Comparing the Developmental Biology and Brood Size of Four Sclerodermus Species (Hymenoptera: Bethylidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Inoculation of Host Larvae with Parasitoids
2.3. Examining the Behavior of Female Parasitoids
2.4. Brood Size and Female Percentage of Parasitoid F2 Offspring
2.5. Body Size and Longevity of F2 Female Offspring
2.6. Statistical Analyses
3. Results
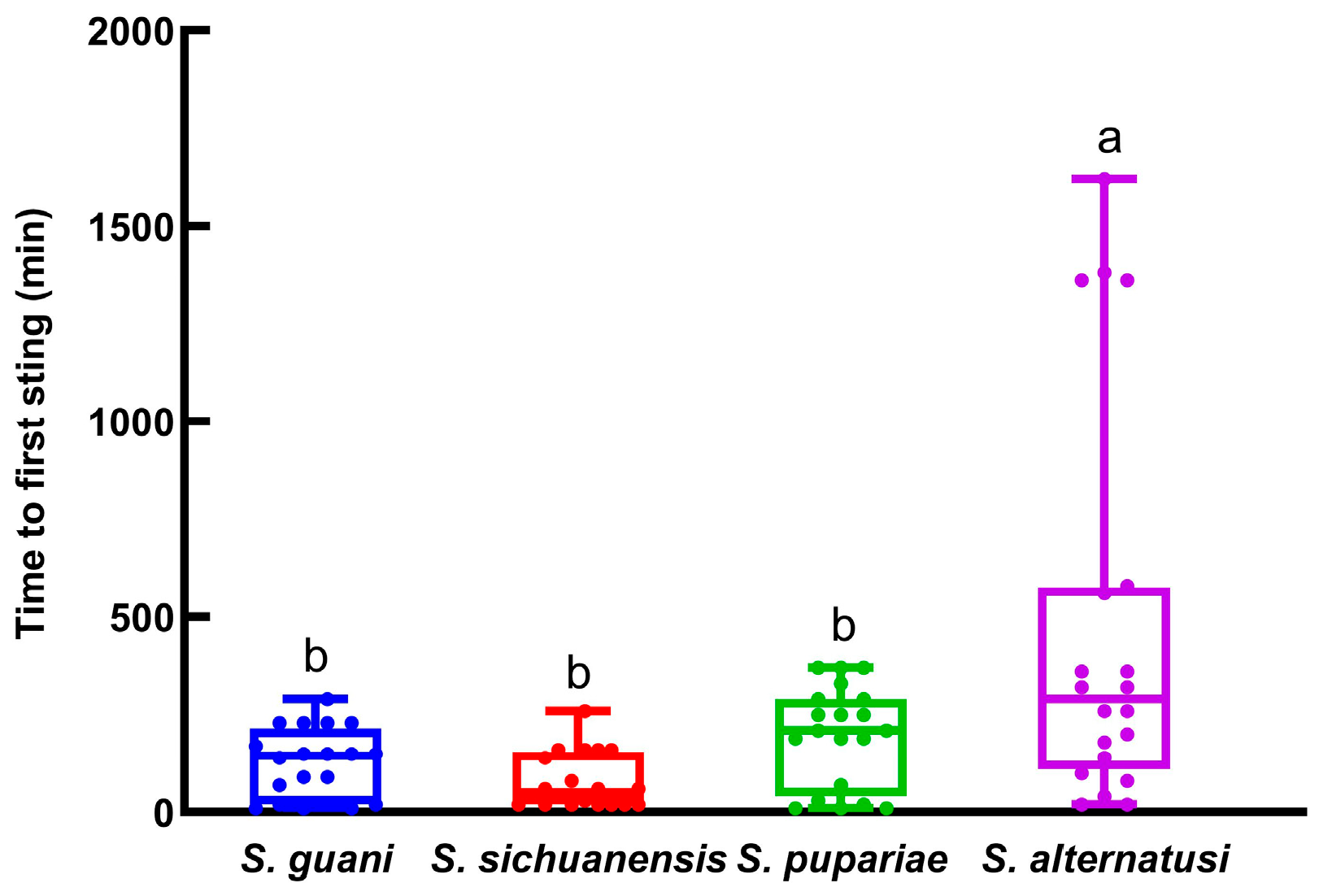
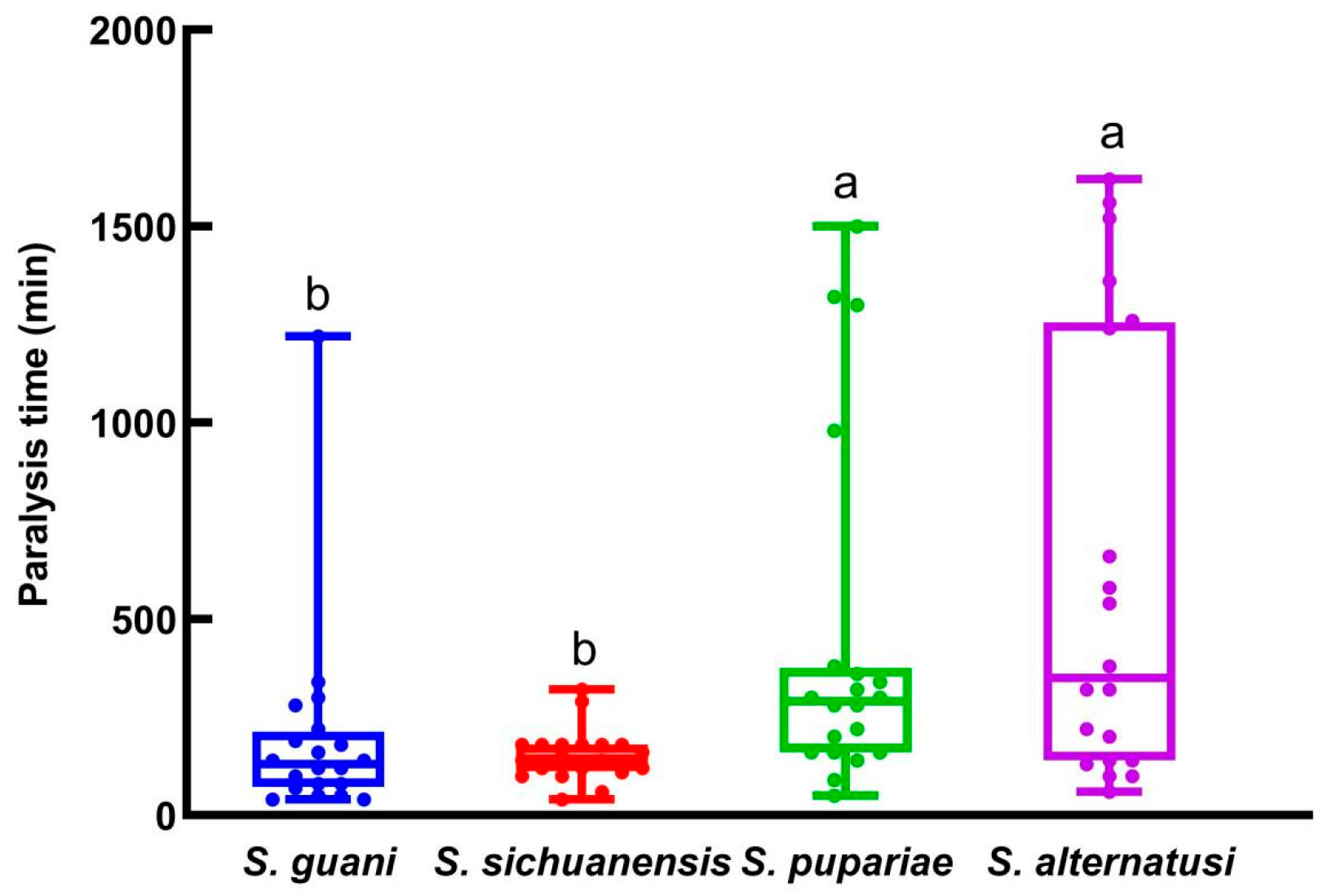
3.1. The Adaption Time, Host Paralysis Time and Pre-Oviposition Period
3.2. The Egg, Larval and Pupal Stages of the Four Sclerodermus Parasitoids
3.3. Brood Size of the Four Sclerodermus Parasitoids
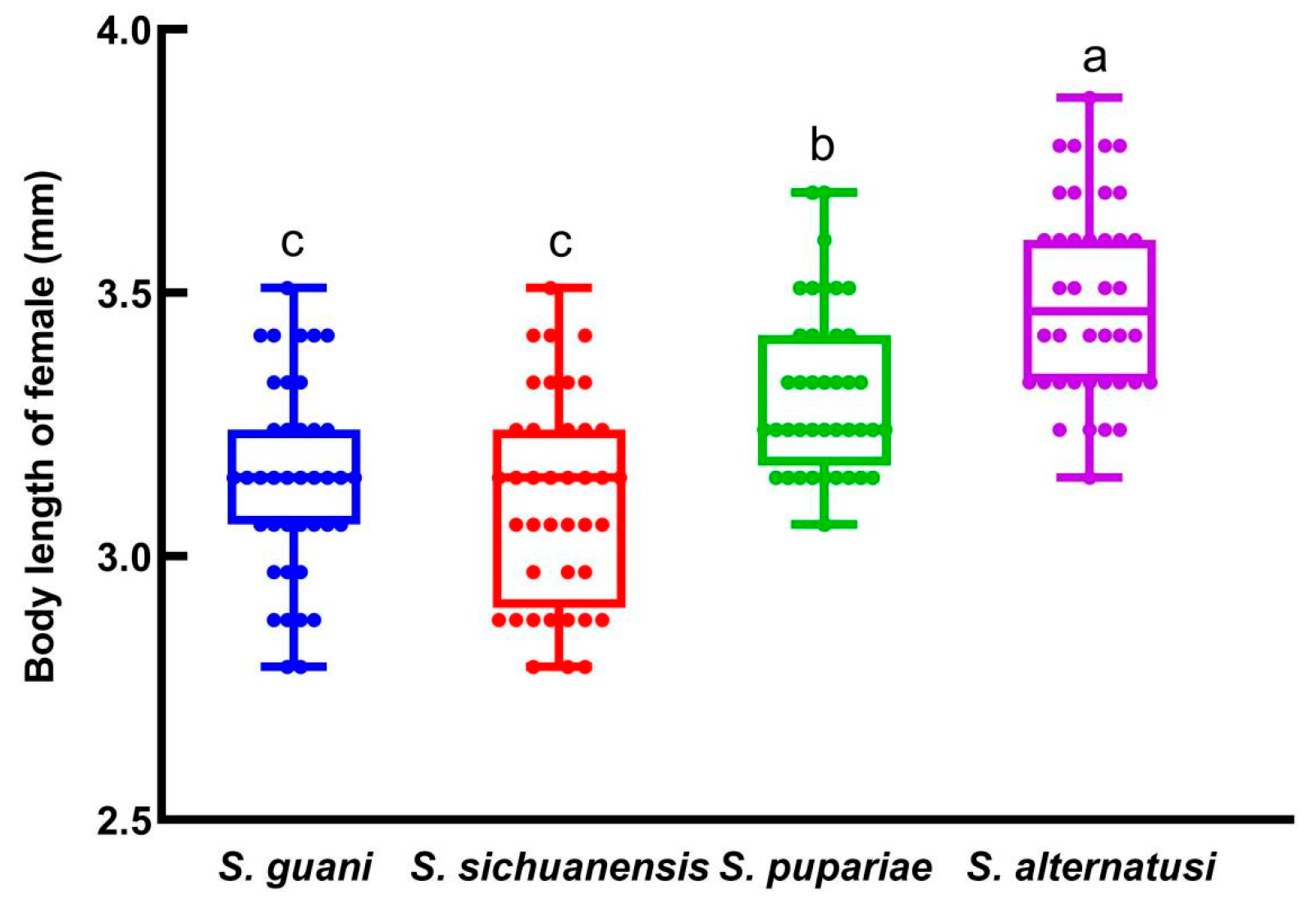
3.4. Body Length of F2 Female Offspring
3.5. Proportion of Winged Females in F2 Offspring
3.6. Survival of F2 Parasitoid Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| L:D | Light time/dark time |
| RH | Relative Humidity |
| ANOVA | Analysis of variance |
| LSD | Least significant difference |
| SE | Standard Error |
References
- Bijay, S.; Anju, P.; Samikshya, A. The impact of climate change on insect pest biology and ecology: Implications for pest management strategies, crop production, and food security. J. Agric. Food Res. 2014, 14, 100733. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Xiao, G.R.; Wu, J. A new species of Sclerodermus from China (Hymenoptera, Bethylidae). Sci. Silvae Sin. 1983, 19, 81–84. (In Chinese) [Google Scholar]
- Tian, S.Z.; Zhang, Z.X. Observe the larvae instars and form of Sclerodermus guani. J. Shandong For. Sci. Technol. 1984, 53, 65–66. (In Chinese) [Google Scholar]
- Zhang, W.G. Studies on Biology and Host Selection of Sclerodermus guani Xiao et Wu; Shandong Agricultural University: Taian, China, 2004. (In Chinese) [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Cao, L.M.; Yao, Y.X.; Tang, Y.L. Re-description of Sclerodermus guani and revision of the Genus (Hymenoptera: Bethylidae) in China. Chin. J. Biol. Control 2014, 30, 1–12. [Google Scholar]
