Interspecific Competition Between Eotetranychus sexmaculatus Riley and Oligonychus biharensis Hirst (Acari: Tetranychidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mite Collection and Rearing
2.2. Investigations on the Damage Caused by Spider Mites on Rubber Trees
2.3. Mite Development and Reproduction in Single and Mixed Populations
2.4. Interspecific Competition Under Various Temperatures
2.5. Data Analysis
- (1)
- R = /T × 100%, where R is the rate of leaf injury, X represents the total number of leaves with mite hazards, i is (1) the total number of leaves damaged by E. sexmaculatus alone, (2) the total number of leaves damaged by E. sexmaculatus alone and in combination, (3) the total number of leaves damaged by O. biharensis alone, and (4) the total number of leaves damaged by O. biharensis alone and in combination, respectively. T denotes the total number of leaves assessed [21].
- (2)
- = , where D represents the development duration, x is the number of developmental days, i represents egg, larvae, protonymph instar, deutonymph instar and egg–adult, respectively (the same below). Similarly, n denotes the total number of spider mites at different developmental stages. Generation time represents the total developmental time from egg to adult mite [5].
- (3)
- = Ci/N × 100%, where S is the survival rate, C represents the number entering the next stage, and N denotes the number completing previous stage [5].
- (4)
- = , where F represents fecundity (eggs/female), f represents the total number of eggs laid on that day, and n denotes the total number of adult female mites on that day. Finally, i represents days [5].
- (5)
- = /N, where L denotes lifespan, d is the survival days of female adult mites on that day, n represents the number of female adult mites, and N indicates the total number of female adult mites [5].
- (6)
- R0 = Nt/N0, rm = lnR0/t, where R0 represents the net increase rate, Nt represents population size at time t, and N0 indicates population size at the initial time, rm represents the intrinsic rate of increase, t represents the number of days of investigation time [22].
