Stridulating Species of Aphids of the Genus Uroleucon (Hemiptera: Aphididae) with Descriptions of a New Species from Iran †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Taxonomy
2.2. Scanning Electron Microscopy
3. Results
3.1. Taxonomy
Review of Stridulating Species of the Aphid Genus Uroleucon
- Uroleucon (Uromelan) adenophorae (Matsumura, 1918)
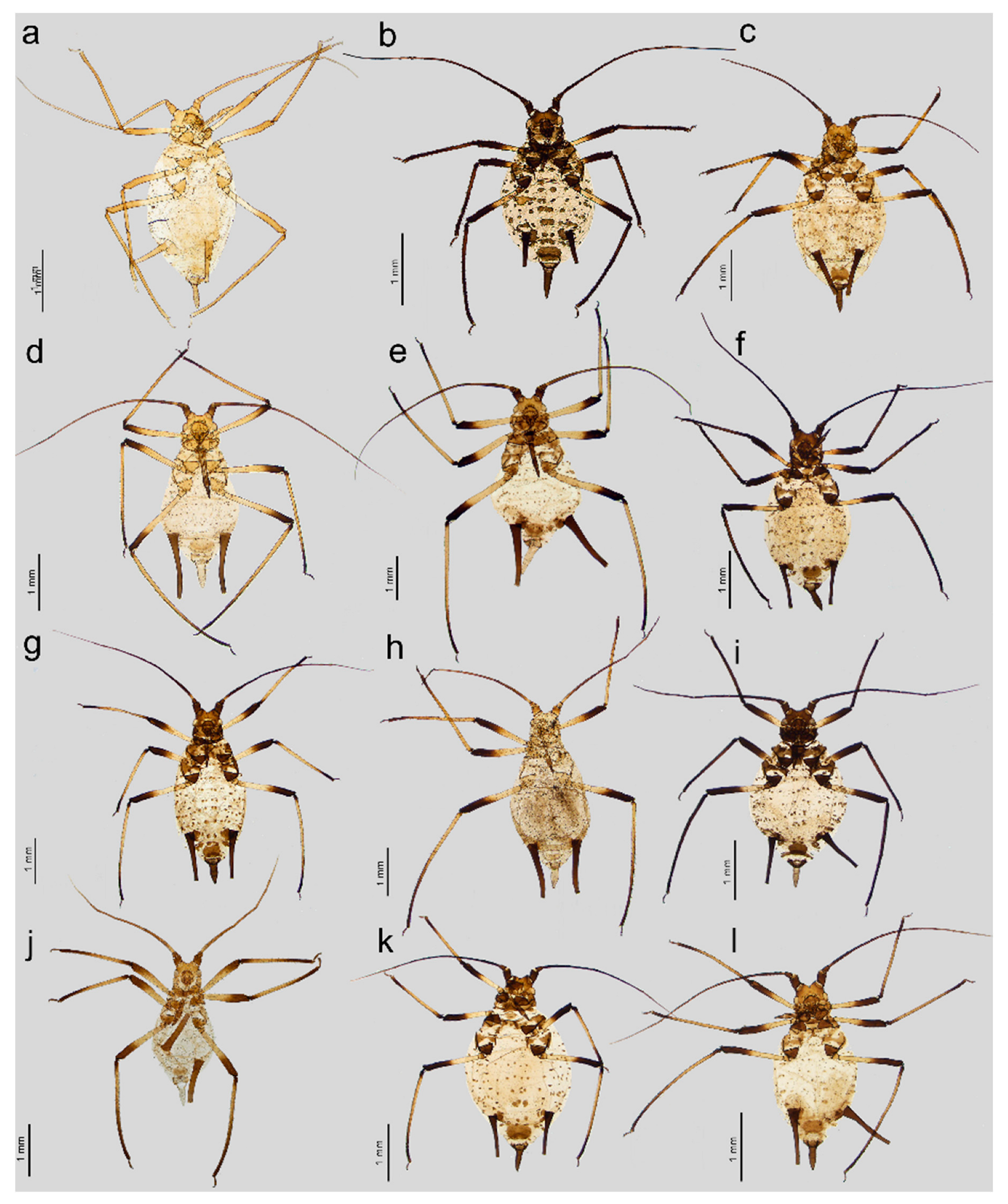
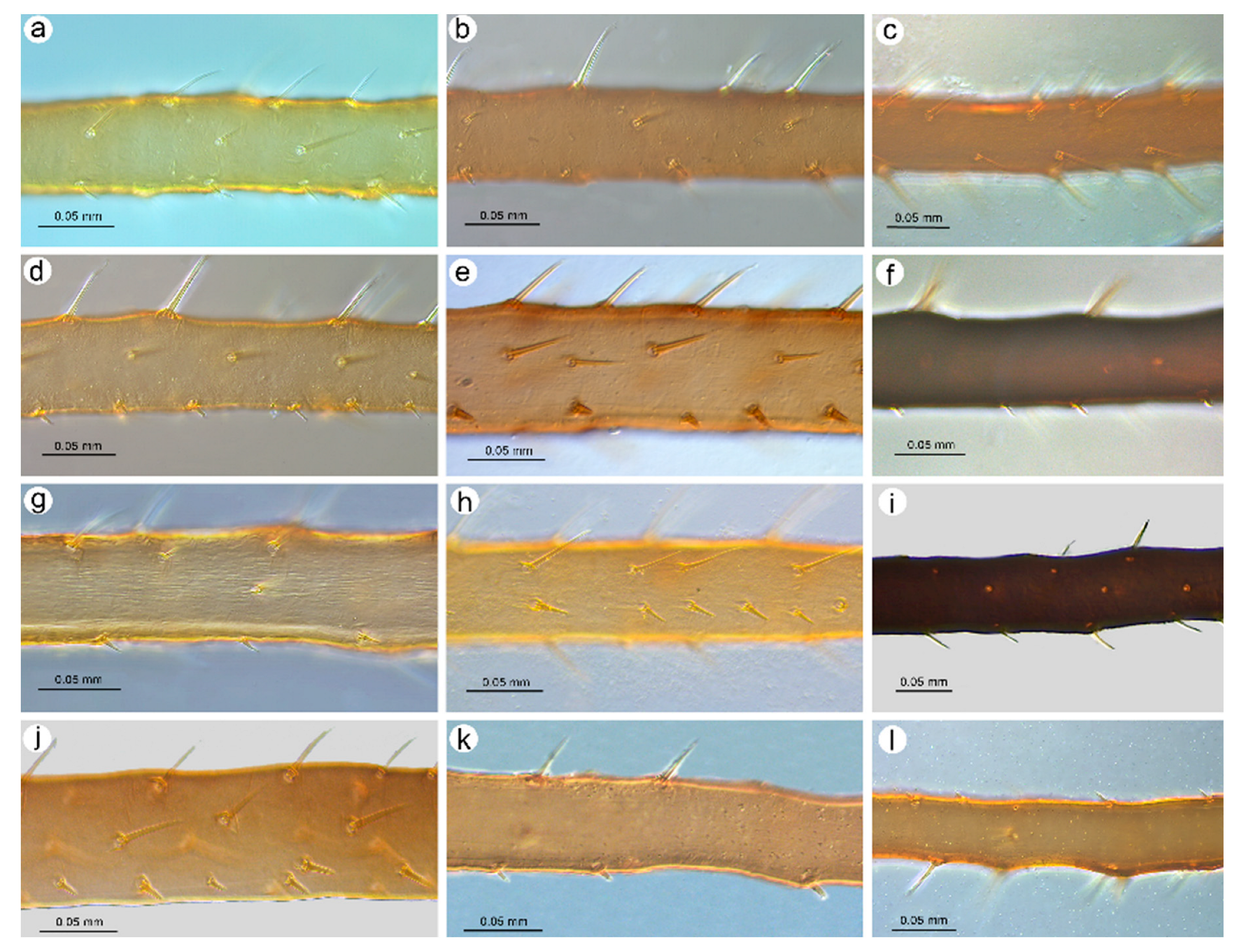
- Apterous viviparous female. Color in life: shiny dark brown to black [2]. Morphological characters: Head slightly sclerotized, light brown, ANT uniformly light brown, femora light brown with pale distal halves, and tibiae light brown with slightly paler inner distal parts (Figure 1a). Peg-like setae on the inner side of the tibiae are rather long, similar to the other setae, and pointed (Figure 2a and Figure 3a). Abdomen pale with light brown to brown scleroites, light brown SIPH, postsiphuncular sclerites, and cauda (Figure 4a).