- Xiao, G.R. Two new species of the genus Sclerodermus from China. Forest Res. 1995, 8, 1–5. (In Chinese) [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Yao, Y.X.; Gould, J.R.; Cao, L.M. A new species of Sclerodermus Latreille (Hymenoptera: Bethylidae) parasitizing Agrilus planipennis (Coleoptera: Buprestidae) from China with a key to Chinese species. Ann. Entomol. Soc. Am. 2012, 105, 619–627. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, X.Y.; Duan, Z.D.; Zhang, Y.L.; Zhang, Y.N.; Cao, L.M.; Wei, K. Sclerodermus alternatus (Hymenoptera: Bethylidae), a new species from China, parasitizing Monochamus alternatus (Coleoptera: Cerambycidae). Zool. Syst. 2024, 49, 258–266. [Google Scholar] [CrossRef]
- Zhou, N.; Yao, S.Z.; Hu, D.F.; Song, S.X.; Zai, J.L. Advances in artificial propagation and applied study of Sclerodermus guani. Arid Zone Res. 2005, 22, 569–575. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Z.J.; Yang, W.; Zeng, C.H.; Yang, D.M.; Ye, W.J. A preliminary study of the bionomics of Sclerodermus sichuanensis (Hymenoptera: Bethylidae). Sci. Slilvae Sin. 1997, 33, 475–480. (In Chinese) [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N.; Zhang, Y.L. Research advances of Chinese major forest pests by integrated management based on biological control. Chin. J. Biol. Control 2018, 34, 163–183. (In Chinese) [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.Y.; Li, M.L.; Yang, Z.Q.; Zeng, F.X.; Wang, H.Y.; Bai, L.; Liu, S.J.; Sun, J. Biology and mass rearing of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae), an important ectoparasitoid of the emerald ashborer, Agrilus planipennis (Coleoptera: Buprestidae) in China. Acta Entomol. Sin. 2008, 51, 46–54. (In Chinese) [Google Scholar] [CrossRef]
