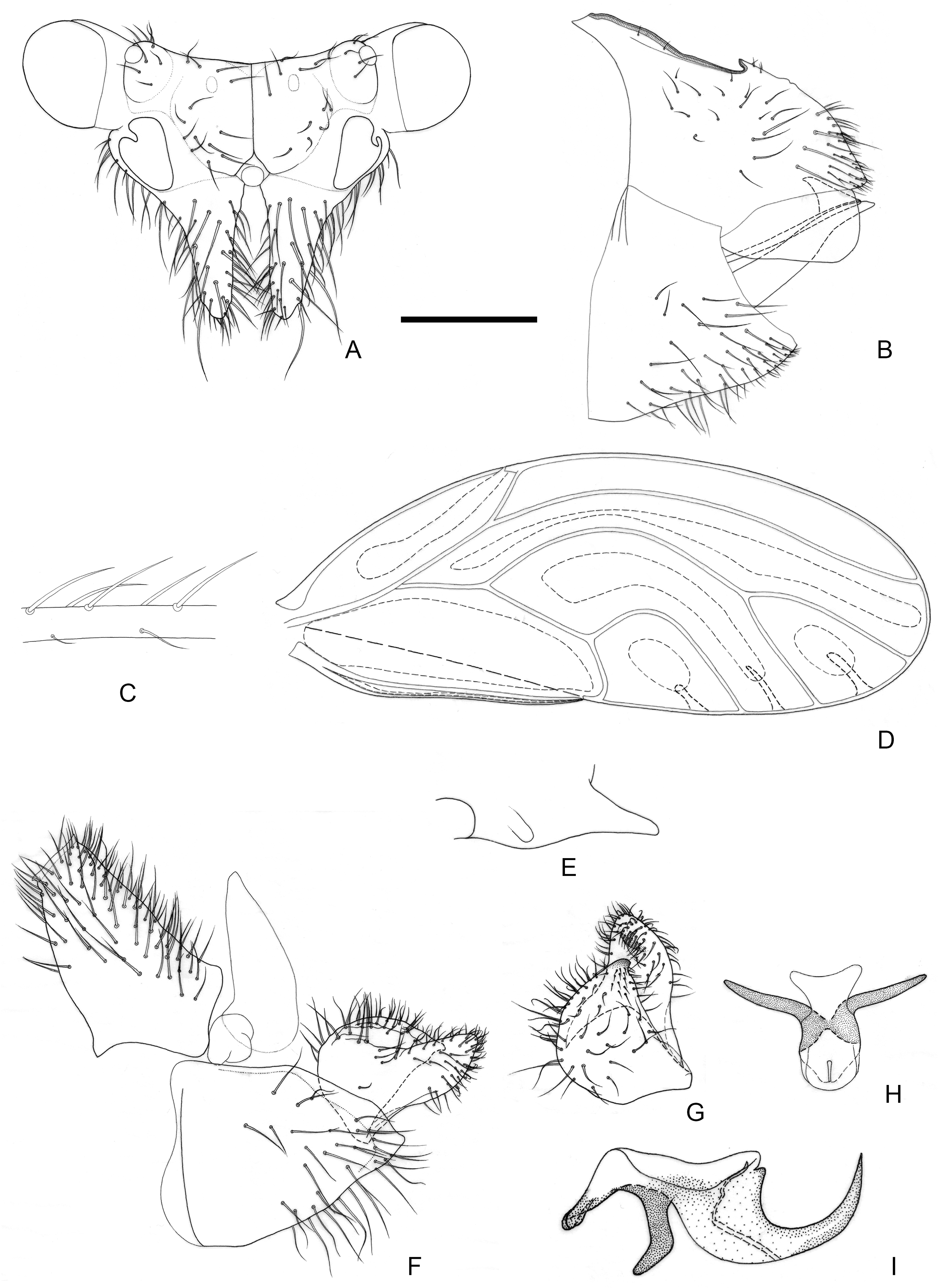
3.1.1. Epipsylla crotalariae Yang & Li, 1984
Figure 2.
Epipsylla crotalariae Yang & Li, 1984. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus. Scale bar (mm): (A) 0.3; (B) 0.15; (C) 0.1; (D) 0.6; (E) 0.15; (F) 0.15; (G) 0.1; (H) 0.1.
Figure 2.
Epipsylla crotalariae Yang & Li, 1984. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus. Scale bar (mm): (A) 0.3; (B) 0.15; (C) 0.1; (D) 0.6; (E) 0.15; (F) 0.15; (G) 0.1; (H) 0.1.
Epipsylla cortalariae Yang & Li [
25]: 251; Yang & Li [
38] (citation); Hodkinson [
39] (list); Li [
27] (redescription).
Material examined: Holotype: male, China, Yunnan, Longchuan, Husa, 24°46′72″ N, 97°89′32″ E, alt. 1430 m, 29 April 1981, Fasheng Li leg. on Crotalaria retusa; dry-mounted; CAU. Paratype: one female, same information as holotype.
Diagnosis: Adult. Body dark green. Head bearing 1 + 1 white longitudinal stripe on both sides, surrounding lateral ocelli and extended to postocular sclerite, with black edge inside, black side mixed with partial outside black edge of middle stripe (
Figure 2A). Genal processes thick and long, apex rounded and blunt, bearing setae (
Figure 2A). Metatibia without basal spine, apex with an open, dense crown of 7–8 strongly sclerotized apical spurs. Forewing oval, widest in the middle; pterostigma very small (
Figure 2D); fore margin with conspicuous dark long setae, gradually getting shorter toward wing apex (
Figure 2C); surface spinules absent except for cell cu
2, radular spinules forming narrow stripes in the middle of cells m1, m2, and cu1 along wing margin (
Figure 2D). Meracanthus slender, uniform thickness, apex rounded and blunt (
Figure 2E). Male terminalia: Proctiger 1.7 times as long as paramere (
Figure 2F). Paramere as in
Figure 2G; outer lobe evenly rounded apically, with sclerotized area on inner surface extended, covering apical third almost entirely; inner lobe short, apex strongly sclerotized, angular, extending to distal third of outer lobe. Basal segment of aedeagus almost entirely straight, gradually narrowing to apex which is thin and curved (
Figure 2F). Distal segment of aedeagus as in
Figure 2H; 1.2 times as long as basal segment; base forming short, subacute dorsal horn; ventro-lateral sclerotized processes almost in apical third, long, tortile; ventral process subapically, divided in apical half; apical dilatation sub-oval; sclerotized end tube near aedeagal apex. Female terminalia: Overall high and long. Dorsal part of apical half of proctiger strongly raised, and apex bearing a tuft of fine setae (
Figure 2B).
Measurements (mm): Male (n = 1): BL 4.38; AL 4.48; HW 0.87; TW 0.87; WL 3.37. Female (n = 1): BL 4.77; AL 4.78; HW 0.97; TW 0.88; WL 3.63 (from Li 2011).