- 2.
- Uroleucon (Uromelan) campanulae (Kaltenbach, 1843)
- Apterous viviparous female. Color in life: They are rather shiny with a dark brown head and pronotum and are brown on the rest of their body with dark ANT. Femora are dark brown to black with yellow to pale proximal halves. Tibiae, SIPH, and cauda dark brown to black.
- Distribution: This is a common Palaearctic species that is rather widely distributed in Europe (with Germany as the terra typica), western Siberia, and southwest and central Asia [3].
- 3.
- Uroleucon (Uromelan) carthami (Hille Ris Lambers, 1948)
- Apterous viviparous female. Color in life: shiny blackish-brown or almost black with only femora pale on the proximal halves. Pigmentation on slide: Head sclerotized, brown. ANT brown with slightly lighter very basal part of ANT III. Pronotum sclerotized, brown. Femora dark with yellow proximal halves. Fore and middle tobiae yellow to light brown with darker proximal and distal ends. (Figure 1c). III TIBIAE brown with a lighter proximal part (Figure 2c). Peg-like setae of different lengths, from short to long, with slightly blunt and pointed apices (Figure 3c). Abdomen yellow with well-developed scleroites at setal bases, which all are of the same size. SIPH with postsiphucular sclerites, cauda brown (Figure 4c).
- 4.
- Uroleucon (Uroleucon) caspicum (Rezwani and Lampel, 1990)
- Apterous viviparous female. Color in life: shiny dark brown with dark ANT, legs, and SIPH. Cauda pale [22]. Pigmentation on slide: Head sclerotized, light brown, and thorax rather membranous. ANT brown with lighter basal part of ANT III. Femora yellow with brown distal ends. Fore and middle tibiae light brown with brown distal and proximal ends (Figure 1d). III TIBIAE yellow with dark knee area and proximal halves (Figure 2d). Peg-like setae short, fine, and pointed (Figure 3d). Abdomen pale with poorly visible scleroites at setal bases. SIPH uniformly brown with very poorly visible postsiphuncular sclerites. Cauda pale (Figure 4d).
- 5.
- Uroleucon (Uroleucon) cirsicola (Holman, 1962)
- Apterous viviparous female. Color in life: dark brassy brown, with often black ANT, legs mainly pale brown, and SIPH black on basal half but brown distally. Cauda yellow (Holman, 1962). Pigmentation on slide: Head sclerotized, prothorax, and mesothorax sclerotized and brown. ANT brown with darker ANT I and II and lighter basal part of ANT III. Femora yellow with dark distal ends. Fore and middle tibiae yellow with brown distal and proximal ends (Figure 1e). III TIBIAE yellow with brown knee area and proximal end (Figure 2e). Peg-like setae very short, slightly bulky at bases with rounded apices (Figure 3e). Abdomen pale with well-visible small scleroites at setal bases. SIPH brown with slightly lighter apical ends and well-developed postsiphuncular sclerites. Cauda pale (Figure 4e).
- 6.
- Uroleucon (Uroleucon) fuchuense (Shinji, 1942)
- Apterous viviparous female. Color in life: Body shiny red with dark ANT, tibiae, and SIPH. Femora black with pale proximal parts. Cauda pale with slightly dusky very distal end [27]. Pigmentation on slide: Head sclerotized, brown. ANT dark, almost black. Femora yellow with dark to black distal halves. Tibiae yellow to light brown with dark knee areas and distal halves. Peg-like setae short and very short, but the difference in size is visible, straight with slightly pointed apices. Abdomen pale with small indistinct scleroites at setal bases, dark brown SIPH, and pale brown cauda.
- Remarks. Kanturski and Lee [27] gave detailed descriptions and figured the sense pegs of this species based on oviparous females.
- 7.
- Uroleucon (Uromelan) jaceae (Linnaeus, 1758)
- Apterous viviparous female. Color in life: shiny, and the color varies from reddish brown to blackish brown with a visible row of dorsal spots on the abdomen (especially in lighter individuals). ANT are black as well as the legs, in addition to the greenish proximal parts of the femora. SIPH and cauda black. Pigmentation on slide: Head and thorax sclerotized and brown. ANT dark brown to black. Femora yellow with dark distal halves (Figure 1f). Tibiae including III TIBIAE dark to black with only very slightly lighter proximal parts (Figure 2f). Peg-like setae very short, straight with slightly rounded or pointed apices (Figure 3f). Abdomen yellow with well-developed scleroites at setal bases, of which the spinal ones often are larger than the rest. SIPH dark brown with well-developed, dark postsiphuncular sclerites. Cauda brown (Figure 4f).
- 8.
- Uroleucon (Uromelan) minor (Börner, 1940)
- Apterous viviparous female. Color in life: brown with black dorsal spots, legs yellow banded with black ANT, SIPH, and cauda (Blackman and Eastop, 2024). Pigmentation on slide: Head, pro and mesothorax sclerotized and brown. ANT dark brown to black with lighter basal part of ANT III. Femora yellow with dark distal halves. Tibiae including III TIBIAE yellow or light brown, with the knee area and distal ends dark (Figure 1g) or III TIBIAE dark with very slightly lighter proximal part (Figure 2g). Peg-like setae short, fine, and with clearly pointed apices (Figure 3g). Abdomen yellow with well-developed scleroites at setal bases, of which the spinal ones on ABD V and VI often are larger than the rest. SIPH dark brown with well-developed, dark postsiphuncular sclerites. Some sclerotization can be noted and treated as antesiphuncular sclerites, but those are rather fused spinal and marginal scleroites. Cauda brown (Figure 4g).