- (7)
- αij = , where αij represents interspecific competition coefficient, αij denotes the competition coefficient of species i and j sharing the same resource, and Pi and Pj represent the proportions of species i and j in each resource sequence [23].
3. Results
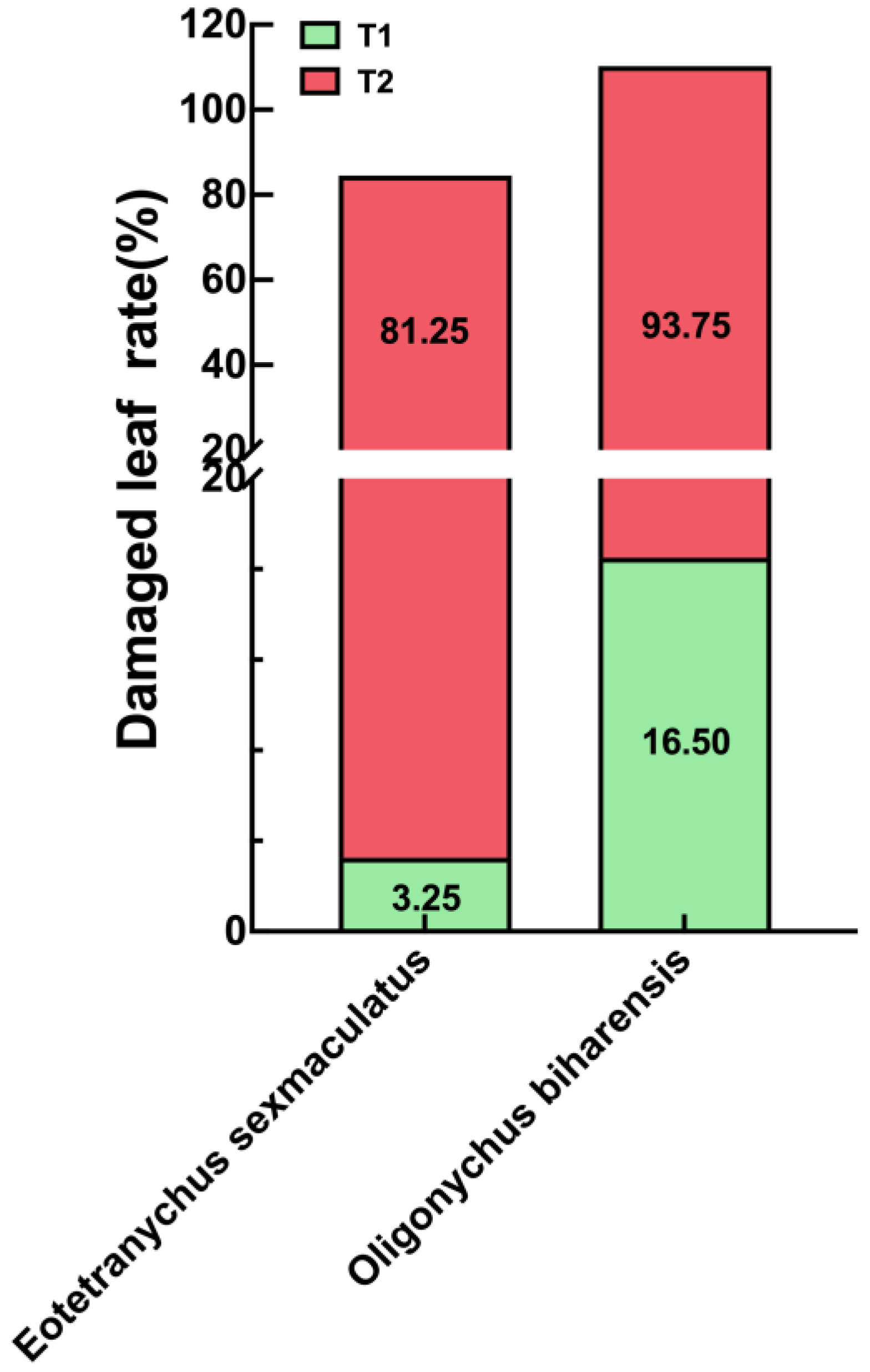
3.1. Spider Mite Damage in Rubber Plantations
3.2. Effects of Mixed Populations on Spider Mite Development
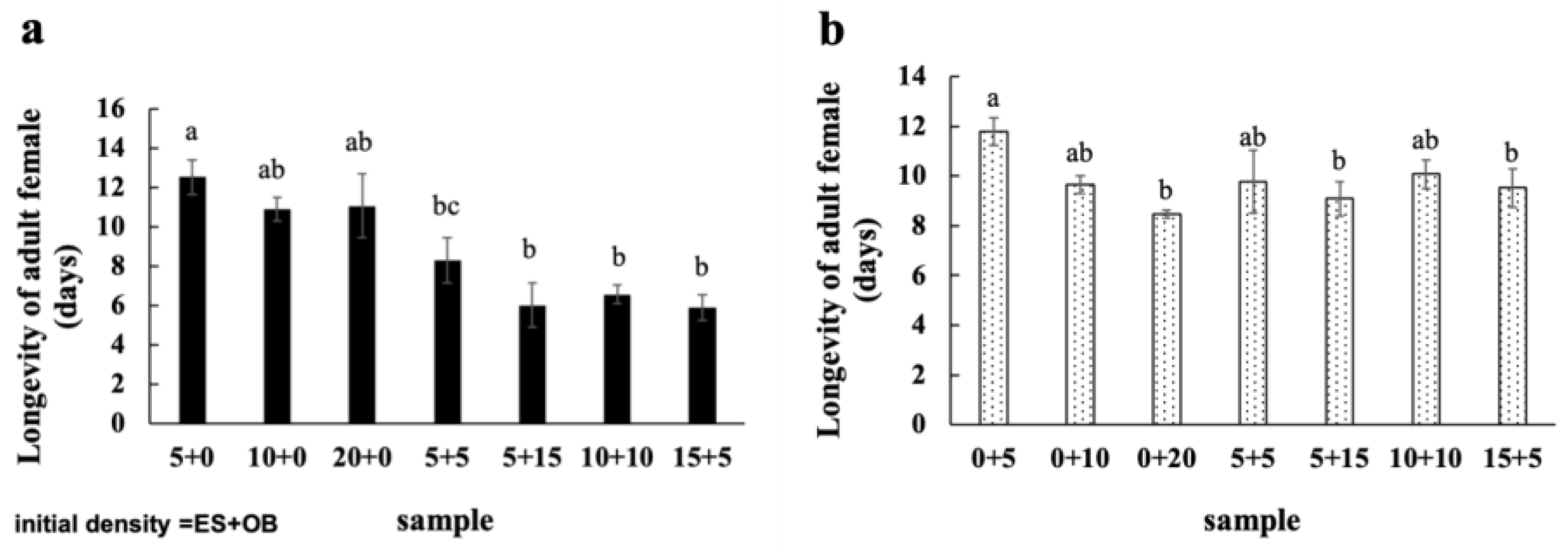
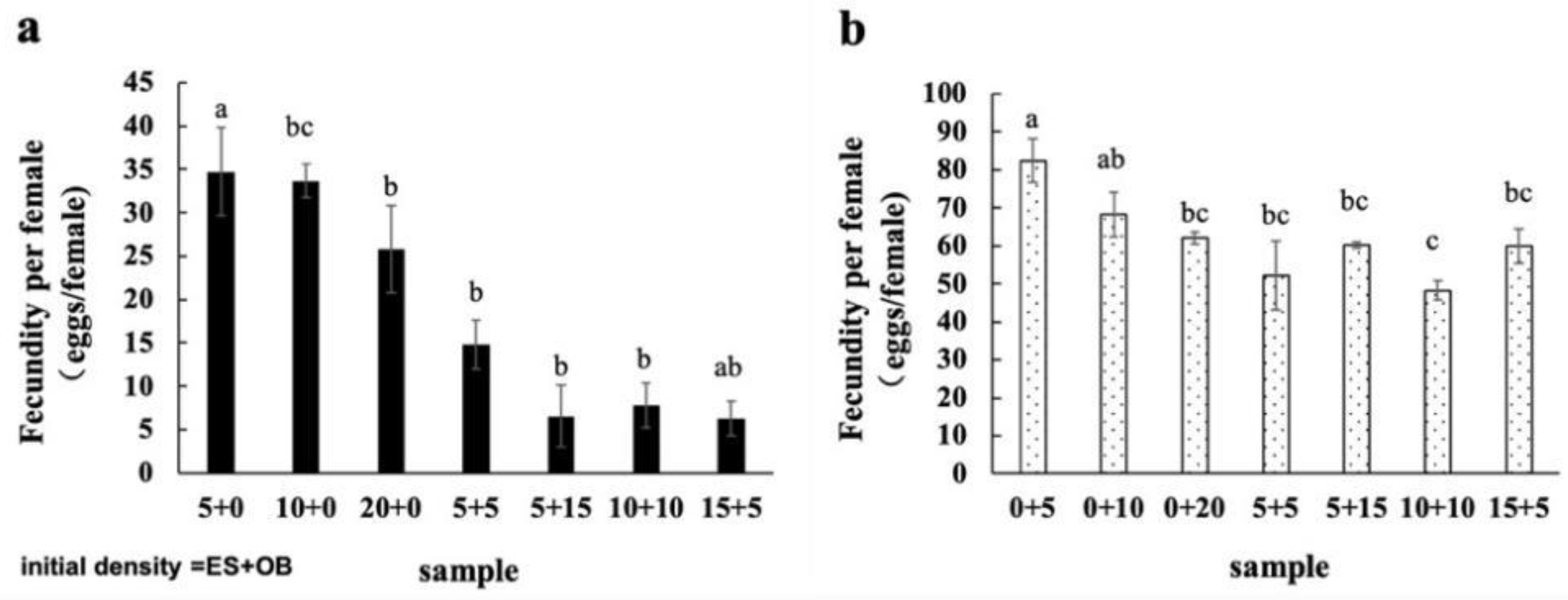
3.3. Effects of Mixed Populations on Spider Mite Reproduction
3.4. Interspecific Competition Between Mite Species at Different Temperatures
3.5. Intrinsic Rate of Increase Under Different Temperatures
3.6. Interspecific Competition Coefficients Under Different Temperatures
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, H.H.; Li, P.Z.; Xu, T.W.; Wu, Q.Q.; Zhang, F.P.; Peng, Z.Q. Preliminary evaluation of the control effect of two predatory mite species on Eotetranychus sexmaculatus in rubber trees in Hainan Province, China. Syst. Appl. Acarol. 2021, 26, 2287–2296. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Chen, J.Y.; Lu, T.F.; Niu, L.M.; Jia, J.J.; Ye, Z.P.; Fu, Y.G. Genetic diversity of three major spider mites damaging rubber trees. Syst. Appl. Acarol. 2022, 27, 2025–2037. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Q.; Wu, C.L.; Liu, Y.; Fang, Y.J. Reference gene validation in Eotetranychus sexmaculatus (Acari: Tetranychidae) feeding on mite-susceptible and mite-resistant rubber tree germplasms. Exp. Appl. Acarol. 2020, 82, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, J.A.; Céspedes, V.; Coccia, C.; Green, A.J. An experimental test of interspecific competition between the alien boatman Trichocorixa verticalis and the native corixid Sigara lateralis (Hemiptera, Corixidae). Aquat. Invasions 2020, 15, 318–334. [Google Scholar] [CrossRef]
