Nontarget Catches of Green and Brown Lacewings (Insecta: Neuroptera: Chrysopidae, Hemerobiidae) Collected by Light- and Volatile-Baited Traps in the Transcarpathian Lowland (W Ukraine)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
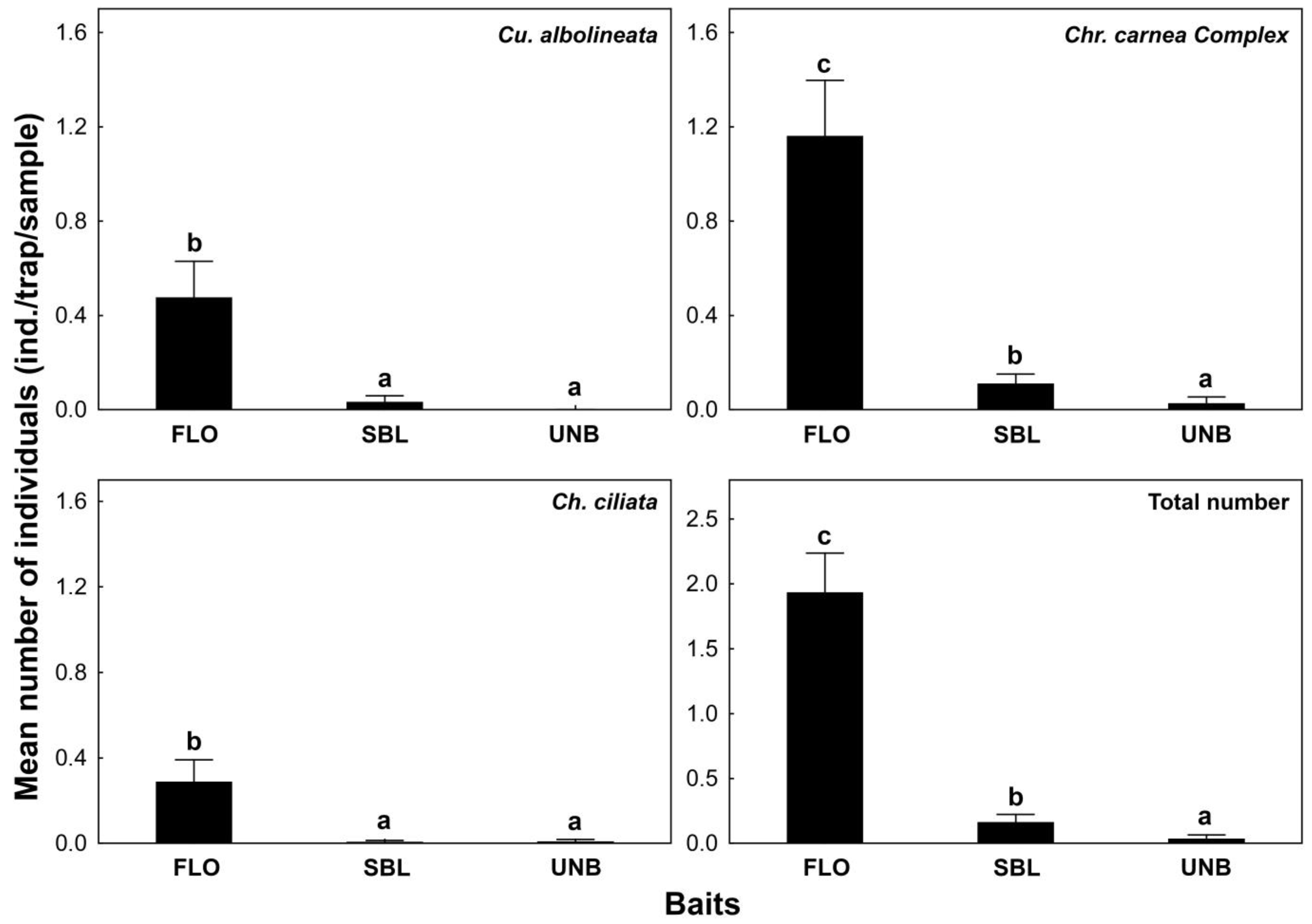
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seredyuk, G.V. Neuropterous (Insecta: Neuroptera) Primeval Beech Forests Uholka Array CBR. Sci. Bull. Uzhhorod Univ. Ser. Biol. 2013, 35, 91–98. [Google Scholar]
- Seredyuk, G.V. Green Lacewings (Insecta: Neuroptera, Chrysopidae) Fauna of Ukraine. Proc. State Nat. Hist. Mus. 2015, 31, 141–148. [Google Scholar]
- Seredyuk, G.V. Neuropterida (Insecta: Neuroptera) of Ukrainian Carpathians. Ukr. Entomol. J. 2016, 11, 46–65. [Google Scholar]
- Seredyuk, G.V. Neuropterous Subfamily Nothochrysinae (Insecta: Neuroptera, Chrysopidae) Fauna of Ukraine. Proc. State Nat. Hist. Mus. 2016, 32, 155–162. [Google Scholar]
- Seredyuk, G.V. Insecta Neuroptera of the Galician National Park. Proc. State Nat. Hist. Mus. 2019, 35, 119–124. [Google Scholar] [CrossRef]
- Seredyuk, G.V. Altitude and Biotope Distribution of Species of a Number of Neuroptera Fauna of the Ukrainian Carpathians and the Transcarpathian Lowlands. Proc. State Nat. Hist. Mus. 2020, 36, 147–158. [Google Scholar] [CrossRef]
- Biodiversity of Ukraine—The Information Resource for Ukraine Biota Diversity. State Museum of Natural History, National Academy of Science of Ukraine. Available online: http://dc.smnh.org/ (accessed on 6 December 2024).