- 9.
- Uroleucon (Uroleucon) monticola (Takahashi, 1935)
- Apterous viviparous female. Color in life: shiny green, dusky around bases of SIPH, with distal halves of femora and tibiae brown-black, black SIPH, and a contrastingly pale yellow cauda (Blackman and Eastop, 2024). Pigmentation on slide: Head slightly sclerotized, yellow to pale brown. ANT brown with yellow basal parts of ANT I, ANT III, and light brown distal part of ANT V. Femora yellow or light brown with dark distal halves (Figure 1h). Tibiae including III TIBIAE light brown also in the knee area with darker distal halves (Figure 2h). Peg-like setae rather long and robust with slightly bulky basal parts and pointed (Figure 3h). Abdomen pale to yellow with very few indistinct scleroites at setal bases, which in general view are invisible. SIPH brown with lighter postsiphuncular sclerites. Cauda pale or yellow (Figure 4h).
- 10.
- Uroleucon (Uromelan) phyteuma (Bozhko, 1950)
- Apterous viviparous female. Color in life: aphids are slightly shiny and uniformly black (Holman, 1969). Morphological characters: Head sclerotized, brown to dark brown, ANT uniformly brown, femora brown to dark with pale distal parts of fore and middle tibiae and pale proximal halves of III FEMORA (Figure 1i). Tibiae brown to dark uniformly (Figure 2i). Peg-like setae on the inner side of tibiae rather long, similar to the other setae, and pointed (Figure 3i). Abdomen pale with brown scleroites at setal bases and characteristically large marginal tubercles on ABD TERG I-IV. SIPH dark brown with brown postsiphuncular sclerites and fused, which may be confused with antesiphuncular sclerites. Cauda light brown (Figure 4i).
- 11.
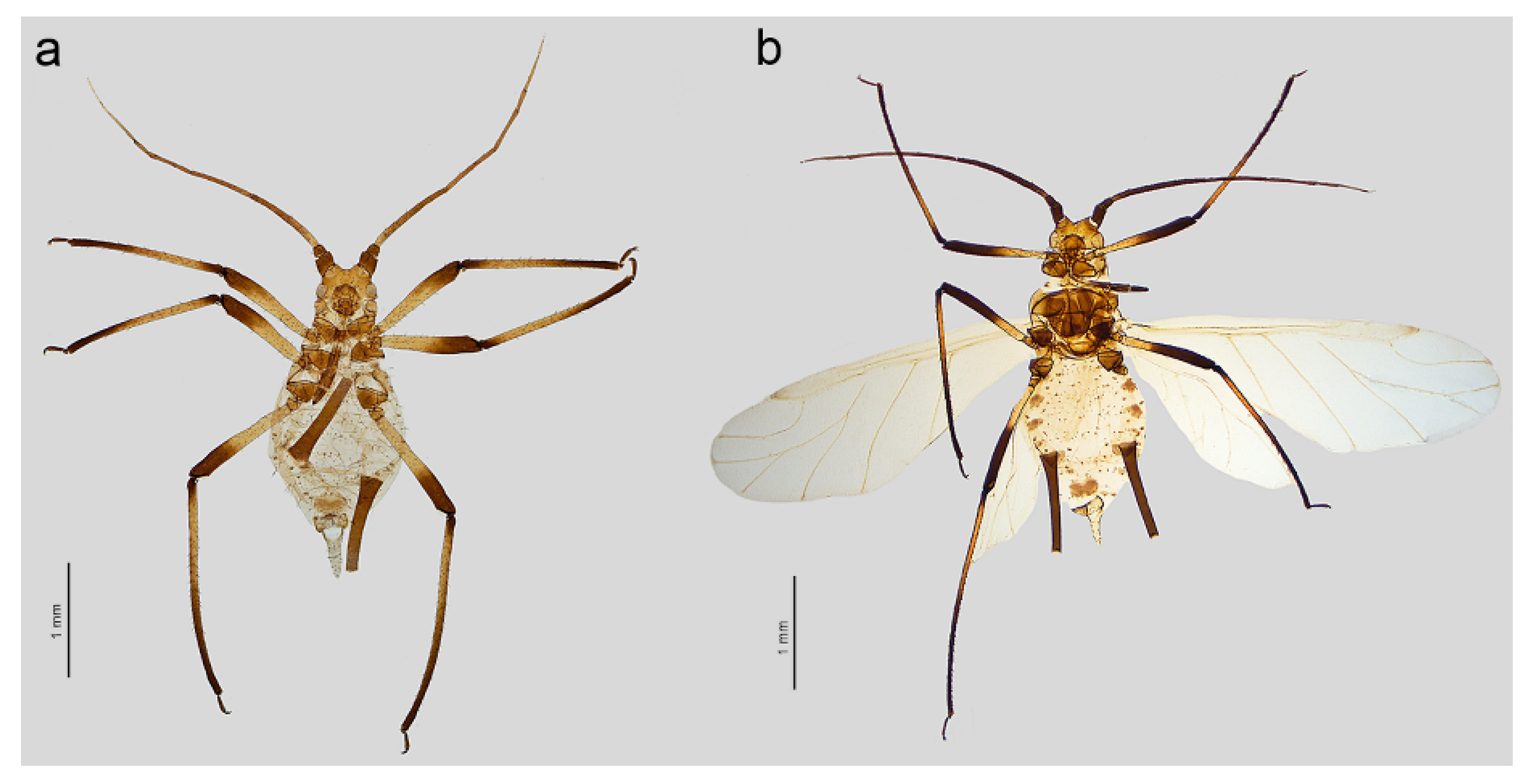
- Uroleucon (Uroleucon) remaudierei sp. nov.
- Description. Apterous viviparous female (n = 18).
- Alate viviparous female (n = 5)
- Types: Holotype. Apterous viviparous female (apt.), Iran, Gajereh, 2300 m, 14.07.1955, Michauxia leavigata, G. Remaudière leg., i767 (apt 4), IECA. Paratypes. Apt., other data same as in holotype, i767 (apt 3), IECA; alate viviparous female (al.), other data same as in holotype, i767 (al 3), IECA; apt., other data ast in holotype, 24911, MNHN; apt., other data as in holotype, 24913, MNHN; four apt., Fasham, 1800 m, 08.09.1972, Mindium laevigatum (=Michauxia laevigata), G. Remaudière leg., i3699 (apt. 23–26), IECA; 2 al., i3699 (al. 7–8), IECA; 2 al., i3699 (al. 9–10), IECA; 2 apt., i3699 (apt. 21–22), IECA; 4 apt., 25 Km E from Sanandaj, 2800 m, 15.08.1955, Asyneuma persicum, G. Remaudière leg., i1048 (apt. 9–12), IECA; apt., Kuh-e Dinar, 3300 m, 13.09.1955, i1112 (apt. 14), IECA, IECA; apt., i1112 (apt. 15), IECA; i1112 (apt. 10), DZUS; apt., i1112 (apt. 11–13), ISIZU.