- Zhang, G.F.; Lovei, G.L.; Hu, M.; Wan, F.H. Asymmetric consequences of host plant occupation on the competition between the whiteflies Bemisia tabaci cryptic species MEAM1 and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2014, 70, 1797–1807. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, S.; Guo, K.; Zhang, F.; Qiao, H.L.; Chen, J.M.; Yang, M.K.; Zhu, X.; Xu, R.; Xu, C.Q. Plant-mediated competition facilitates a phoretic association between a gall mite and a psyllid vector. Exp. Appl. Acarol. 2018, 76, 325–337. [Google Scholar] [CrossRef]
- Denno, R.F.; McClure, M.S.; Ott, J.R. Interspecific interactions in phytophagous insects: Competition reexamined and resurrected. Annu. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Mortazavi, N.; Fathipour, Y.; Talebi, A.A. Interactions between two-spotted spider mite, Tetranychus urticae and greenhouse whitefly, Trialeurodes vaporariorum on strawberry. Syst. Appl. Acarol. 2017, 22, 2083–2092. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Throne, J.E.; Nakas, C.T. Competition among Species of Stored-Product Psocids (Psocoptera) in Stored Grain. PLoS ONE 2014, 9, e102867. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Lin, C.H.; Lin, Y.P.; Tseng, C.T. Temperature-dependent life history of Eutetranychus africanus (Acari: Tetranychidae) on papaya. Syst. Appl. Acarol. 2020, 25, 479–490. [Google Scholar]
- Araujo, F.G.D.; Lima, E.L.D.; Costa, E.; Daud, R.D. Influence of natural vegetation conservation on the distribution of mites in rubber tree crops. Syst. Appl. Acarol. 2022, 27, 1629–1647. [Google Scholar] [CrossRef]
- Ferraz, J.C.B.; Neto, A.V.G.; Frana, S.M.D.; Silva, P.R.R.; Lima, D.B.D. Temperature-dependent development and reproduction of Oligonychus punicae (Acari: Tetranychidae) on Eucalyptus. Syst. Appl. Acarol. 2021, 26, 918–927. [Google Scholar]
- Liu, S.S.; Barro, P.J.D.; Xu, J.; Luan, J.B.; Zang, L.S.; Ruan, Y.M.; Wan, F.H. Asymmetric Mating Interactions Drive Widespread Invasion and Displacement in a Whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Chen, W.M.; Fu, Y.G.; Zhang, F.P.; Peng, Z.Q. Effect of different varieties of litchi on the development and reproduction of Oligonychus biharensis (Hirst). Syst. Appl. Acarol. 2005, 10, 11–16. [Google Scholar] [CrossRef]