- Varga, Z. Geographical Patterns of Biodiversity in the Palearctic and in the Carpathian Basin. Acta Zool. Acad. Sci. Hung. 1995, 41, 71–92. [Google Scholar]
- Varga, Z. The Zoogeography of the Carpathian Basin. In Növény, Állat, Élőhely; Láng, I., Bedő, Z., Csete, L., Eds.; Magyar Tudománytár III., MTA Társadalomkutató Központ: Budapest, Hungary, 2003; pp. 89–119. [Google Scholar]
- James, D.G. Field Evaluation of Herbivore-Induced Plant Volatiles as Attractants for Beneficial Insects: Methyl Salicylate and the Green Lacewing, Chrysopa nigricornis. J. Chem. Ecol. 2003, 29, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.; Szentkirályi, F.; Vuts, J.; Letardi, A.; Tabilio, M.R.; Jaastad, G.; Knudsen, G. Optimization of a Phenylacetaldehyde-Based Attractant for Common Green Lacewings (Chrysoperla carnea s.l.). J. Chem. Ecol. 2009, 35, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Koczor, S.; Szentkirályi, F.; Pickett, J.; Birkett, M.; Tóth, M. Aphid Sex Pheromone Compounds Interfere with Attraction of Common Green Lacewings to Floral Bait. J. Chem. Ecol. 2015, 41, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Koczor, S.; Szentkirályi, F.; Tóth, M. New Perspectives for Simultaneous Attraction of Chrysoperla and Chrysopa Lacewing Species for Enhanced Biological Control. Sci. Rep. 2019, 9, 10303. [Google Scholar] [CrossRef]
- Szanyi, S.; Attila, M.-G.; Lajos, K.; Tímea, S.; Zoltán, V.; Miklós, T.; Antal, N. Nyírségi Macroheterocera Együttesek Vizsgálata Illatanyagcsapdák Alkalmazásával. Erdészettudományi Közlemények 2019, 9, 51–68. [Google Scholar] [CrossRef]
- Nagy, A.; Szarukán, I.; Szalárdi, T.; Szanyi, S.; Jósvai, J.K.; Tóth, M. Addition of 4-Oxoisophorone Improves Performance of Bisexual Lure for Autographa gamma (L.) (Lepidoptera: Noctuidae). J. Appl. Entomol. 2022, 146, 328–334. [Google Scholar] [CrossRef]
- Dövényi, Z. Inventory of Microregions in Hungary; MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 2010; p. 876. [Google Scholar]
- Nagy, A.; Ősz, A.; Tóth, M.; Rácz, I.A.; Kovács, S.; Szanyi, S. Nontarget Catches of Traps with Chemical Lures May Refer to the Flower-Visitation, Probable Pollination, and Feeding of Bush Crickets (Ensifera: Tettigoniidae). Ecol. Evol. 2023, 13, e10249. [Google Scholar] [CrossRef]
- Nagy, A.; Szarukán, I.; Gém, F.; Nyitrai, R.; Füsti-Molnár, B.; Némerth, A.; Kozák, L.; Molnár, A.; Katona, K.; Szanyi, S.; et al. Preliminary Data on the Effect of Semi-Synthetic Baits for Noctuidae (Lepidoptera) on the Non-Target Lepidoptera Species. Acta Agrar. Debreceniensis 2015, 66, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Katona, P.; Molnár, A.; Rádai, Z.; Tóth, M.; Szanyi, K.; Szanyi, S. Wide Range of Brachyceran Fly Taxa Attracted to Synthetic and Semi-Synthetic Generic Noctuid Lures and the Description of New Attractants for Sciomyzidae and Heleomyzidae Families. Insects 2023, 14, 705. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Nagy, A.; Gém, F.; Nyitrai, R.; Kecskés, Z.; Krakkó, L.; Jósvai, J.K.; Bélai, I. Félszintetikus “Biszex” Csalétkek Kártevő Rovarok Nőstényeinek és Hímjeinek Fogására. Növényvédelem 2015, 51, 197–205. [Google Scholar]
- Aspöck, H.; Aspöck, U.; Hölzel, H. Die Neuropteren Europas. In Eine Zusammenfassende Darstellung der Systematik, Ökologie Und Chorologie der Neuropteroidea (Megaloptera, Raphidioptera, Planipennia) Europas; Goecke & Evers: Krefeld, Germany, 1980; Volume 1–2, p. 850. [Google Scholar]
- Thierry, D.; Cloupeau, R.; Jarry, M.; Canard, M. Discrimination of the West-Palearctic Chrysoperla Steinmann species of the carnea Stephens group by means of claw morphology (Neuroptera, Chrysopidae). Acta Zool. Fenn. 1998, 209, 255–262. [Google Scholar]
- Ketskeméty, L.; Izsó, L.; Könyves Tóth, E. Bevezetés az IBM SPSS Statistics Programrendszerbe; Artéria Stúdió Kft: Budapest, Hungary, 2011; p. 580. [Google Scholar]