Fifth-instar immature unknown.
Host plant: Adults were collected on Crotalaria retusa L. (Fabaceae), which is a possible host.
Distribution: China (Yunnan).
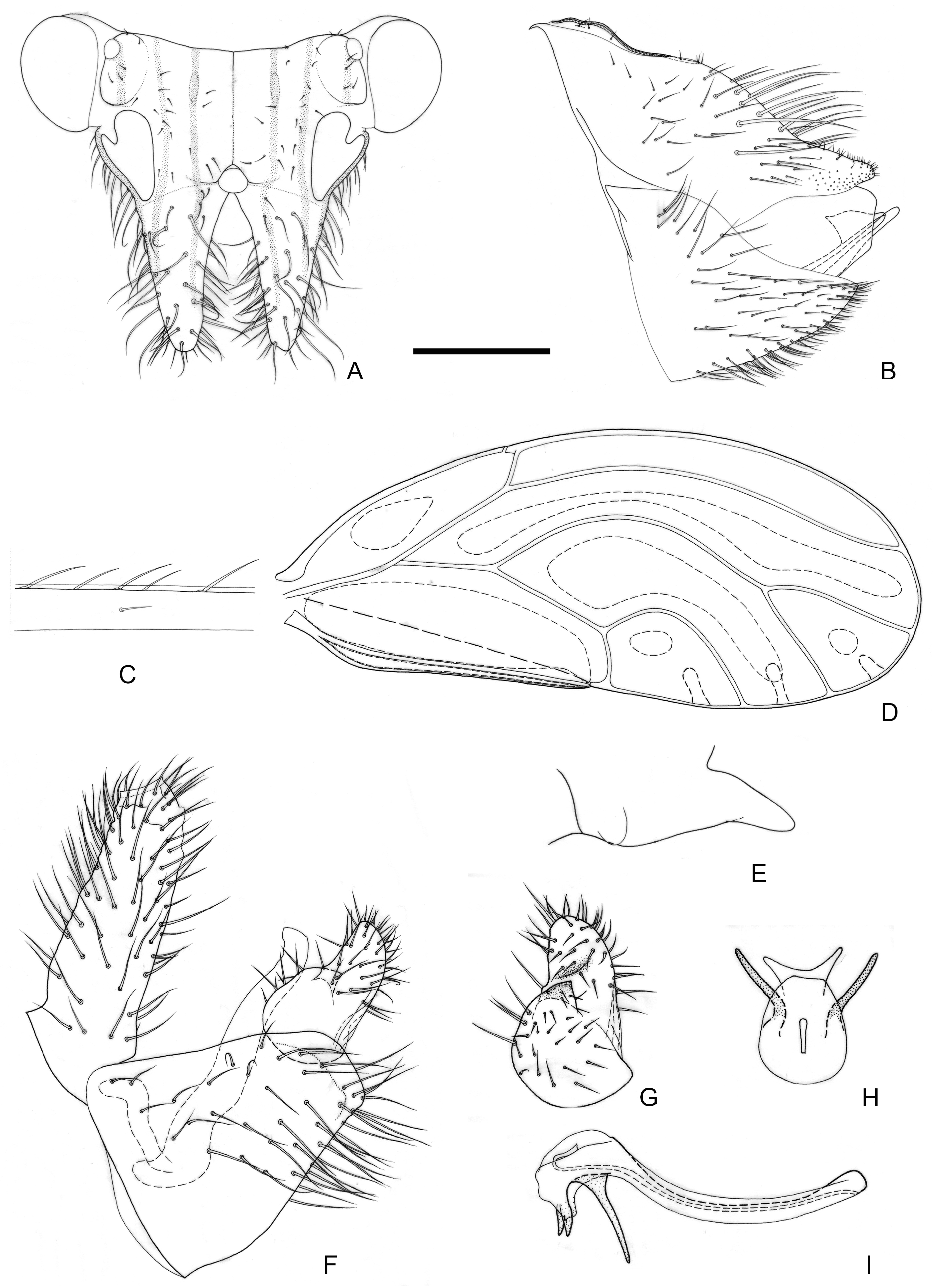
3.1.2. Epipsylla guangxiana Yang & Li, 1983
Figure 3.
Epipsylla guangxiana Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.1; (G) 0.1; (H) 0.1; (I) 0.1.
Figure 3.
Epipsylla guangxiana Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.1; (G) 0.1; (H) 0.1; (I) 0.1.
Epipsylla guangxiana Yang & Li [
24]: 314; Hodkinson [
39] (list); Li [
27] (description).
Epipsylla whitfordiodendritis Yang & Li [
24]: 315; Hodkinson [
39] (list); Li et al. [
12] (synonymy).
Material examined: Holotype: holotype of E. guangxiana: male, China, Guangxi, Tianlin, Langping, 24°48′45″ N, 106°36′54″ E, alt. 1500 m, 29 May 1982, Fasheng Li & Ceekun Yang leg. on Padbruggea filipes; dry-mounted; CAU; holotype of E. whitfordiodendritis: same information as E. guangxiana. Paratypes: paratypes of E. guangxiana: four females (dry-mounted) and two immatures (in 75% ethanol), same information as holotype; paratypes of E. whitfordiodendritis: eight males, ten females, same information as holotype.
Diagnosis: Adult. Body green. Vertex bearing 1 + 1 white stripe with black edge in middle, extending forward but not reaching apex of genal processes; head bearing 1 + 1 white longitudinal stripe on both sides, with black edge inside. (
Figure 3A). Genal processes thick and long, apex rounded and blunt, bearing slender setae (
Figure 3A). Metatibia without basal spine, apex with an open, dense crown of eight strongly sclerotized apical spurs. Forewing bearing three rows (dorsal surface, ventral surface, and anterior margin) of long setae in vein C + Sc (
Figure 3C); surface spinules absent apart from base of cell cu
2, radular spinules forming narrow stripes in the middle of cells r
2, m
1, m
2 and cu
1 along wing margin (
Figure 3D). Meracanthus slender, apex somewhat acute, overall decurved (
Figure 3E).
Male terminalia: Proctiger 1.4–1.5 times as long as paramere (
Figure 3F). Paramere as in
Figure 3G; outer lobe irregularly rounded apically, with sclerotized area on inner surface restricted to area near apex; inner lobe short, apex strongly sclerotized, angular, extending to distal quarter of outer lobe. Basal segment of aedeagus weakly sinuous, gradually narrowing to apex (
Figure 3F). Distal segment of aedeagus as in
Figure 3H,I; 1.2 times as long as basal segment; base forming short, blunt dorsal tubercle; ventro-lateral sclerotized processes in apical quarter, moderately long, irregularly weakly curved; ventral process subapically, divided in apical quarter; apex not dilated; sclerotized end tube near aedeagal apex. Female terminalia: Proctiger narrower in apical half, apex rounded and blunt, with sparse tiny setae (
Figure 3B).