- Kaimal, S.G.; Sheeja, U.M.; Ramani, N. Ultrastructural elucidation of leaf damage on cassava induced by Oligonychus biharensis (Hirst) (Acari: Tetranychidae). Int. J. Acarol. 2011, 37, 108–113. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.X.; Chen, X.; Lin, J.Z. Responses to stimuli from Oligonychus biharensis Hirst (Acari: Tetranychidae) on loquat leaves by Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae). Int. J. Acarol. 2008, 34, 175–181. [Google Scholar] [CrossRef]
- Lu, F.P.; Chen, Z.S.; Lu, H.; Liang, X.; Zhang, H.Y.; Li, Q.; Chen, Q.; Huang, H.S.; Hua, Y.W.; Tian, W.M. Effects of resistant and susceptible rubber germplasms on development, reproduction and protective enzyme activities of Eotetranychus sexmaculatus (Acari: Tetranychidae). Exp. Appl. Acarol. 2016, 69, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.P.; Zhu, J.H.; Li, L.; Han, D.Y.; Niu, L.M.; Chen, J.Y.; Fu, Y.G. Effect of Rubber Leaves on the Selectivity and Growth Stages of Eotetranychus sexmaculatus at Different Growing Period. Chin. J. Trop. Crops 2016, 37, 153–157. [Google Scholar]
- Zhang, F.P.; Fu, Y.G.; Jin, Q.A.; Zhang, J.B. Development and fecundity of Oligonychus biharensis on three southern fruit crops. J. Fruit Sci. 2007, 24, 185–188. [Google Scholar]
- Wang, W.H.; Wan, S.L.; Zheng, L.J.; Zhang, F.P.; Chen, J.Y. Evaluation of the temperature adaptation of three rubber tree pest mites based on their two-sex life table. Insects 2024, 116, 22138. [Google Scholar] [CrossRef]
- Zhang, T.F.; Zhao, T.P.; Li, X.D.; Rao, Y.F.; Yang, J.B.; Yang, Z.J. Study on the field control effect of Arma chinensis (Fallou) on Spodoptera frugiperda (J. E. Smith). Yunnan Agric. 2023, 8, 74–76. [Google Scholar]
- Li, Y.; Wei, X.; Zhang, J.; Xiang, L.; Feng, H.Z. Interspecific competition between Tetranychus urticae and Tetranychus truncates on cotton. Acta Agric. Bor.-Oci. Sin. 2015, 24, 172–178. [Google Scholar]
- May, R.M. Some notes on estimating the competition matrix. Ecology 1975, 56, 737–741. [Google Scholar] [CrossRef]
- Ntiri, E.S.; Calatayud, P.A.; Berg, J.V.D.; Schulthess, F.; Ru, B.P.L. Influence of Temperature on Intra- and Interspecific Resource Utilization within a Community of Lepidopteran Maize Stemborers. PLoS ONE 2016, 11, e0148735. [Google Scholar] [CrossRef]