- Diagnosis and taxonomical comments. The new species belongs to subgenus Uroleucon Mordvilko, 1914, with a row of short thick peg-like setae on the hind tibia ventrally. The following species from the same subgenus have a row of short thick peg-like setae: U. caspicum, U. cirsicola, U. fuchuense, and U. monticola. The apterous viviparous females of Uroleucon remaudierei sp. nov. are distinguished from those of U. caspicum by (1) the number of setae on the cauda: cauda with 13–18 setae in the new species, while there are 24–41 setae in U. caspicum; (2) the URS L/HT II L ratio: 0.97–1.07 in the new species and 1.20–1.46 in U. caspicum; and (3) the SIPH L/BL ratio: 0.27–0.32 in the new species and 0.33–0.44 in U. caspicum [3,22].
- Etymology. We have the pleasure of naming the new species to honor Georges Remaudière—for years an outstanding aphidologist and author of numerous aphid taxa. The name of the species was also the intention of Jaroslav Holman.
- Host plant and biology. Uroleucon remaudierei has been found on two plant species: Asyneuma persicum and Michauxia laevigata (Campanulaceae). Its sexual morphs and life cycle are unknown.
- 12.
- Uroleucon (Uromelan) riparium (Stroyan, 1955)
- Apterous viviparous female. Color in life: dark bronze-brown with black antennae, SIPH, cauda, tarsi, apices of femora, and tibiae [3]. Pigmentation on slide: Head and prothorax sclerotized, brown, antennae dark brown. Femora yellow or pale with dark distal halves (Figure 1k). Tibiae including hind tibiae yellow or light brown with dark knee areas and distal ends (Figure 2k). Peg-like setae very short, robust, with rounded or slightly pointed apices (Figure 3k). Abdomen yellow or pale with well-developed and visible dark scleroites at setal bases, of which the spinal ones on ABD TERG V are larger than the others. SIPH dark brown with well-developed, dark postsiphuncular sclerites. Cauda brown (Figure 4k).
- 13.
- Uroleucon (Uromelan) siculum (Barbagallo and Stroyan, 1982)
- Apterous viviparous female. Color in life: dark brown, with mainly black appendages (Blackman and Eastop, 2024). Pigmentation on slide: Head and prothorax sclerotized, brown, antennae brown with slightly lighter ANT III. Femora yellow or pale with dark distal halves (Figure 1l). Tibiae including III TIBIAE light brown with dark knee areas and distal ends (Figure 2l). Peg-like setae short, straight, with clearly pointed apices (Figure 3l). Abdomen yellow or pale with well-developed and visible light brown scleroites at setal bases. SIPH brown with well-developed, brown postsiphuncular sclerites. Cauda brown (Figure 4l).