Measurements (mm): Male (n = 3); BL 3.76–3.90; AL 4.85–4.88; HW 0.85–0.86; TW 0.70–0.71; WL 3.01–3.12. Female (n = 3): BL 4.13–4.27; AL 5.03–5.54; HW 0.89–0.90; TW 0.72–0.75; WL 3.33–3.34.
Fifth-instar *immature: Abdominal margin bearing a pair of sectasetae. Outer circumanal ring comprising more than six rows of oval pores laterally which, between the anterior and posterior margins, consist of subrectangular pores.
Host plant: Padbruggea filipes (Dunn) Craib (Fabaceae).
Distribution: China (Guangxi).
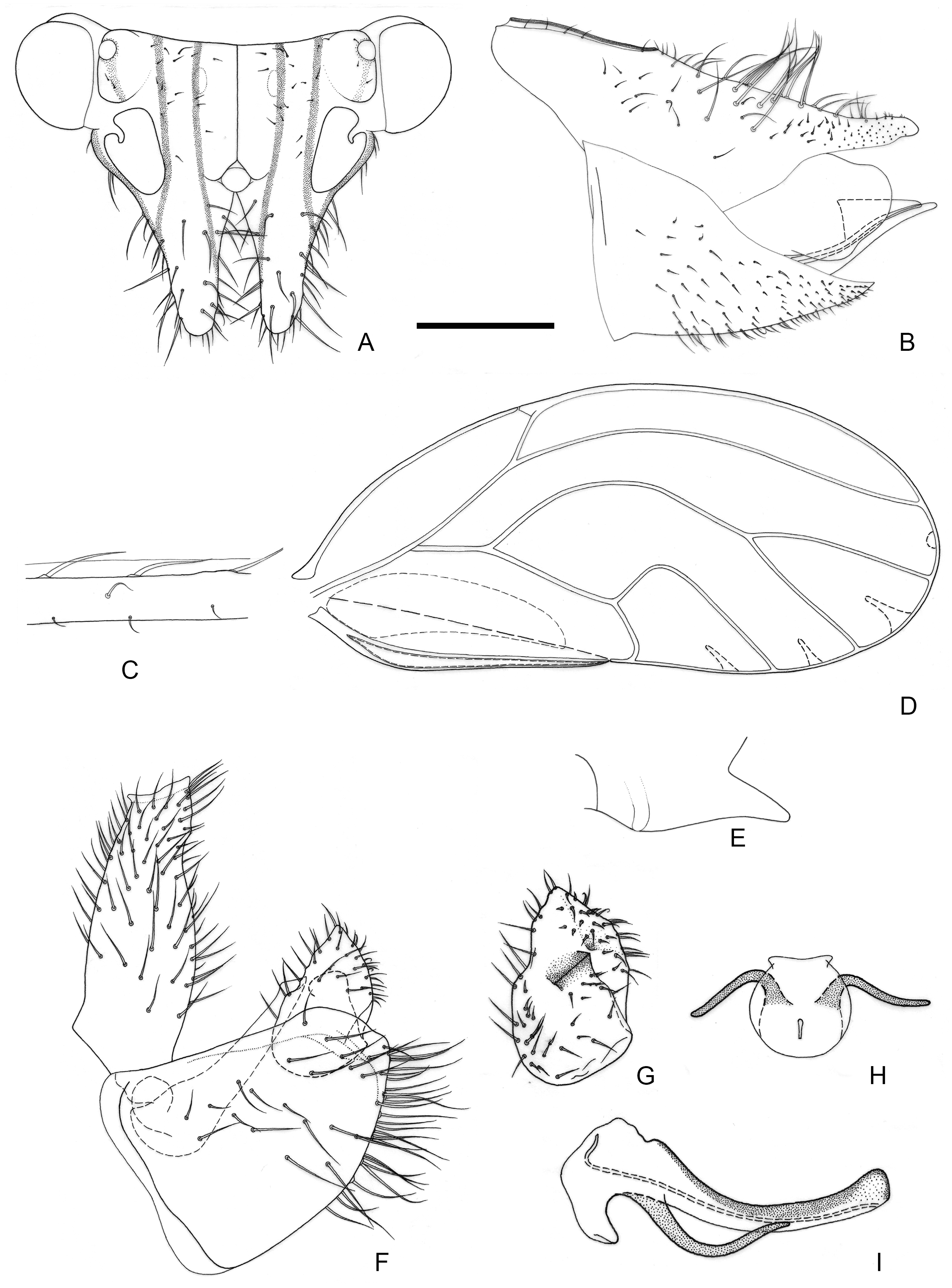
3.1.3. Epipsylla hainanana Yang & Li, 1983
Figure 4.
Epipsylla hainanana Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.25; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.1; (H) 0.15; (I) 0.1.
Figure 4.
Epipsylla hainanana Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.25; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.1; (H) 0.15; (I) 0.1.
Epipsylla hainanana Yang & Li [
24]: 308; Hodkinson [
39] (list); Li [
40] (list); Li [
27] (redescription). Li et al. [
12] (redescription).
Material examined: Holotype: male, China, Hainan, Lingshui, Diaoluoshan, Nanxi, 18°70′31″ N, 109°83′88″ E, 27 April 1965, Sikong Liu leg. dry-mounted; CAU. Paratypes: six males, seven females, same information as holotype.
Diagnosis: Adult. Body greyish-green. Head without longitudinal stripes (
Figure 4A). Mesopraescutum dark green in anterior half, separated from the background area by a black edge; mesoscutum bearing 2 + 2 dark green stripes with black edge on both sides. Genal processes slightly longer than vertex, base separated, apex more acute with dense long setae (
Figure 4A). Metatibia without basal spine, apex with an open, dense crown of seven strongly sclerotized apical spurs. Forewing elongated in shape, bearing three rows (dorsal surface, ventral surface, and anterior margin) of long setae in anterior margin (
Figure 4C); range of surface spinules relatively large and radular spinules slender, degenerated in r2 (
Figure 4D). Meracanthus tapered evenly toward apex, apex somewhat acute (
Figure 4E). Male terminalia: Proctiger 1.4–1.5 times as long as paramere (
Figure 4F). Paramere as in
Figure 4G; outer lobe irregularly triangular, blunt apically, with sclerotized area on inner surface forming transversely oblique ridge in distance from margin; inner lobe large, triangular, sclerotized apex, angular, extending to distal quarter of outer lobe. Basal segment of aedeagus almost straight, gradually narrowing from broad base to narrow apex (
Figure 4F). Distal segment of aedeagus as in
Figure 4H,I; 1.3 times as long as basal segment; base forming long, falci-form pointed process; ventro-lateral sclerotized processes in apical third, short, irregularly curved, blunt; ventral process apical, not divided into apical processes; apex not dilated; sclerotized end tube proximal of aedeagal middle. Female terminalia: Whole short and tall. Dorsal surface of proctiger plumped up, with a row of long setae (
Figure 4B).