- Yan, W.T.; Qiu, G.S.; Zhou, Y.S.; Zhang, H.J.; Zhang, P.; Liu, C.L.; Zheng, Y.C. Interspecific domino effects of three major pernicious mites in apple orchard. J. Fruit Sci. 2010, 27, 815–818. [Google Scholar]
- Zhang, Q.C.; Yan, W.J.; Wang, J.G. Laboratory Assays of Density-Dependent Interspecific and Intraspecific Competition between Aphis gossypii and Acyrthosiphon gossypii (Hemiptera: Aphididae). J. Entomol. Sci. 2022, 57, 530–547. [Google Scholar]
- Kishi, S.; Nishida, T.; Tsubaki, Y. Reproductive interference determines persistence and exclusion in species interactions. J. Anim. Ecol. 2009, 78, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Quellhorst, H.; Athanassiou, C.G.; Bruce, A.; Scully, E.D.; Morrison, W.R., III. Temperature-Mediated Competition Between the Invasive Larger Grain Borer (Coleoptera: Bostrichidae) and the Cosmopolitan Maize Weevil (Coleoptera: Curculionidae). Environ. Entomol. 2020, 49, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Pascual, S.; Callejas, C. Intra-and interspecific competition between biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) from Spain. Bull. Entomol. Res. 2004, 94, 369–375. [Google Scholar] [CrossRef]
- Gamelon, M.; Vriend, S.J.G.; Engen, S.; Adriaensen, F.; Dhondt, A.A.; Evans, S.R.; Matthysen, E.; Sheldon, B.C.; Saether, B.E. Accounting for interspecific competition and age structure in demographic analyses of density dependence improves predictions of fluctuations in population size. Ecol. Lett. 2019, 22, 797–806. [Google Scholar] [CrossRef]
- Kim, T.N.; Underwood, N.; Inouye, B.D. Insect herbivores change the outcome of plant competition through both inter-and intraspecific processes. Ecology 2013, 94, 1753–1763. [Google Scholar] [CrossRef]
- Bujang, N.S.; Lee, C.Y. Interspecific competition between the Smooth cockroach Symploce pallens and the German cockroach Blattella germanica (Dictyoptera: Blattellidae) under different food and water regimes. Trop. Biomed. 2010, 27, 103–114. [Google Scholar] [PubMed]