- Mahzoum, A.M.; Villa, M.; Benhadi-Marín, J.; Pereira, J.A. Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control. Agronomy 2020, 10, 1511. [Google Scholar] [CrossRef]
- Ranjbar Aghdam, H.; Nemati, Z. Modeling of the Effect of Temperature on Developmental Rate of Common Green Lacewing, Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae). J. Biol. Pest Control 2020, 30, 145. [Google Scholar] [CrossRef]
- Nabli, H.; Bailey, W.C.; Necibi, S. Beneficial Insect Attraction to Light Traps with Different Wavelengths. Biol. Control 1999, 16, 185–188. [Google Scholar] [CrossRef]
- Deutsch, B.; Paulian, J.A.; Thierry, D.; Canard, M. Quantifying Biodiversity in Ecosystems with Green Lacewing Assemblages. Agron. Sustain. Dev. 2005, 25, 337–343. [Google Scholar] [CrossRef]
- Koczor, S.; Szentkirályi, F.; Birkett, M.A.; Pickett, J.A.; Voigt, E.; Tóth, M. Attraction of Chrysoperla carnea Complex and Chrysopa spp. Lacewings (Neuroptera: Chrysopidae) to Aphid Sex Pheromone Components and a Synthetic Blend of Floral Compounds in Hungary. Pest Manag. Sci. 2010, 66, 1374–1379. [Google Scholar] [CrossRef] [PubMed]

| Species | 2014 | 2015 | 2016 | SUM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FLO | SBL | UNB | FLO | SBL | Light | UNB | FLO | SBL | UNB | ||
| Chrysopa perla (Linnaeus, 1758) | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 14 |
| Chrysopa nigricostata (Brauer, 1850) | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Chrysopa phyllochroma Wesmael, 1841 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 |
| Chrysopa walkeri (McLachlan, 1893) | 0 | 0 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 17 |
| Chrysoperla carnea Complex | 22 | 6 | 0 | 39 | 6 | 13 | 3 | 112 | 5 | 0 | 206 |
| Chrysopidia ciliata Wesmael, 1841 | 0 | 0 | 0 | 37 | 1 | 0 | 1 | 6 | 0 | 0 | 45 |
| Cunctochrysa albolineata Killington, 1935 | 71 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 76 |
| Apertochrysa prasina Burmeister, 1839 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Hemerobius humulinus Linnaeus, 1758 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| Micromus variegatus (Fabricius, 1793) | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 10 |
| Total number of individuals | 94 | 12 | 0 | 76 | 8 | 30 | 4 | 118 | 5 | 0 | 378 |
| Total number of species | 3 | 3 | 0 | 2 | 3 | 7 | 2 | 2 | 1 | 0 | 10 |
| Sdiff | 0 | 0 | 0 | 0 | 1 | 6 | 0 | 1 | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szanyi, K.; Nagy, A.; Ősz, A.; Ábrahám, L.; Molnár, A.; Tóth, M.; Szanyi, S. Nontarget Catches of Green and Brown Lacewings (Insecta: Neuroptera: Chrysopidae, Hemerobiidae) Collected by Light- and Volatile-Baited Traps in the Transcarpathian Lowland (W Ukraine). Insects 2025, 16, 74. https://doi.org/10.3390/insects16010074
Szanyi K, Nagy A, Ősz A, Ábrahám L, Molnár A, Tóth M, Szanyi S. Nontarget Catches of Green and Brown Lacewings (Insecta: Neuroptera: Chrysopidae, Hemerobiidae) Collected by Light- and Volatile-Baited Traps in the Transcarpathian Lowland (W Ukraine). Insects. 2025; 16(1):74. https://doi.org/10.3390/insects16010074
Chicago/Turabian StyleSzanyi, Kálmán, Antal Nagy, Aletta Ősz, Levente Ábrahám, Attila Molnár, Miklós Tóth, and Szabolcs Szanyi. 2025. "Nontarget Catches of Green and Brown Lacewings (Insecta: Neuroptera: Chrysopidae, Hemerobiidae) Collected by Light- and Volatile-Baited Traps in the Transcarpathian Lowland (W Ukraine)" Insects 16, no. 1: 74. https://doi.org/10.3390/insects16010074
APA StyleSzanyi, K., Nagy, A., Ősz, A., Ábrahám, L., Molnár, A., Tóth, M., & Szanyi, S. (2025). Nontarget Catches of Green and Brown Lacewings (Insecta: Neuroptera: Chrysopidae, Hemerobiidae) Collected by Light- and Volatile-Baited Traps in the Transcarpathian Lowland (W Ukraine). Insects, 16(1), 74. https://doi.org/10.3390/insects16010074