Fifth-instar immature and host plant unknown.
Distribution: China (Hainan).
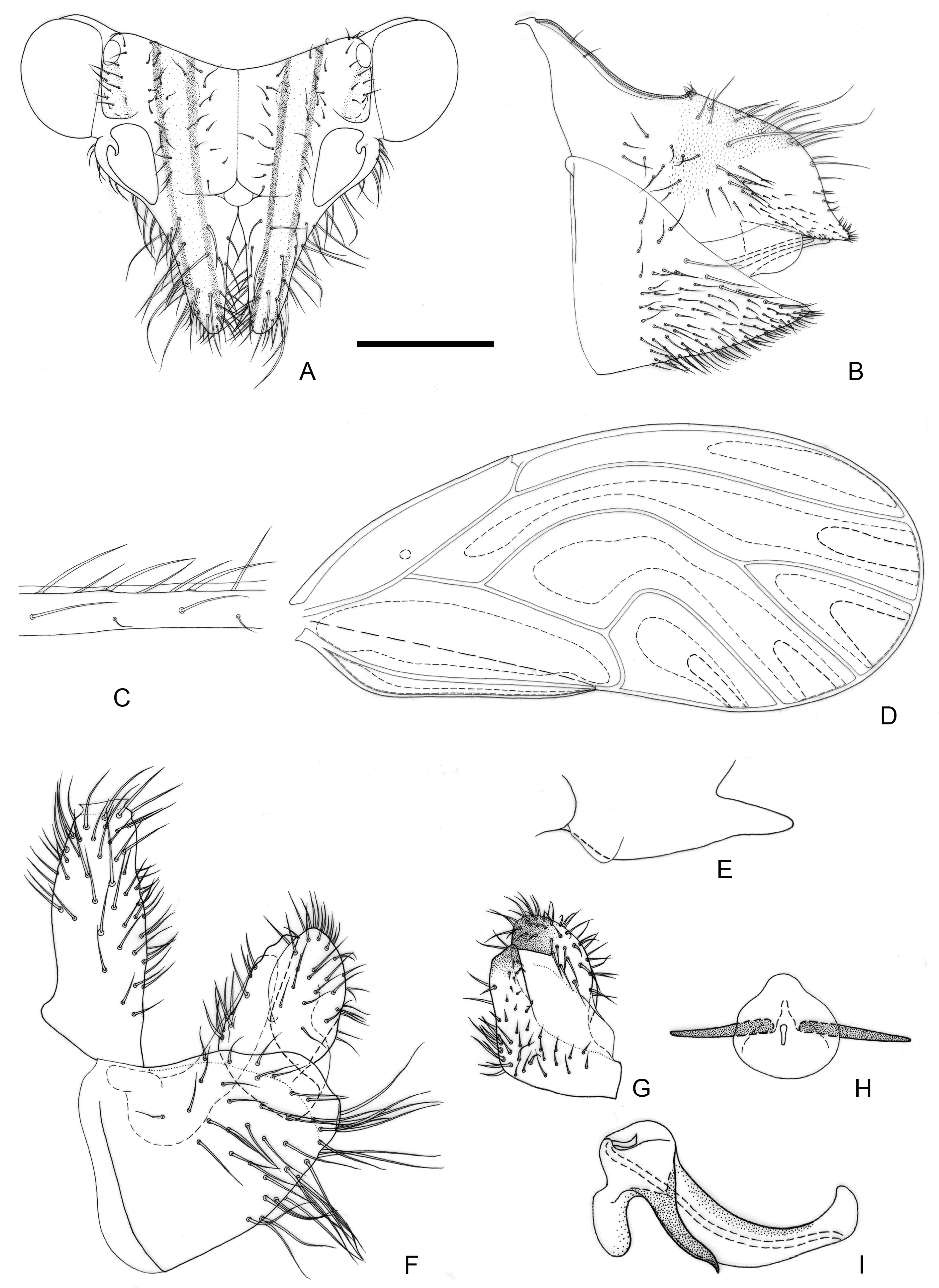
3.1.4. Epipsylla liui Yang & Li, 1983
Figure 5.
Epipsylla liui Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.1; (H) 0.2; (I) 0.1.
Figure 5.
Epipsylla liui Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.1; (H) 0.2; (I) 0.1.
Epipsylla liui Yang & Li [
24]: 309; Hodkinson [
39] (list); Li [
40] (list); Li [
27] (redescription).
Material examined: Paratypes: three males, two females, Hainan, Lingshui, Diaoluoshan, Nanxi, 18°69′78″ N, 109°84′69″ E, 27 April 1965, Sikong Liu leg. dry-mounted; CAU.
Diagnosis: Adult. Body yellowish brown. Vertex bearing 1 + 1 white stripe with black edge in middle part, slightly wider in genal processes, extended forward but not reaching apex of genal processes; head bearing 1 + 1 short white longitudinal stripe on both sides, with black edge inside, black edge surrounding most of lateral ocelli; external margin of antennal insertion bearing a black edge, extended to basal two-fifths of genal processes, not mixed with middle longitudinal stripe; another black stripe on posterior base of genal processes, extending to about two-fifths of genal processes (
Figure 5A). Genal processes slightly longer than vertex, whole curved inward, setae short (
Figure 5A). Metatibia without basal spine, apex with an open, dense crown of seven strongly sclerotized apical spurs. Forewing bearing relatively short and sparse setae at anterior margin (
Figure 5C); range of somewhat large surface spinules and relatively wide and short radular spinules, that of r
2 disappeared (
Figure 5D). Meracanthus decurved, apex somewhat acute (
Figure 5E).
Male terminalia: Proctiger 2 times as long as paramere (
Figure 5F). Paramere as in
Figure 5G; outer lobe irregularly rounded apically, with sclerotized area on inner surface forming oblique ridge in apical third of paramere; inner lobe short, apex strongly sclerotized, pointed, extending to the middle of outer lobe. Basal segment of aedeagus weakly sinuous, only weakly narrowing to apex (
Figure 5F). Distal segment of aedeagus as in
Figure 5H,I; 1.1 times as long as basal segment; base forming at most inconspicuous dorsal tubercle; ventro-lateral sclerotized processes subapical, relatively short and almost straight; ventral process subapically, divided in the middle; apex weakly dilated; sclerotized end tube near aedeagal apex. Female terminalia: Whole taller than long. Proctiger rounded and plump in basal half, apical expanding area short (
Figure 5B).
Fifth-instar immature and host plant unknown.
Distribution: China (Hainan).
3.1.5. Epipsylla millettiae Li, Yang & Burckhardt, 2015
Figure 6.