| Species | Initial Density | Egg | Larva | Protonymph Instar | Deutonymph Instar | Generation Time |
|---|---|---|---|---|---|---|
| E. sexmaculatus | 0 + 5 | 4.93 ± 0.16 a | 2.28 ± 0.23 a | 1.73 ± 0.14 cd | 1.57 ± 0.19 abc | 10.49 ± 0.11 b |
| 0 + 10 | 4.00 ± 0.00 b | 2.00 ± 0.00 a | 2.82 ± 0.09 ab | 1.10 ± 0.19 c | 9.92 ± 0.18 bc | |
| 0 + 20 | 4.13 ± 0.08 b | 2.37 ± 0.13 a | 1.22 ± 0.17 d | 2.29 ± 0.33 a | 9.99 ± 0.31 bc | |
| 5 + 5 | 4.36 ± 0.18 b | 2.20 ± 0.33 a | 1.67 ± 0.26 cd | 1.20 ± 0.20 bc | 9.50 ± 0.22 c | |
| 5 + 15 | 4.33 ± 0.21 b | 2.53 ± 0.18 a | 2.93 ± 0.21 ab | 2.21 ± 0.36 ab | 12.00 ± 0.45 a | |
| 10 + 10 | 5.41 ± 0.19 a | 2.24 ± 0.15 a | 2.39 ± 0.20 bc | 2.62 ± 0.37 a | 12.67 ± 0.33 a | |
| 15 + 5 | 4.43 ± 0.24 b | 2.48 ± 0.45 a | 3.33 ± 0.58 a | 2.60 ± 0.56 a | 12.84 ± 0.31 a | |
| F, df1, df2, p value | 8.308, 6, 35, p < 0.001 | 0.532, 6, 35, p < 0.001 | 7.728, 6, 34, p < 0.001 | 3.414, 6, 34, 0.015 | 22.269, 6, 34, 0.432 | |
| O. biharensis | 0 + 5 | 4.63 ± 0.20 bc | 1.97 ± 0.22 ab | 1.68 ± 0.23 c | 2.99 ± 0.53 a | 11.27 ± 0.56 ab |
| 0 + 10 | 5.00 ± 0.03 ab | 2.11 ± 0.12 ab | 2.41 ± 0.37 bc | 1.39 ± 0.25 b | 10.94 ± 0.06 abc | |
| 0 + 20 | 4.95 ± 0.02 ab | 1.26 ± 0.13 c | 2.50 ± 0.32 bc | 1.68 ± 0.23 b | 10.38 ± 0.20 bc | |
| 5 + 5 | 4.10 ± 0.28 c | 1.60 ± 0.13 bc | 2.04 ± 0.38 c | 1.83 ± 0.22 b | 9.57 ± 0.28 c | |
| 5 + 15 | 5.38 ± 0.16 a | 1.74 ± 0.13 bc | 3.64 ± 0.25 a | 1.17 ± 0.17 b | 11.93 ± 0.05 a | |
| 10 + 10 | 5.43 ± 0.29 a | 1.98 ± 0.26 ab | 3.96 ± 0.41 a | 1.65 ± 0.37 b | 12.08 ± 0.91 a | |
| 15 + 5 | 4.48 ± 0.23 bc | 2.33 ± 0.21 a | 3.39 ± 0.49 ab | 2.05 ± 0.51 ab | 12.21 ± 0.22 a | |
| F, df1, df2, p value | 5.689, 6, 35, p < 0.001 | 3.949, 6, 35, 0.004 | 6.113, 6, 32, p < 0.001 | 3.041, 6, 32, 0.018 | 4.688, 6, 33, 0.001 | |
| Spider Mite | Factor | df | F | p |
|---|---|---|---|---|
| E. sexmaculatus | Temperature | 2 | 5.100 | 0.007 |
| Initial density | 3 | 40.163 | p < 0.001 | |
| Rearing time | 7 | 3.679 | 0.001 | |
| Temperature and initial density | 6 | 3.032 | 0.008 | |
| Temperature and rearing time | 9 | 2.126 | 0.031 | |