- Duan, J.J.; Ulyshen, M.D.; Bauer, L.S.; Gould, J.; van Driesche, R. Measuring the impact of biotic factors on populations of immature emerald ash borers (Coleoptera: Buprestidae). Ecol. Econ. 2010, 39, 1513–1521. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z.Q.; Wang, X.Y.; Hou, Y.X. Molecular identification of sibling species of Sclerodermus (Hymenoptera: Bethylidae) that parasitize buprestid and cerambycid beetles by using partial sequences of Mitochondrial DNA Cytochrome Oxidase Subunit 1 and 28s Ribosomal RNA gene. PLoS ONE 2015, 10, e0119573. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N.; Wu, C.J.; Ma, S.F.; Lu, Z.G. Functional response of the parasitoid Sclerodermus sp. (Hymenoptera: Bethylidae) to the third instar of host Monochamus alternatus (Coleoptera: Cerambycidae). Acta Entomol. Sin. 2012, 55, 426–434. (In Chinese) [Google Scholar] [CrossRef]
- Stiling, P.; Cornelissen, T. What makes a successful biocontrol agent? A meta-analysis of biological control agent performance. Biol. Control 2005, 34, 236–246. [Google Scholar] [CrossRef]
- Stilmant, D.; van Bellinghen, C.; Hance, T.; Boivin, G. Host specialization in habitat specialists and generalists. Oecologia 2008, 156, 905–912. [Google Scholar] [CrossRef]
- Tang, Y.L.; Wang, L.N.; Zhang, Y.L.; Wu, S.Y.; Wang, X.Y.; Yang, Z.Q. Effect of different parasitoid colonies on the mass rearing of Sclerodermus alternatusi (Hymenoptera: Bethylidae). Sci. Slilvae Sin. 2020, 56, 97–103. (In Chinese) [Google Scholar] [CrossRef]
- Tang, Y.L.; Wang, L.N.; Dai, H.L.; He, J.M.; Kang, K.; Zhang, Y.L.; Cao, L.M. Effect of low temperature storage duration on breeding of Sclerodermus alternatusi Yang (Hymenoptera: Bethylidae). Chin. J. Biol. Control 2022, 38, 1361–1366. (In Chinese) [Google Scholar] [CrossRef]
- Tang, Y.L.; Wang, L.N.; Chen, Y.; Kang, K.; Zeng, B.P.; Yang, Z.Q.; Wei, K. Parasitism ability and offspring development of Sclerodermus alternatusi Yang (Hymenoptera: Bethylidae) in different female oviposition times. Chin. J. Biol. Control 2023, 39, 499–506. (In Chinese) [Google Scholar] [CrossRef]