Epipsylla millettiae Li, Yang & Burckhardt, 2015. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.1; (H) 0.15; (I) 0.1.
Figure 6.
Epipsylla millettiae Li, Yang & Burckhardt, 2015. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.1; (H) 0.15; (I) 0.1.
Epipsylla millettiae Li et al. [
12]: 137.
Material examined. Paratypes: five males, five females, China, Guangdong, Zhanjiang, Southern Aisa Subtropical Botanical Garden, 21°16′31″ N, 110°27′63″ E; 19 April 2013, Bin Li & Meng Jiao leg. on Millettia pachyloba; dry-mounted; IEGU.
Diagnosis: Adult. Body green. Head bearing 1 + 1 light greenish yellow longitudinal stripes with black edge in middle part, extended forward and not reaching genal processes; head bearing 1 + 1 white longitudinal stripes on both sides, with black edge on inner margin; a narrow black band extended forward along outer edge of antennal insertion on genal side, retracting back to middle of genal processes (
Figure 6A). Genal processes stout, apex rounded and blunt, densely elongated setae (
Figure 6A). Metatibia without basal spine, apex with an open, dense crown of 7–8 strongly sclerotized apical spurs. Forewing bearing two rows (anterior margin and ventral surface) of long seta along vein C + Sc (
Figure 6C); range of surface spinules small and radular spinules degenerated in r2 (
Figure 6D). Meracanthus relatively slender, apex somewhat acute, whole slightly curved downward (
Figure 6E). Male terminalia: Proctiger 1.1 times as long as paramere (
Figure 6F). Paramere as in
Figure 6G; outer lobe irregularly, narrowly triangular, blunt apically, with sclerotized area on inner surface covering area near apex; inner lobe long, apex strongly sclerotized, forming anteriorly directed hook, extending to apical quarter of outer lobe. Basal segment of aedeagus mostly straight, slightly curved basally and apically, relatively evenly narrowing to apex (
Figure 6F). Distal segment of aedeagus as in
Figure 6H,I; 1.2 times as long as basal segment; base forming slightly curved, long dorsal process; ventro-lateral sclerotized processes in the middle, relatively short and curved; ventral process apical, not divided distally; apex broad, truncated; sclerotized end tube at aedeagal apex. Female terminalia: Dorsal outline of proctiger, in lateral view, weakly sinuate; apical expanded area short, dorsal surface plump, apex rounded and blunt, with sparse fine setae (
Figure 6B).
Measurements (mm): Male (n = 4): BL 3.00–3.55; AL 3.59–3.82; HW 0.72–0.78; TW 0.59–0.71; WL 2.39–2.71. Female (n = 3): BL 3.28–3.73; AL 3.62–3.94; HW 0.73–0.78; TW 0.63–0.73; WL 2.56–2.92.
Fifth-instar immature unknown.
Host plant: Adults were collected on Millettia pachyloba Drake (Fabaceae), which is a likely host.
Distribution: China (Guangdong).
3.1.6. Epipsylla mucunae Yang & Li, 1984
Figure 7.
Epipsylla mucunae Yang & Li, 1984. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.15; (C) 0.1; (D) 0.6; (E) 0.15; (F) 0.15; (G) 0.1; (H) 0.15; (I) 0.1.
Figure 7.
Epipsylla mucunae Yang & Li, 1984. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.15; (C) 0.1; (D) 0.6; (E) 0.15; (F) 0.15; (G) 0.1; (H) 0.15; (I) 0.1.
Epipsylla mucunae Yang & Li [
25]: 253; Hodkinson [
39] (list); Yang & Li [
38] (list); Li [
27] (redescription).
Epipsylla ruiliana Yang & Li [
25]: 25; Hodkinson [
39] (list); Li et al. [
12] (synonym).
Epipsylla yunnanica Yang & Li [
25]: 255; Hodkinson [
39] (list); Li et al. [
12] (synonym).
Material examined: Holotype: E. mucunae: male, China, Yunnan, Ruili, Guangshuang, alt. 750 m, 23°94′38″ N, 97°78′11″ E, 1 May 1981, Fasheng Li leg. on Mucuna macrocarpa; dry-mounted; CAU; holotype of E. ruiliana: same information as E. mucunae; holotype of E. yunnanica: same information as E. mucunae. Paratypes: paratypes of E. mucunae: four males, same information as holotype; paratypes of E. ruiliana: three males, two females, same information as holotype; paratypes of E. yunnanica: four males, nine females, same information as holotype.
Diagnosis: Adult. Body yellow. Head bearing 1 + 1 white stripe with black edge in middle part, extended forward and reaching apex of genal processes, black edge incontinuity at anterior margin of vertex; vertex bearing 1 + 1 white longitudinal stripe on both sides, with black edge inside, surrounding most of lateral ocelli; outside of antennal insertion bearing 1 + 1 black edge, extended to about basal one-quarter of genal processes (
Figure 7A). Genal processes slender, sometimes slightly curved inward, apex relatively acute and covered by long setae (
Figure 7A). Metatibia without basal spine, apex with an open, dense crown of 7–8 strongly sclerotized apical spurs. Setae of anterior margin of forewing somewhat short (
Figure 7C), range of surface spinules relatively large but away from end of each cell, range of radular spinules relatively small, that of r2 shrinking (
Figure 7D). Meracanthus thin, apex somewhat acute, middle part curved downward obviously (
Figure 7E).
Male terminalia: Proctiger 1.5–1.6 times as long as paramere (
Figure 7F). Paramere as in
Figure 7G; outer lobe irregularly, broadly triangular, blunt apically, inner surface with sclerotized band along postero-apical margin; inner lobe short and broad, triangular, strongly sclerotized apically, extending to the apical third of outer lobe. Basal segment of aedeagus mostly straight, slightly bent in the middle, relatively evenly narrowing to apex (
Figure 7F). Distal segment of aedeagus as in
Figure 7H,I; 1.1 times as long as basal segment; base forming inconspicuous dorsal tubercle; ventro-lateral sclerotized processes in apical quarter, relatively long and slightly curved; ventral process apical, divided just at the apex; apex irregularly rounded, hardly inflated; sclerotized end tube at aedeagal apex. Female terminalia: Dorsal surface of proctiger relatively straight, distribution area extended to proximal portion of long setae, end bearing little fine setae (
Figure 7B).
Measurements (mm): Male (n = 2): BL 3.54–3.72; AL 4.39–4.53; HW 0.83–0.84; TW 0.67–0.68; WL 2.81–2.86. Female (n = 4): BL 3.84–4.02; AL 4.81–4.82; HW 0.86–0.87; TW 0.70–0.74; WL 3.08–3.17.
Fifth-instar immature unknown.
Host plant: Adults were collected on Mucuna macrocarpa Wall. (Fabaceae), which is a likely host.
Distribution: China (Yunnan).
