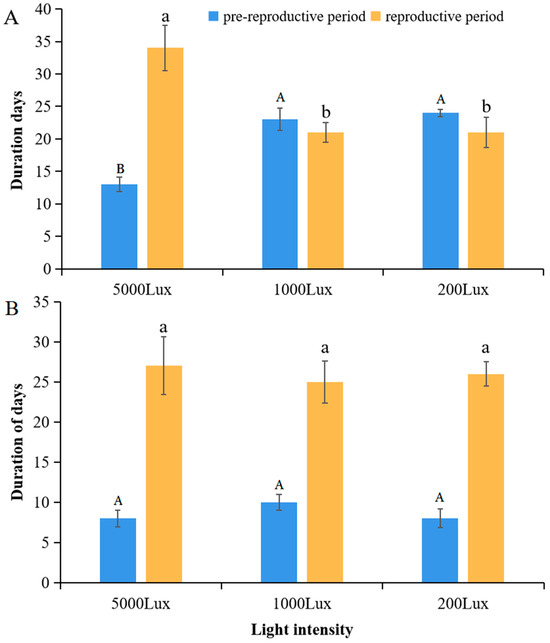
Figure 2.
Developmental timing responses to light intensity at (A) 12 °C and (B) 22 °C. Data represent mean ± SEM (n = 3 independent experimental replicates; each replicate included 30 pooled aphids from a single plant). Different letters indicate significant differences among light intensities within the same temperature (Tukey’s HSD test, p < 0.05).
3.3. Light-Enhanced Fecundity Under Cold Stress
A highly significant interaction was seen between temperature and light intensity on the daily fecundity of A. pisum (F2,12 = 62.91, p < 0.0001). Under cold stress (12 °C), fecundity increased significantly with light intensity, reaching 90.2 ± 5.1 nymphs/female/day at 5000 lux—markedly higher than values observed at 1000 lux (55.3 ± 4.2 nymphs/female/day; p < 0.01) and 200 lux (38.1 ± 3.5 nymphs/female/day; p < 0.001), representing a 2.4-fold increase under high illumination (Figure 3A). In contrast, at the optimal temperature (22 °C), fecundity remained consistently elevated (95.2–108.4 nymphs/female/day) across all light treatments with no statistically significant differences (p > 0.05) (Figure 3B). These results indicate that temperature may play a role in activating carotenoid-associated reproductive benefits.
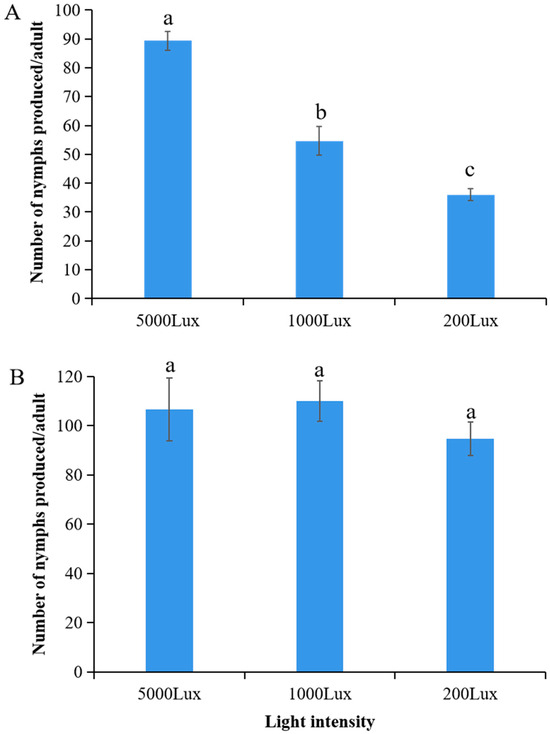
Figure 3.
Fecundity of A. pisum under varying light intensities at (A) 12 °C and (B) 22 °C. Data represent mean ± SEM (n = 3 independent experimental replicates; each replicate included 30 pooled aphids from a single plant). Different lowercase letters indicate significant differences among light intensities within the same temperature (Tukey’s HSD test, p < 0.05).
3.4. Light-Dependent Survival and Reproductive Strategies
Survival and fecundity patterns of A. pisum exhibited striking light-associated plasticity specifically under cold stress (12 °C) but not at optimal temperatures (22 °C). At 12 °C, high light intensity (5000 lux) resulted in stable early survival with low pre-reproductive mortality (7%) and sustained reproduction (peak mx = 8.1 nymphs/day during days 15–42). In contrast, under low light (200 lux), populations showed progressive mortality throughout the experiment, with higher early mortality (27%) and compressed reproductive windows (peak mx = 3.2 nymphs/day, days 27–32) (Figure 4). This light-dependent demographic plasticity was abolished at 22 °C, where all treatments maintained high early survival and showed no significant differences in reproductive output across light intensities (Figure 5). The temperature-gated transition between these demographic states suggests carotenoid-associated energy allocation is specifically activated under cold stress.