| Initial density and rearing time | 18 | 4.547 | p < 0.001 | |
| Temperature and initial density and rearing time | 21 | 0.579 | 0.927 | |
| O. biharensis | Temperature | 2 | 7.308 | 0.001 |
| Initial density | 3 | 45.195 | p < 0.001 | |
| Rearing time | 7 | 271.637 | p < 0.001 | |
| Temperature and initial density | 6 | 0.927 | 0.477 | |
| Temperature and rearing time | 9 | 3.269 | 0.001 | |
| Initial density and rearing time | 18 | 7.011 | p < 0.001 | |
| Temperature and initial density and rearing time | 21 | 0.721 | 0.806 |
| Temperature | Mixed Population | ||
|---|---|---|---|
| 20 ES vs. 10 OB | 15 ES vs. 15 OB | 10 ES vs. 20 OB | |
| 27 °C | 0.4157 ± 0.04 a | 0.6466 ± 0.07 a | 0.5388 ± 0.42 b |
| 30 °C | 0.5152 ± 0.06 a | 0.3927 ± 0.04 b | 0.5484 ± 0.16 b |
| 33 °C | 0.4976 ± 0.05 a | 0.5441 ± 0.05 ab | 0.6591 ± 0.05 a |
| F, df1, df2, p | 6.493, 2, 6, 0.032 | 5.975, 2, 6, 0.037 | 1.017, 2, 6, 0.417 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhang, Y.; Shi, X.; Gan, W.; Zhang, F.; Fu, Y.; Liu, Y.; Chen, J.; Ye, Z. Interspecific Competition Between Eotetranychus sexmaculatus Riley and Oligonychus biharensis Hirst (Acari: Tetranychidae). Insects 2025, 16, 96. https://doi.org/10.3390/insects16010096
Zheng L, Zhang Y, Shi X, Gan W, Zhang F, Fu Y, Liu Y, Chen J, Ye Z. Interspecific Competition Between Eotetranychus sexmaculatus Riley and Oligonychus biharensis Hirst (Acari: Tetranychidae). Insects. 2025; 16(1):96. https://doi.org/10.3390/insects16010096
Chicago/Turabian StyleZheng, Lijiu, Yong Zhang, Xia Shi, Wei Gan, Fangping Zhang, Yueguan Fu, Ya Liu, Junyu Chen, and Zhengpei Ye. 2025. "Interspecific Competition Between Eotetranychus sexmaculatus Riley and Oligonychus biharensis Hirst (Acari: Tetranychidae)" Insects 16, no. 1: 96. https://doi.org/10.3390/insects16010096
APA StyleZheng, L., Zhang, Y., Shi, X., Gan, W., Zhang, F., Fu, Y., Liu, Y., Chen, J., & Ye, Z. (2025). Interspecific Competition Between Eotetranychus sexmaculatus Riley and Oligonychus biharensis Hirst (Acari: Tetranychidae). Insects, 16(1), 96. https://doi.org/10.3390/insects16010096