3.1.7. Epipsylla nadana Yang & Li, 1983
Figure 8.
Epipsylla nadana Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.15; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.12; (H) 0.2; (I) 0.1.
Figure 8.
Epipsylla nadana Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.15; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.12; (G) 0.12; (H) 0.2; (I) 0.1.
Epipsylla nadana Yang & Li [
24]: 312; Hodkinson [
39] (list); Li [
27] (redescription).
Material examined: Holotype: male, China, Hainan, Danzhou, Nada, 19°51′37″ N, 109°55′02″ E, 10 December 1974, Fasheng Li leg. dry-mounted; CAU. Paratypes: three males, one female, same information as holotype.
Diagnosis: Adult. Head and thorax yellowish brown and abdomen green. Head bearing 1 + 1 white stripe with black edge in middle part, extended forward but not reaching apex of genal processes; vertex bearing 1 + 1 white longitudinal stripe on both sides, with black edge inside, surrounding most of lateral ocelli; outside of antennal insertion bearing 1 + 1 black edge, extended to about basal one-third of genal processes (
Figure 8A). Genal processes stout, obviously longer than median suture, apex somewhat rounded and blunt, slightly skewing outward. Metatibia without basal spine, apex with an open, dense crown of eight strongly sclerotized apical spurs. Setae of anterior margin of forewing somewhat short (
Figure 8C), range of surface spinules relatively large but away from end of each cell, range of radular spinules large as well, that of r2 away from end (
Figure 8D). Meracanthus stout and short, apex somewhat rounded and blunt, whole curved downward obviously (
Figure 8E). Male terminalia: Proctiger 2.1–2.2 times as long as paramere (
Figure 8F). Paramere as in
Figure 8G; broad at base, narrower, lamellar in apical two-thirds; outer lobe irregular, lamellar, blunt apically, inner surface with sclerotized patch in apical third; inner lobe very short, subtriangular, strongly sclerotized apically, extending to the middle of outer lobe. Basal segment of aedeagus mostly straight, slightly curved basally and apically, weakly narrowing to apex (
Figure 8F). Distal segment of aedeagus as in
Figure 8H,I; 1.4 times as long as basal segment; base forming inconspicuous dorsal tubercle; ventro-lateral sclerotized processes in apical quarter, moderately long, only weakly curved; ventral process apical, divided near the apex into two short processes; apex irregularly rounded, weakly inflated; sclerotized end tube near aedeagal apex. Female terminalia: proctiger evenly tapered toward end, dorsal margin sloped down evenly and end hairless (
Figure 8B).
Measurements (mm): Male (n = 2): BL 3.55–3.71; AL 4.14–4.31; HW 0.72–0.76; TW 0.64–0.66; WL 2.80–2.95. Female (n = 1): BL 3.36; AL 3.45; HW 0.74; TW 0.59; WL 2.69.
Fifth-instar immature and host plant unknown.
Distribution: China (Hainan).
3.1.8. Epipsylla puerariae Yang & Li, 1983
Figure 9.
Epipsylla puerariae Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.2; (B) 0.15; (C) 0.1; (D) 0.5; (E) 0.18; (F) 0.15; (G) 0.1; (H) 0.12; (I) 0.1.
Figure 9.
Epipsylla puerariae Yang & Li, 1983. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.2; (B) 0.15; (C) 0.1; (D) 0.5; (E) 0.18; (F) 0.15; (G) 0.1; (H) 0.12; (I) 0.1.
Epipsylla puerariae Yang & Li [
24]: 310; Hodkinson [
39] (list); Li [
27] (redescription).
Material examined: Holotype: male, China, Hainan, Danzhou, Nada, 19°53′12″ N, 109°54′71″ E, 10 December 1974, Fasheng Li leg. on Pueraria montana; dry-mounted; CAU. Paratypes: 15 males, 13 females, 1 immature (in 75% ethanol), same information as holotype.
Diagnosis: Adult. Body green. Head bearing 1 + 1 light stripe with black edge in middle part, extended forward and wider when reaching genal processes, but not reaching apex of genal processes, outside black edge mixed with black edge of antennal insertion in apex; vertex bearing light longitudinal stripe on both sides, with black edge inside, surrounding most of lateral ocelli (
Figure 9A). Genal processes relatively short and thin, straight, apex somewhat rounded and blunt, setae short (
Figure 9A). Metatibia without basal spine, apex with an open, dense crown of eight strongly sclerotized apical spurs. Forewing oval, widest in middle, surface spinules present only in cell cu
2, radular spinules of r
2 degenerated and located at end (
Figure 9D). Meracanthus relatively thin, apex somewhat acute, whole slightly curved downward (
Figure 9E). Male terminalia: Proctiger 1.5 times as long as paramere (
Figure 9F). Paramere as in
Figure 9G; irregularly lanceolate; outer lobe irregularly triangular, subacute apically, inner surface with sclerotized area bearing a small tubercle postero-subapically; inner lobe short, subtriangular, strongly sclerotized apically, extending slightly beyond the middle of outer lobe. Basal segment of aedeagus almost straight, distinctly narrowing to apex (
Figure 9F). Distal segment of aedeagus as in
Figure 9H,I; 1.4 times as long as basal segment; base forming inconspicuous dorsal tubercle; ventro-lateral sclerotized processes in apical quarter, moderately long, curved; ventral process apical, inconspicuously divided near the apex; apex irregularly rounded, weakly inflated; sclerotized end tube near aedeagal apex. Female terminalia: Proctiger bearing sparse setae on both sides, apical half relatively short, apex hairless (
Figure 9B).
Measurements (mm): Male (n = 4): BL 3.05–3.20; AL 3.26–3.46; HW 0.69–0.73; TW 0.56–0.62; WL 2.40–2.49. Female (n = 3): BL 3.28–3.54; AL 3.48–3.74; HW 0.72–0.75; TW 0.60–0.64; WL 2.54–2.71.
Fifth-instar immature: Abdominal margin lacking sectasetae or lanceolate setae. Outer circumanal ring comprising five rows of subrectangular pores laterally.
Host plant: Pueraria montana (Lour.) Merr. (Fabaceae).
Distribution: China (Hainan).
3.1.9. Epipsylla suni Luo, Burckhardt & Cai, sp. nov.
Figure 10.
Epipsylla suni Luo, Burckhardt & Cai, sp. nov. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.15; (G) 0.12; (H) 0.12; (I) 0.1.
Figure 10.
Epipsylla suni Luo, Burckhardt & Cai, sp. nov. (A) Head (antennae removed); (B) female terminalia in lateral view; (C) setae on vein C + Sc; (D) forewing; (E) meracanthus; (F) male terminalia in lateral view (ignoring distal segment of aedeagus and phallobase); (G) inner surface of paramere; (H) distal segment of aedeagus in dorsal view; (I) distal segment of aedeagus in lateral view. Scale bar (mm): (A) 0.25; (B) 0.2; (C) 0.1; (D) 0.5; (E) 0.15; (F) 0.15; (G) 0.12; (H) 0.12; (I) 0.1.