- Tang, Y.L.; Wang, L.N.; Liu, F.; Kang, K.; Zeng, B.P.; Wang, X.Y.; Wei, K. Comparison of parasitic behavior and progeny development between winged and wingless female sdults of Sclerodermus pupariae (Hymenoptera: Bethylidae). Sci. Slilvae Sin. 2023, 59, 128–136. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.Y.; Wei, K.; Yang, Z.Q.; Jennings, D.E.; Duan, J.J. Effects of biotic and abiotic factors on phenotypic partitioning of wing morphology and development in Sclerodermus pupariae (Hymenoptera: Bethylidae). Sci. Rep. 2016, 6, 26408. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X.Y.; Yang, Z.Q.; Duan, J.J. Effects of photoperiod and light intensity on wing dimorphism and development in the parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Moore, M.E.; Kester, K.M.; Kingsolver, J.G. Rearing temperature and parasitoid load determine host and parasitoid performance in Manduca sexta and Cotesia congregata. Ecol. Entomol. 2020, 45, 79–89. [Google Scholar] [CrossRef]
- Wang, L.N.; Situ, Y.H.; Zhang, J.; Kang, K.; Xiao, Z.J.; Wang, S.B.; Wei, K.; Tang, Y.L. The thermal adaptability of Sclerodermus guani Xiao et Wu (Hymenoptera: Bethylidae), an important parasitoid of long-horned beetles in China. Biology 2025, 14, 1234. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X.Y.; Yang, Z.Q.; Chen, R. Optimal temperatures for artificial rearing of parasitoid, Sclerodermus pupariae (Hymenoptera: Bethylidae). Chin. J. Biol. Control 2019, 35, 343–349. (In Chinese) [Google Scholar] [CrossRef]
- Kang, K.; Wang, L.M.; Xiao, Z.J.; Wang, S.B.; Wei, K.; Wang, X.Y.; Zhang, Y.L.; Tang, Y.L. Effects of photoperiod on the developmental duration and reproduction of Sclerodermus sichuanensis. Insects 2025, 16, 701. [Google Scholar] [CrossRef]
- Wei, K.; Tang, Y.L.; Wang, X.Y.; Cao, L.M.; Yang, Z.Q. The developmental strategies and related profitability of an idiobiont ectoparasitoid Sclerodermus pupariae vary with host size. Ecol. Entomol. 2014, 39, 101–108. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Yang, C.P.; Zhou, Z.J.; Yang, W. The survival rate of Scleroderma sichuanensis in different storage conditions. Chin. Bu. Entomol. 2006, 43, 673–677. (In Chinese) [Google Scholar]