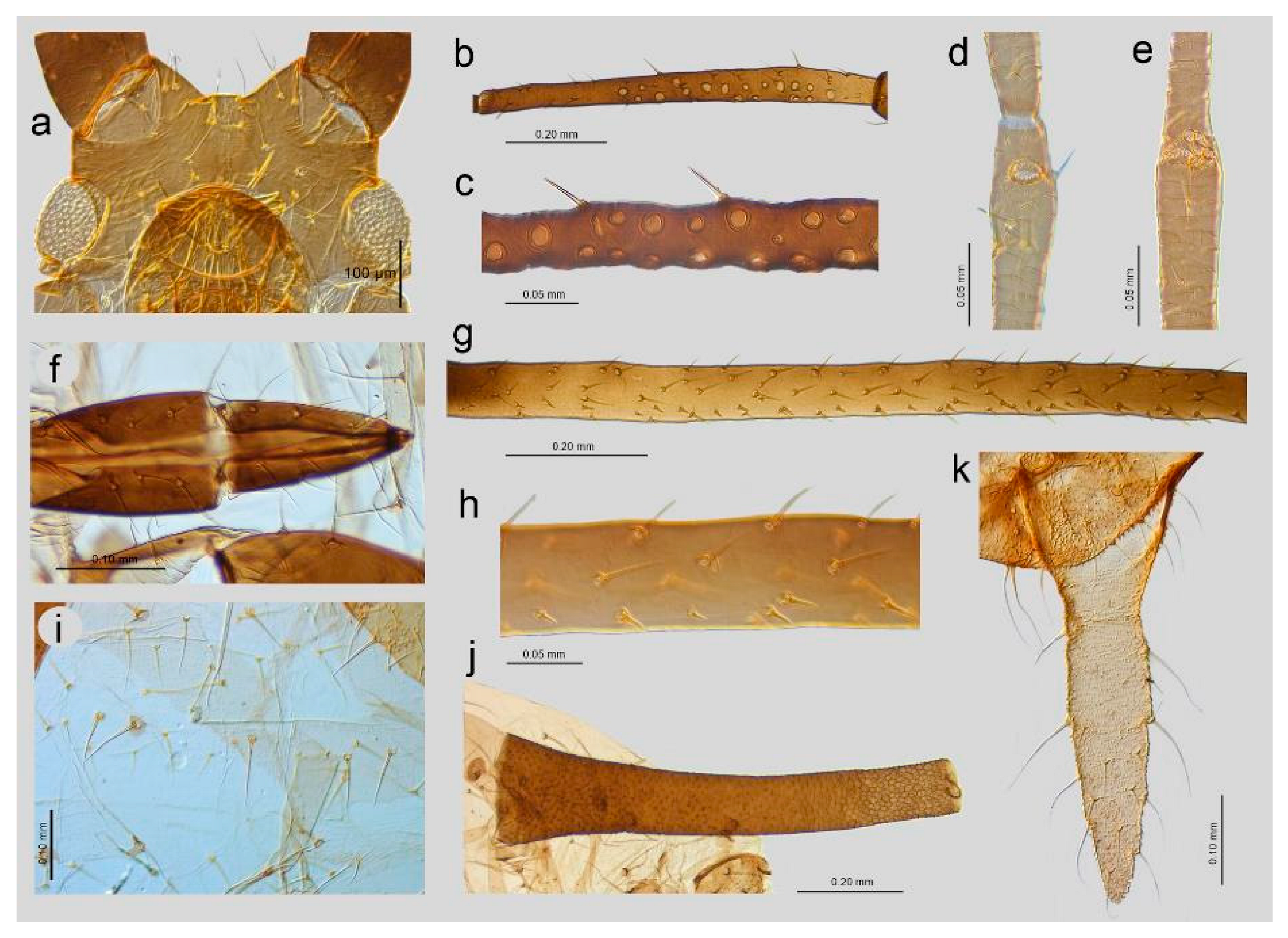
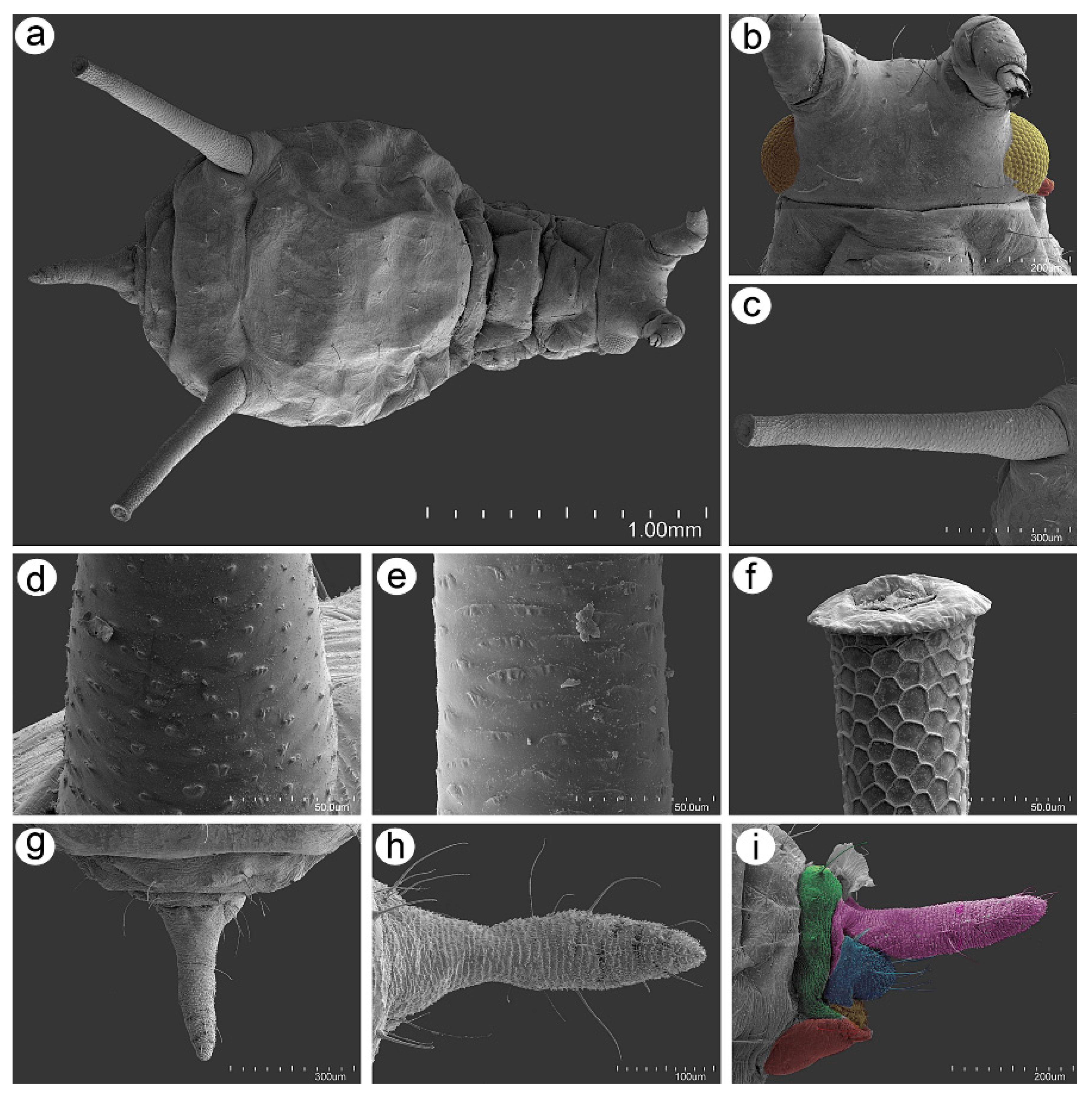
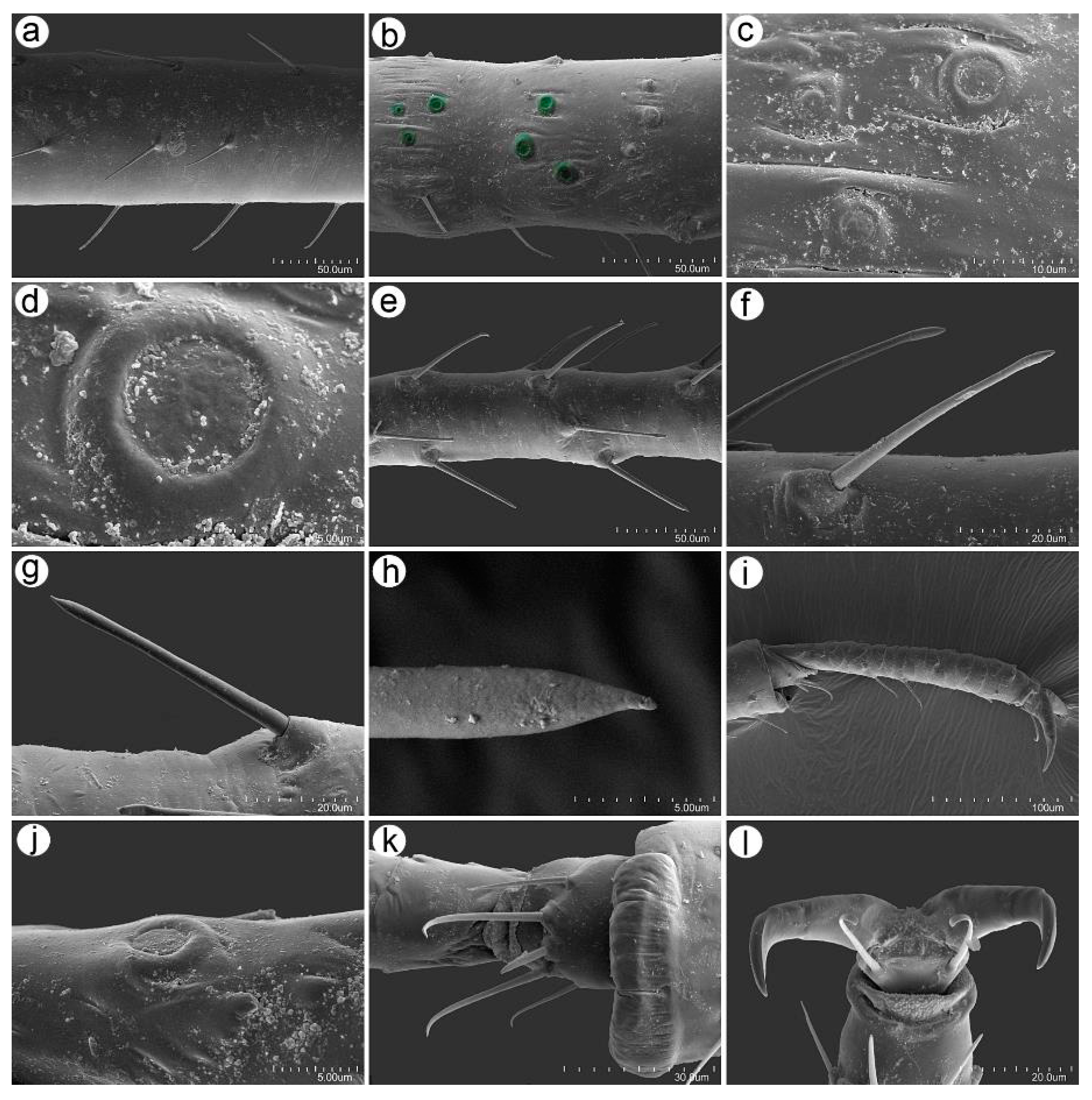
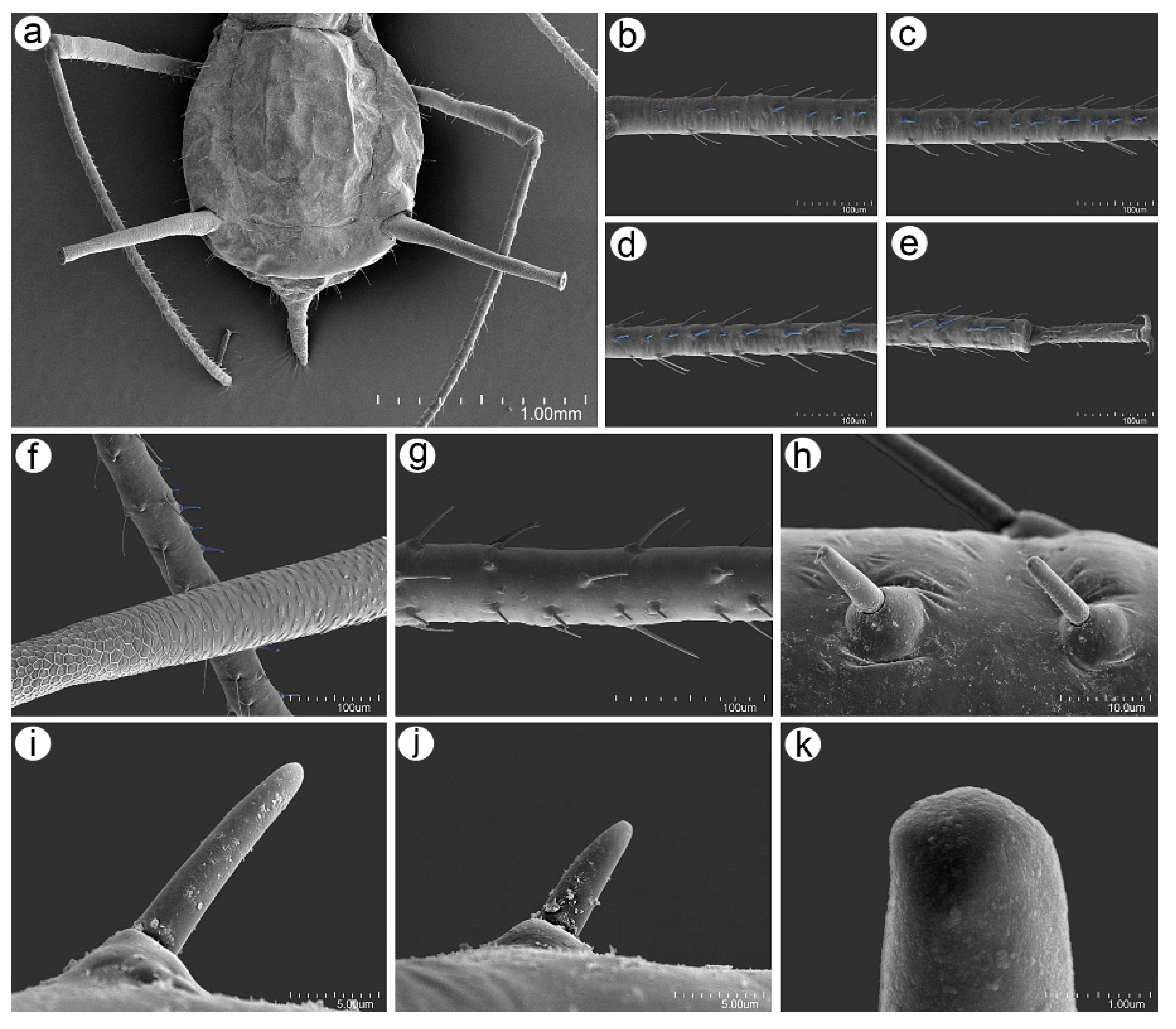
3.2. Notes on the SEM Morphology of Uroleucon remaudierei sp. nov.—Representative of Stridulating Aphids
3.2.1. General Characters
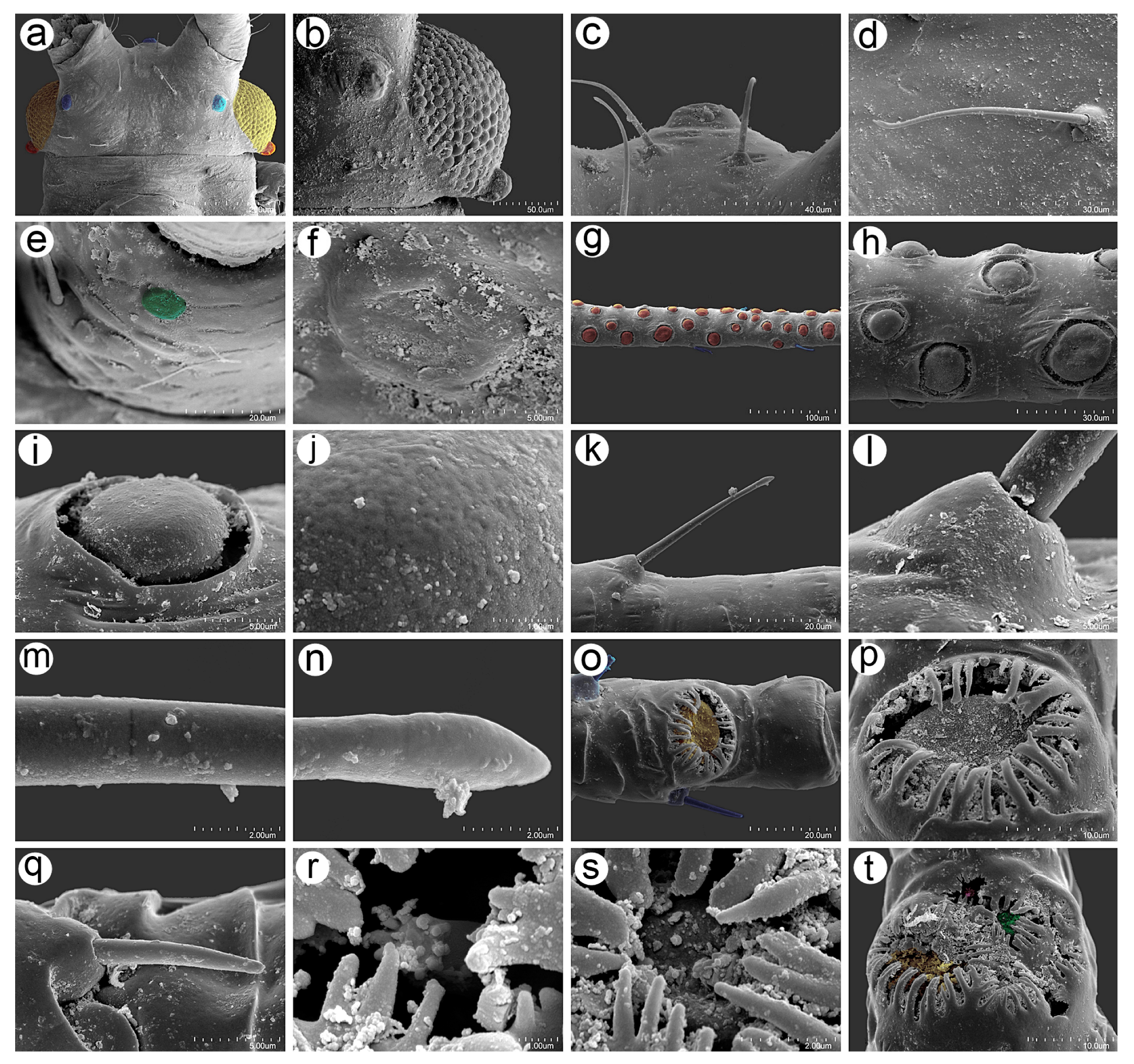
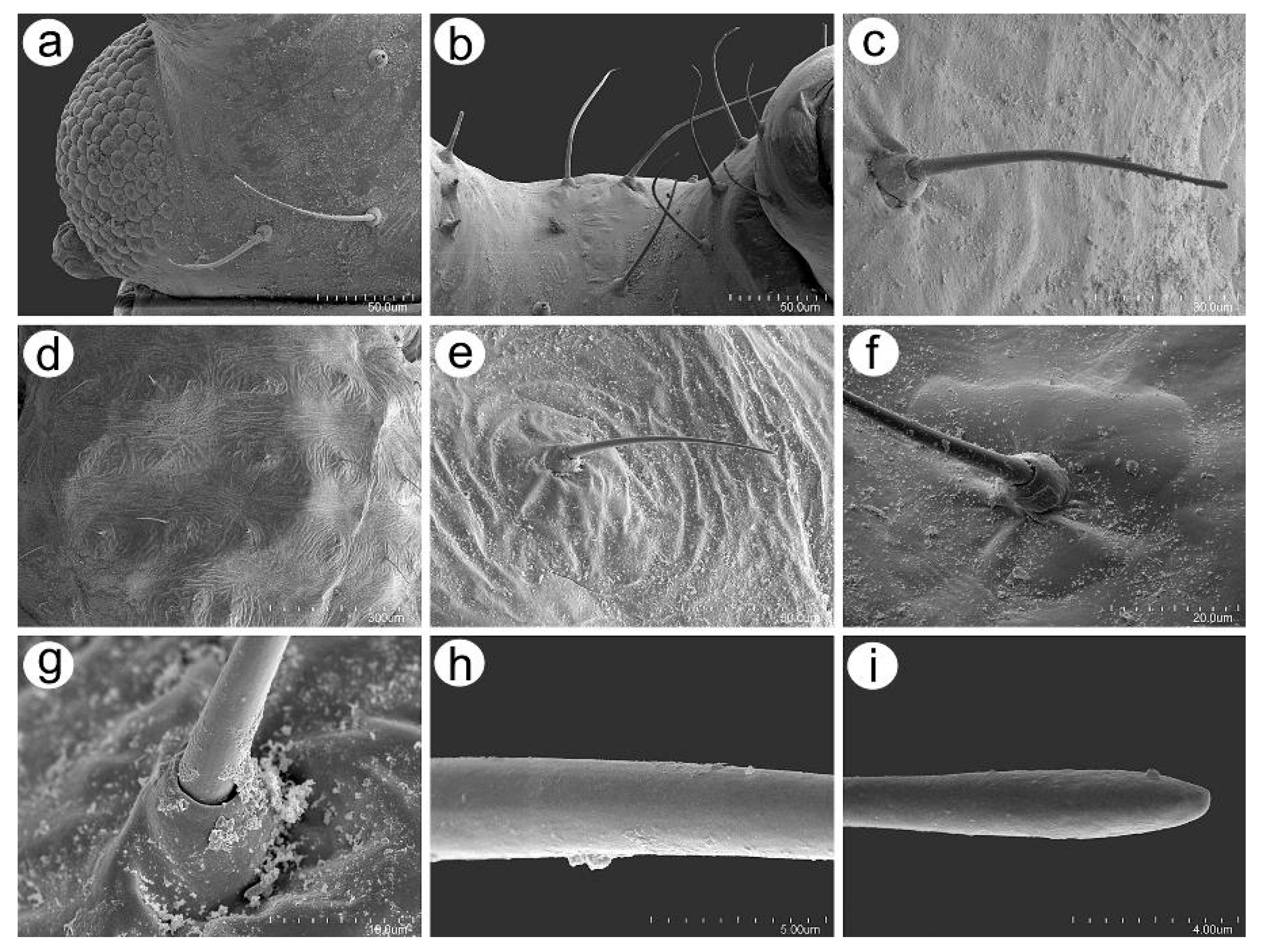
3.2.2. Antennal Sensilla
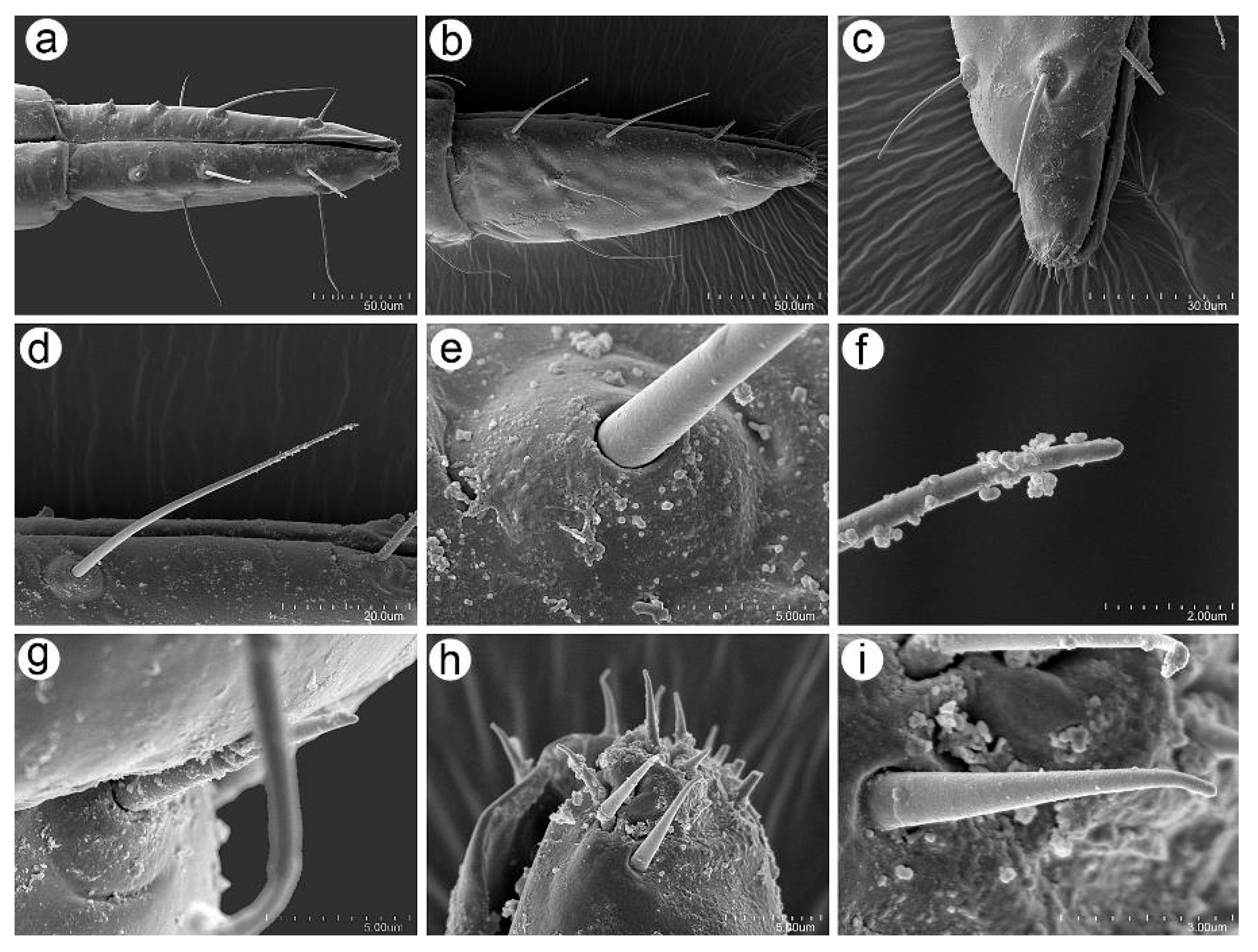
3.2.3. Mouthparts and Body Sensilla
3.2.4. Legs Sensilla and the Stridulatory Apparatus
3.3. Identification Key for Campanulaphis and Uroleucon Aphid Species Living on Campanulaceae Based on Apterous Viviparous Females
- 1.
- Cauda pale or dusky ……………………………………………………………………… 2
- –
- Cauda dark like SIPH …………………………………………………………………….. 5
- 2.
- URS 0.70–1.07 × HT II ……………………………………………………………………... 3
- –
- URS 1.10–1.30 × HT II …………………………………………………………………….. 4
- 3.
- Cauda with 13–18 setae. Hind tibia with a ventral row of short thick peg-like setae. On Asyneuma persicum and Michauxia laevigata. In Iran …………………………………………………………… Uroleucon remaudierei sp. nov.
- –