Figure 11.
Epipsylla suni Luo, Burckhardt & Cai, sp. nov., fifth-instar immature. (A) Habitus, dorsal aspect on the left half, ventral aspect on the right half; (B) dorsal view of circumanal pore field; (C) ventral view of circumanal pore field; (D) blunt lanceolate seta located in margin of abdomen; (E) tarsal arolium. Scale bar (mm): (A) 0.4; (B) 0.12; (C) 0.12; (D) 0.03; (E) 0.03.
Figure 11.
Epipsylla suni Luo, Burckhardt & Cai, sp. nov., fifth-instar immature. (A) Habitus, dorsal aspect on the left half, ventral aspect on the right half; (B) dorsal view of circumanal pore field; (C) ventral view of circumanal pore field; (D) blunt lanceolate seta located in margin of abdomen; (E) tarsal arolium. Scale bar (mm): (A) 0.4; (B) 0.12; (C) 0.12; (D) 0.03; (E) 0.03.
Diagnosis: Adult. Body green overall. White longitudinal stripes of anterior part of body bearing narrow black edge on both sides (
Figure 1C,D). Vertex yellowish brown in middle part and gradually darker to both sides, white longitudinal stripes of lateral margin bearing brown edge inside (
Figure 10A). Genal processes slender and straight, gradually thinned to end, slightly away, apex somewhat acute (
Figure 10A). Metatibia without basal spine, apex with an open, dense crown of eight strongly sclerotized apical spurs. Forewing membranous, oval, widest in apical third, anterior margin strongly arced; radular spinules thick in middle of end of cell and gathered into a long strip area, and spreading around to form small particles (
Figure 10D); setae of anterior margin of forewing of different length (
Figure 10C). Meracanthus relatively long, straight, apex somewhat acute, pointed to back and upward (
Figure 10E). Male terminalia: Proctiger 1.4 times as long as paramere (
Figure 10F). Paramere as in
Figure 10G; irregularly lanceolate; outer lobe irregularly rounded, blunt apically, inner surface with sclerotized area apically; inner lobe very long, lamellar, obliquely truncate and strongly sclerotized apically, extending inner apex of outer lobe. Basal segment of aedeagus almost straight, apart from weakly curved basally; distinctly narrowing to apex (
Figure 10F). Distal segment of aedeagus as in
Figure 10H,I; 1.0 times as long as basal segment; base forming short dorsal process; ventro-lateral sclerotized processes in apical quarter, moderately long, hardly curved; ventral process subapical, not divided apically; apex irregularly rounded, weakly inflated; sclerotized end tube near aedeagal apex. Female terminalia: Dorsal surface of proctiger strongly rounded with a cluster of short setae on apical part (
Figure 10B).
Fifth-instar immature: Abdominal margin bearing 1 + 1 lanceolate setae. Outer circumanal ring composed of each an inner and outer row of slit-like pores enclosing a band of numerous irregular pores.
Etymology: The name comes from the last name of the scholar, Ziqiang Sun, who accompanied the collector (Xinyu Luo) during the collection of the type specimens.
Type material: Holotype: male, China, Yunnan, Ruili, Nanjingli, 24°09′01″ N, 97°83′71″ E, 18 April 2014, Xinyu Luo leg. on Fabaceae sp.; dry-mounted; CAU. Paratypes: 39 males, 61 females, 12 larvae, same data as for holotype; 37 males, 44 females, 25 larvae, Yunnan, Ruili, Nanjingli, 24°09′01″ N, 97°83′71″ E, 12 April 2024, Zhixin He & Rongzhen Xu leg. on Derris taiwaniana; in 95% ethanol; 2 males, 2 females, same data as for holotype, NZMC; 2 males, 2 females, same data as for holotype, BMNH; 2 males, 2 females, same data as for holotype, NHMB.
Description: In addition to diagnosis, this species has the following characteristics:
Coloration: Adult. White longitudinal stripes of anterior part of body bearing narrow black edge on both sides. Pronotum yellowish brown, lateral angle of mesoscutum yellowish brown; lateral plate of each segment of thorax mostly black. Coxa of front and middle legs yellowish brown, rest of segments brown, darker in apex of tibia; coxa and trochanter of hind leg yellowish brown, femur black, tibia and tarsus yellow. Forewing somewhat yellow, veins dark brown. Abdomen completely black.
Structure: Head compressed by pronotum and slightly located below it, inclined about 80° downward with longitudinal axis of body. Surrounding part of antero-lateral angle and lateral ocelli of vertex strongly plump, anterior margin somewhat plump as a whole, boundary with antennal base blurred. Surface of base colored (with black edge) of head scaly with fine structure with relatively long setae, middle stripe area smooth and hairless. Pronotum bearing little long setae in posterior margin, plate of mesothorax and metathorax only bearing fine setae. Pronotum bearing 2 + 2 gentle small protuberances on both sides.
Male terminalia: Proctiger short and simple, not bearing posterior lobe. Sclerotized end of ejaculatory duct extended backward and slightly curved forward. Subgenital plate flat, upper margin bearing little setae, lower surface bearing dense long setae.
Female terminalia: Subgenital plate subtriangular in lateral view; upper margin bearing a row of coarsely long setae, with dense and uniform setae on both sides, gradually shorter form base to end.