- Tang, Y.L.; Wang, X.Y.; Yang, Z.Q.; Jiang, J.; Wei, J.R.; Wei, K.; Lu, J. Studies on control young larvae of Massicus raddei (Coleoptera: Cerambycidae) by applying parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae) and its supercooling point. Chin. J. Biol. Control 2014, 30, 293–299. (In Chinese) [Google Scholar]
- Gao, S.K.; Wei, K.; Tang, Y.L.; Wang, X.Y.; Yang, Z.Q. Effect of parasitoid density on the timing of parasitism and development duration of progeny in Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2016, 97, 57–62. [Google Scholar] [CrossRef]
- Gao, S.K.; Tang, Y.L.; Wei, K.; Wang, X.Y.; Yang, Z.Q.; Zhang, Y.L. Relationships between body size and parasitic fitness and offspring performance of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae). PLoS ONE 2016, 11, e0156831. [Google Scholar] [CrossRef]
- Fisher, R. The Genetical Theory of Natural Selection; Clarendon Press: Oxford, UK, 1930. [Google Scholar]
- King, B.H. Offspring sex ratios in parasitoid wasps. Q. Rev. Biol. 1987, 62, 367–396. [Google Scholar] [CrossRef]
- Tang, X.Y.; Meng, L.; Kapranas, A.; Xu, F.Y.; Hardy, I.C.W.; Li, B. Mutually beneficial host exploitation and ultra–biased sex ratios in quasisocial parasitoids. Nat. Commun. 2014, 5, 4942. [Google Scholar] [CrossRef] [PubMed]
- Boulton, R.A.; Collins, L.A.; Shuker, D.M. Beyond sex allocation: The role of mating systems in sexual selection in parasitoid wasps. Biol. Rev. 2015, 90, 599–627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.S.; Hopper, K.R.; Ode, P.J.; Fuester, R.W.; Chen, J.H.; Heimpel, G.E. Complementary sex determination in Hymenopteran parasitoids and its implications for biological control. Entomol. Sin. 2003, 10, 81–93. [Google Scholar] [CrossRef]
- Roff, D.A. The cost of being able to fly: A study of wing polymorphism in two species of crickets. Oecologia 1984, 63, 30–37. [Google Scholar] [CrossRef]