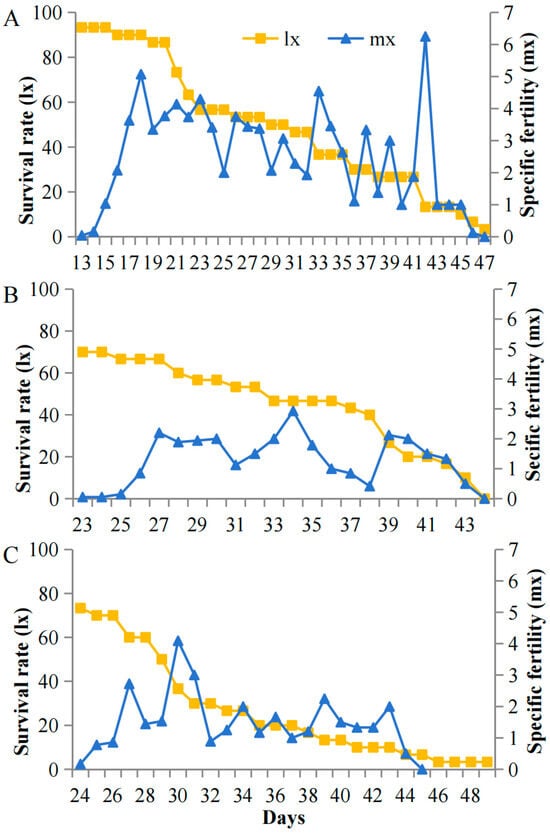
Figure 4.
Survival (lx) and fecundity (mx) schedules of A. pisum at 12 °C under (A) 5000 lux, (B) 1000 lux, and (C) 200 lux.
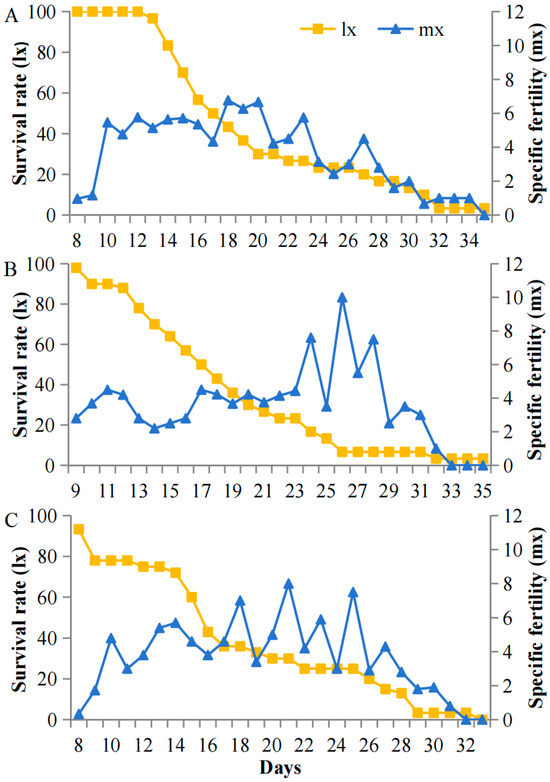
Figure 5.
Survival (lx) and fecundity (mx) schedules at 22 °C under (A) 5000 lux, (B) 1000 lux, and (C) 200 lux.
3.5. Light-Optimized Population Growth Under Cold Stress
A one-way ANOVA revealed a highly significant interaction between temperature and light intensity on all key demographic parameters of A. pisum (for all, p < 0.0001) (Table 1). Under cold stress (12 °C), high light intensity (5000 lux) profoundly enhanced population fitness, resulting in an 87% higher net reproductive rate (R0 = 82.3 ± 5.1 vs. 44.1 ± 3.2 offspring/female; p < 0.001), a 50% faster intrinsic rate of increase (rₘ = 0.21 ± 0.01 vs. 0.14 ± 0.01 day−1; p = 0.002), and a 30% shorter generation time (T = 18.2 ± 0.8 vs. 26.1 ± 1.2 days; p = 0.015) compared to low light conditions (200 lux). In stark contrast, these light-associated demographic advantages were completely abolished at the optimal temperature (22 °C), where no significant differences in R0, rₘ, T, or doubling time were observed across light intensity treatments (p > 0.05). These findings suggest that carotenoid-driven enhancement of population fitness is specifically activated under thermal stress, which is consistent with a sophisticated temperature-gated ecological adaptation in A. pisum.

Table 1.
Life table parameters of A. pisum under different light intensities at 12 °C.