- Cauda with 9–12 setae. Hind tibia without a ventral row of short thick peg-like setae. On species of Adenophora, Campanula, and Platycodon. In Japan, Korea, and Russia (East Siberia) ………………………………………... Uroleucon kikioense (Shinji, 1942)*
- 4.
- First tarsal segments with five setae. Tibiae wholly dark. ANT III with 50–66 secondary rhinaria. Antesiphuncular sclerites present. On undetermined Campanula. In Russia (East Siberia) ………………………………... Uroleucon gredinae Pashtshenko, 2000
- –
- First tarsal segments with 3 (–4) setae. Tibiae pale on basal 0.7. ANT III with 10–30 secondary rhinaria. Antesiphuncular sclerites absent. On species of Campanula. In Tajikistan, Afghanistan, Pakistan, and northern India …………………………………………………… Uroleucon kashmiricum (Verma, 1966)
- 5.
- Large Mtu tubercles present on prothorax and ABD TERG II–IV. On Asyneuma canescens. In Slovakia and Ukraine ……………….. Uroleucon phyteuma (Bozhko, 1950)
- –
- Ordinary Mtu present on prothorax and ABD TERG II–IV …………………………... 6
- 6.
- SIPH 0.85–1.25 × cauda. URS 0.8–1.2 × HT II …………………………………………… 7
- –
- SIPH 1.5–2.6 × cauda. URS 1.0–2.0 × HT II ……………………………………………… 8
- 7.
- ANT III with 30–60 secondary rhinaria. URS with 8–10 accessory setae. On species of Adenophora. In Japan, Mongolia, and Russia (Transbaikalia) …………………………………………………… Uroleucon triphyllae (Miyazaki, 1966)