- Guerra, P.A. Evaluating the life-history trade-off between dispersal capability and reproduction in wing dimorphic insects: A meta-analysis. Biol. Rev. 2011, 86, 813–835. [Google Scholar] [CrossRef]
- Wei, K.; Gao, S.K.; Tang, Y.L.; Wang, X.Y.; Yang, Z.Q. Determination of the optimal parasitoid-to-host ratio for efficient mass-rearing of the parasitoid, Sclerodermus pupariae (Hymenoptera: Bethylidae). J. Appl. Entomol. 2017, 141, 181–188. [Google Scholar] [CrossRef]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Ma, X.F.; Jiang, Y.Q.; Fan, H.W.; Xu, J.Y.; et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef]


| Species | Egg Stage/h | Larval Stage/d | Pupal Stage of Males/d | Pupal Stage of Females/d | Developmental Period of Males/d | Developmental Period of Females/d |
|---|---|---|---|---|---|---|
| S. guani | 85.20 ± 1.20 c | 5.65 ± 0.10 b | 10.95 ± 0.14 b | 11.60 ± 0.13 b | 20.15 ± 0.13 c | 20.80 ± 0.13 c |
| S. sichuanensis | 89.40 ± 1.62 bc | 5.63 ± 0.10 b | 10.98 ± 0.16 b | 11.55 ± 0.16 b | 20.33 ± 0.18 c | 20.90 ± 0.18 c |
| S. pupariae | 92.80 ± 1.47 ab | 5.40 ± 0.09 b | 11.85 ± 0.15 a | 12.53 ± 0.13 a | 21.13 ± 0.14 b | 21.80 ± 0.14 b |
| S. alternatusi | 94.10 ± 1.80 a | 7.10 ± 0.11 a | 10.78 ± 0.18 b | 11.45 ± 0.21 b | 21.80 ± 0.24 a | 22.48 ± 0.25 a |
| df | 3, 79 | 3, 79 | 3, 79 | 3, 79 | 3, 79 | 3, 79 |
| F | 6.693 | 65.474 | 9.601 | 9.749 | 19.072 | 19.573 |
| p | 0.0005 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Species | Number of F2 Male Offspring (n) | Number of F2 Female Offspring (n) | Total Number of F2 Offspring (n) | F2 Female Percentage (%) |
|---|---|---|---|---|
| S. guani | 3.90 ± 0.55 b | 70.85 ± 3.30 a | 74.75 ± 3.34 a | 94.71 ± 0.69 b |
| S. sichuanensis | 2.75 ± 0.44 b | 50.85 ± 3.98 b | 53.60 ± 4.19 b | 94.95 ± 0.66 b |
| S. pupariae | 1.60 ± 0.18 b | 63.60 ± 3.84 a | 65.20 ± 3.92 a | 97.46 ± 0.27 a |
| S. alternatusi | 18.40 ± 2.49 a | 35.30 ± 2.23 c | 53.70 ± 3.12 b | 67.07 ± 3.81 b |
| df | 3, 79 | 3, 79 | 3, 79 | 3, 79 |