4. Discussion
Since 2010, genomic studies have revealed that several arthropods, including insects such as the pea aphid [5] and the goldenrod gall midge (Asteromyia carbonifera) [14], as well as arachnids like the two-spotted spider mite (Tetranychus urticae) [15], possess horizontally acquired carotenoid biosynthesis genes of fungal origin. These genes typically encode lycopene β-cyclase/phytoene synthase (CrtYB) and phytoene desaturase (CrtI), which facilitate the production of carotenoids from simple precursors [10,16,17]. Functional studies have primarily elucidated the roles of these genes in aphids and spider mites, where carotenoids contribute to pigmentation, photoprotection, and energy metabolism [4,6,18,19].
Our study provides compelling evidence that carotenoid-associated solar energy utilization enhances the fitness of A. pisum under low-temperature conditions, offering a novel perspective on the physiological adaptations of aphids to environmental stress. The results suggest that light intensity significantly influences ATP synthesis, development, and reproductive performance in A. pisum at 12 °C, while these effects are negligible at the optimal temperature of 22 °C. These findings align with previous observations that green-morph aphids exhibit enhanced ATP production under cold stress [7] and suggest a potential adaptive role of carotenoids in energy metabolism during thermal challenges.
4.1. Carotenoid-Associated ATP Synthesis and Light Dependency
The observed increase in ATP content under high light intensity (5000 lux) at 12 °C supports the hypothesis that A. pisum utilizes carotenoids for photo-induced electron transfer, effectively converting solar energy into biochemical energy when ambient temperatures are suboptimal. This light-harvesting mechanism may compensate for reduced metabolic efficiency and contribute to the decrease of enzymatic activity and membrane fluidity under low temperatures, as evidenced by the 240% higher ATP levels at 5000 lux compared to 200 lux. The absence of this response at 22 °C indicates that this energy-capturing system is temperature-gated and likely suppressed under thermally optimal conditions where metabolic demand is fully met through conventional respiration. These results corroborate earlier findings that carotenoid-derived ATP synthesis in aphids is most active in cold-adapted green morphs [7,20].
4.2. Enhanced Reproductive Fitness Under Cold Stress
Life table analyses further revealed that high light intensity at 12 °C markedly enhanced reproductive performance through multiple pathways: shortening the pre-reproductive period by 46%, extending reproductive duration by 62%, and increasing net reproductive rate (R0) and intrinsic rate of increase (rm). These light-associated improvements in fecundity and developmental timing suggest that enhanced energy availability under high light mitigates cold-induced metabolic constraints, enabling sustained reproduction despite suboptimal temperatures. The improved early survival under high light further indicates that light-enhanced ATP production supports essential physiological functions under cold stress. This observation illustrates how light availability can directly shape demographic trajectories and life history strategies under thermal challenges.
4.3. Ecological and Evolutionary Implications
The temperature-specific effects of light on A. pisum fitness highlight a sophisticated adaptation to seasonal environments. The ability to harness solar energy for ATP synthesis may provide a selective advantage in early spring or late autumn, when temperatures are low but sunlight is abundant. This plasticity could explain the predominance of green morphs in cold climates and their rapid population growth under favorable light conditions [7]. Our results are closely consistent with the known photo-physical properties of carotenoids, especially their ability to associate with light-driven electron transfer, which further supports the proposed mechanism [5,7].
5. Conclusions
Our results underscore the importance of light as a critical environmental factor that interacts with temperature to shape aphid life history strategies. The light-dependent enhancement of energy metabolism and reproductive output under cold stress provides new insights into the physiological mechanisms underlying insect resilience to climate variability. Understanding these light-associated adaptations could improve predictive models of aphid population dynamics and inform the development of targeted management strategies in temperate agroecosystems.