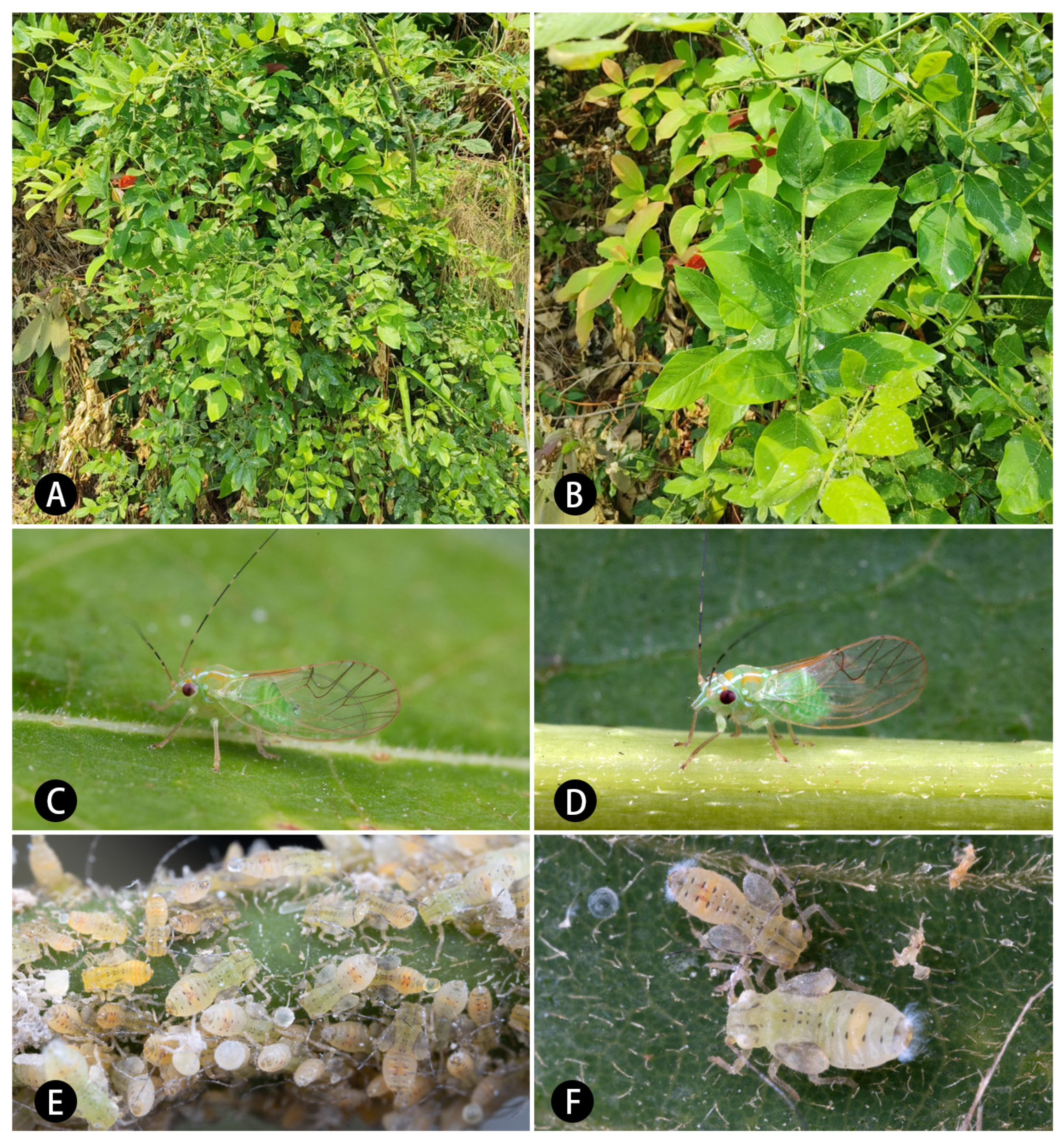
Fifth-instar immature: Surface of membranous part of body covered with small granular structures, setae attached to back of body basically simple. Antennae developed, protruding forward (
Figure 1E,F and
Figure 11A). Antennal with seven segments, segments III and V with an apical rhinarium, respectively, and segment VII bearing two rhinaria in middle part. Ocular seta and postorbital seta long. Sclerites of dorsal side of thorax small and fragmented, central sclerites obvious, brown, and lateral sclerites relatively weak. Outer margin of hindwing pad bearing setae of varying length, anterior half bearing two small holes. Hindwing pad bearing two relatively long setae apically. Each leg relatively long, femur covered by densely short setae, tibiotarsus of middle and hind legs bearing a long seta in base of dorsal surface and proximal part. Tarsal arolium (
Figure 11E) shaped as fishtail, with broad short spine and developed unguitractor; narrow as a whole, rough thickening area of palm narrow, not extended to outer margin. Dorsal sclerites of each segment of abdomen small, brown in middle part; ventral surface with fragmentary, less sclerotized sclerites. Anal plate barely sclerotized on ventral side, somewhat sclerotized on dorsal side. Anus located on the terminal of abdomen. Circumanal pore rings (
Figure 11B,C) extending on both side of abdomen. Outer circumanal pore ring relatively thin in middle part and strongly widened on both sides, outermost ring as a series of long fissured pores, innermost ring as a series of longer fissured pores, and middle part as closely arranged irregular pores; inner circumanal pore ring narrow in middle and wide on both sides, and composed of small pores with irregular shape and loose arrangement. Abdomen bearing 3 + 3 setae in anterior middle of margin of dorsal surface (
Figure 11A), and 1 + 1 short, unsegmented in middle, and round and blunt apically lanceolate setae (
Figure 11D).
Measurements (mm): Male (n = 4): BL 3.09–3.38; AL 3.73–3.76; HW 0.75–0.80; TW 0.59–0.67; WL 2.52–2.66. Female (n = 4): BL 3.24–3.66; AL 3.52–4.32; HW 0.78–0.84; TW 0.65–0.69; WL 2.60–2.90. Fifth-instar larvae (n = 4): BL 1.76–1.94; AL 1.87–2.11; HW 0.72–0.76; FL 0.63–0.67.
Host plant:
Derris taiwaniana (Hayata) Z. Q. Song (
Figure 1A,B).
Distribution: China (Yunnan).
Comments: The new species is similar with
E. hainanana in large range of surface spinules and length ratio of genal processes/vertex, but they are different in shape and position of paramere’s sclerotized area (
Figure 4G versus
Figure 10G) and shape of distal segment of aedeagus (
Figure 4F versus
Figure 10F). It is similar with
E. viridis in anterior margin of sclerotized area of parameres visible and female terminalia, but they are different in forewing with surface spinules or not and shape of male proctiger. The type locality of new species is close to that of
E. mucunae, but they differ from length ratio of genal processes/vertex (
Figure 7A versus
Figure 10A), range of surface spinules and radular spinules (
Figure 7D versus
Figure 10D) and shape of sclerotized area of paramere (
Figure 7G versus
Figure 10G) obviously. It also differs from
E. bilineata in the distribution of surface spinules and radular spinules, and differs with
E. pulchra in shape of male proctiger obviously.
Figure 12.
Habitus of adults. (A,B) E. crotalariae; (C,D) E. guangxiana; (E,F) E. hainanana; (G,H) E. millettiae. (A,C,E,G) Dorsal view; (B,D,F,H) lateral view. Scale bar: 1 mm.
Figure 12.
Habitus of adults. (A,B) E. crotalariae; (C,D) E. guangxiana; (E,F) E. hainanana; (G,H) E. millettiae. (A,C,E,G) Dorsal view; (B,D,F,H) lateral view. Scale bar: 1 mm.
Figure 13.
Habitus of adults. (A,B) E. mucunae; (C,D) E. nadana; (E,F) E. puerariae; (G,H) E. suni. (A,C,E,G) Dorsal dorsal view; (B,D,F,H) lateral view. Scale bars: 1 mm.
Figure 13.
Habitus of adults. (A,B) E. mucunae; (C,D) E. nadana; (E,F) E. puerariae; (G,H) E. suni. (A,C,E,G) Dorsal dorsal view; (B,D,F,H) lateral view. Scale bars: 1 mm.