| F | 36.949 | 20.781 | 7.736 | 53.388 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Species | Survival Rate of F2 Parasitoid Females over Time (%) | |||||
|---|---|---|---|---|---|---|
| 30 d | 60 d | 90 d | 120 d | 150 d | 180 d | |
| S. guani | 99.61 ± 0.14 a | 97.60 ± 0.42 a | 95.48 ± 0.55 a | 90.22 ± 1.30 a | 44.56 ± 2.23 a | 5.38 ± 1.02 a |
| S. sichuanensis | 97.91 ± 0.54 b | 95.23 ± 0.77 b | 90.83 ± 1.47 b | 85.17 ± 1.94 ab | 41.55 ± 3.57 a | 4.44 ± 1.41 a |
| S. pupariae | 98.74 ± 0.34 ab | 95.74 ± 0.88 b | 91.52 ± 1.76 b | 85.44 ± 2.68 ab | 43.88 ± 3.71 a | 6.68 ± 1.50 a |
| S. alternatusi | 99.03 ± 0.35 a | 95.25 ± 0.59 b | 89.90 ± 0.81 b | 80.49 ± 1.56 b | 43.50 ± 1.24 a | 3.26 ± 0.81 a |
| df | 3, 79 | 3, 79 | 3, 79 | 3, 79 | 3, 79 | 3, 79 |
| F | 3.609 | 2.667 | 3.867 | 4.193 | 0.203 | 1.410 |
| p | 0.017 | 0.0437 | 0.0125 | 0.0084 | 0.8942 | 0.2464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Situ, Y.; Zhang, J.; Wang, L.; Kang, K.; Xiao, Z.; Wang, S.; Wang, X.; Wei, K.; Tang, Y. Comparing the Developmental Biology and Brood Size of Four Sclerodermus Species (Hymenoptera: Bethylidae). Insects 2025, 16, 1012. https://doi.org/10.3390/insects16101012
Situ Y, Zhang J, Wang L, Kang K, Xiao Z, Wang S, Wang X, Wei K, Tang Y. Comparing the Developmental Biology and Brood Size of Four Sclerodermus Species (Hymenoptera: Bethylidae). Insects. 2025; 16(10):1012. https://doi.org/10.3390/insects16101012
Chicago/Turabian StyleSitu, Yuhua, Jie Zhang, Lina Wang, Kui Kang, Zhongjiu Xiao, Shaobo Wang, Xiaoyi Wang, Ke Wei, and Yanlong Tang. 2025. "Comparing the Developmental Biology and Brood Size of Four Sclerodermus Species (Hymenoptera: Bethylidae)" Insects 16, no. 10: 1012. https://doi.org/10.3390/insects16101012
APA StyleSitu, Y., Zhang, J., Wang, L., Kang, K., Xiao, Z., Wang, S., Wang, X., Wei, K., & Tang, Y. (2025). Comparing the Developmental Biology and Brood Size of Four Sclerodermus Species (Hymenoptera: Bethylidae). Insects, 16(10), 1012. https://doi.org/10.3390/insects16101012