Author Contributions
Conceptualization, J.M.; methodology, J.M. and H.L.; software, Z.G., H.L. and Y.D.; validation, Z.G., X.T., R.L. and M.B.; formal analysis, J.M., H.L., Y.D., Z.G., X.T. and R.L.; investigation, J.M., H.L., Y.D. and Z.G.; resources, J.M. and Y.W.; data curation, J.M., H.L., Y.D., Z.G. and X.T.; writing—original draft preparation, J.M. and H.L.; writing—review and editing, Y.D., Z.G., X.T. and M.B.; visualization, J.M. and H.L.; supervision, Y.W.; project administration, J.M. and Y.W.; funding acquisition, J.M. and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Special Fund for Independent Innovation of Henan Academy of Agricultural Sciences (2024ZC062), Henan Provincial Science and Technology Research and Development Plan Joint Fund (242301420135), Natural Science Foundation of Henan (242300420161), and China Agricultural Research System (CARS-03).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oliveira Filho, J.G.; Bertolo, M.R.V.; Fernandes, S.S.; Lemes, A.C.; Silva, G.C.; Bogusz Junior, S.; Azeredo, H.M.C.; Mattoso, H.L.C.; Egea, M.B. Intelligent and active biodegradable biopolymeric films containing carotenoids. Food Chem. 2024, 434, 137454. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.H.; Rajesh banu, J.; Kumar, G. Technological advances in the production of carotenoids and their applications—A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dora, K.C.; Kumar, S.; Saklani, P.; Muthukumar, A.; Ozogul, F.; Harisankar, K.C.; Mutum, R.D.; Celine Hilda Mary, S.; Surasani, V.K.R.; et al. A critical review on technical advances and multifaceted role of carotenoids in human health with special emphasis on metabolic diseases. Phytochem. Rev. 2025. [Google Scholar] [CrossRef]
- Maoka, T.; Kawase, N.; Ueda, T.; Nishida, R. Carotenoids of dragonflies, from the perspective of comparative biochemical and chemical ecological studies. Biochem. Syst. Ecol. 2020, 89, 104001. [Google Scholar] [CrossRef]
- Moran, N.A.; Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 2010, 328, 624–627. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Maoka, T.; Ueda, T.; Takemura, M. Biosynthesis of Carotenoids in Insects: What We Currently Know? In Carotenoids; Campos Chisté, R., de Aguiar Andrade, E.H., de Oliveira, M.S., Eds.; Springer: New York, NY, USA, 2024. [Google Scholar]
- Valmalette, J.; Dombrovsky, A.; Brat, P.; Mertz, C.; Capovilla, M.; Robichon, A. Light-induced electron transfer and ATP synthesis in a carotene synthesizing insect. Sci. Rep. 2012, 2, 579. [Google Scholar] [CrossRef] [PubMed]
- Trissi, N.; Troczka, B.J.; Ozsanlav-Harris, L.; Singh, K.S.; Mallott, M.; Aishwarya, V.; O’Reilly, A.; Bass, C.; Wilding, C.S. Differential regulation of the tor gene homolog drives the red/green pigmentation phenotype in the aphid Myzus persicae. Insect Biochem. Mol Biol. 2023, 153, 103896. [Google Scholar] [CrossRef] [PubMed]
- Lougheed, K. Photosynthesis-like process found in insects. Nature 2012. [Google Scholar] [CrossRef]
- Bryon, A.; Kurlovs, A.H.; Dermauw, W.; Greenhalgh, R.; Riga, M.; Grbić, M.; Tirry, L.; Osakabe, M.; Vontas, J.; Clark, R.M.; et al. Disruption of a horizontally transferred phytoene desaturase abolishes carotenoid accumulation and diapause in Tetranychus urticae. Proc. Natl. Acad. Sci. USA 2017, 114, E5871–E5880. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.M.; Zhang, Y.Y.; Song, Z.R.; Xiong, X.H.; Hong, X.Y. The potential pigmentation-related genes in spider mites revealed by comparative transcriptomes of the red form of Tetranychus urticae. Insect Mol. Biol. 2021, 30, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Wybouw, N.; Pauchet, Y.; Heckel, D.G.; Van Leeuwen, T. Horizontal gene transfer contributes to the evolution of arthropod herbivory. Genome Biol. Evol. 2016, 8, 1785–1801. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Benjamin Cummings: Boston, MA, USA, 1999. [Google Scholar]
- Cobbs, C.; Heath, J.; Stireman, J.O.; Abbot, P. Carotenoids in unexpected places: Gall midges, lateral gene transfer, and carotenoid biosynthesis in animals. Mol. Phylogenet. Evol. 2013, 68, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Altincicek, B.; Kovacs, J.L.; Gerardo, N.M. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 2012, 8, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Dong, C.; Zhang, P.; Roberts, T.H.; Park, R.F. Carotenoid biosynthesis and the evolution of carotenogenesis genes in rust fungi. Fungal Biol. 2021, 125, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, Y.; Wang, Y.; Zhang, Y.; Liu, M.; Zhang, Z.; Zhou, X.; Ren, M. Molecular basis governing diapause and pigmentation in the hawthorn spider mite, Amphitetranychus viennensis. BMC Biol. 2025, 23, 152. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Kawase, N.; Hironaka, M.; Nishida, R. Carotenoids of hemipteran insects, from the perspective of chemo-systematic and chemical ecological studies. Biochem. Syst. Ecol. 2021, 95, 104241. [Google Scholar] [CrossRef]
- Ding, B.Y.; Niu, J.; Shang, F.; Yang, L.; Zhang, W.; Smagghe, G.; Wang, J.J. Parental silencing of a horizontally transferred carotenoid desaturase gene causes a reduction of red pigment and fitness in the pea aphid. Pest Manag. Sci. 2020, 76, 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Feng, Z.; Zhu, J.; Zhang, Y.; Liu, T. Starvation stress causes body color change and pigment degradation in Acyrthosiphon pisum. Front. Physiol. 2019, 10, 197. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).