- –
- ANT III with 11–35 secondary rhinaria. URS with 4–5 accessory setae. On species of Campanula and Jasione. In Europe, western Siberia, and southwest and central Asia ……………………………………………… Uroleucon campanulae (Kaltenbach, 1843)
- 8.
- ANT III 2.8–4.4 × URS …………………………………………………………………….. 9
- –
- ANT III 4.4–6.6 × URS ……………………………………………………………………. 11
- 9.
- Body spindle shaped. URS 1.7–2.0 × HT II. SIPH 5.4–6.4 × HT II. on Campanula peregrina. In Lebanon ……………………………………. Uroleucon sp. (BMNH collection)
- –
- Body oval. URS 1.0–1.45 × HT II. SIPH 2.9–4.2 × HT II ……………………………….. 10
- 10.
- ANT III with 2–18 secondary rhinaria on basal half, and none on ANT IV. On species of Campanula. In Italy, Poland, and the former Yugoslavia …………………………………….. Uroleucon minosmartelli Barbagallo and Patti 1994
- –
- ANT III with 28–45 secondary rhinaria distributed over its entire length, and 0–6 on ANT IV. On species of Campanula. In Kazakhstan …………………………………………... Campanulaphis radicivora Kadyrbekov, 2016
- 11.
- Longest setae on outer side of hind tibia 1.5–2.0 × diameter of tibia at midlength. Longest setae on ANT III 1.2–1.5 × BD III. On species of Campanula and Platycodon grandiflorus. In Japan, Kazakhstan, and Russia (East Siberia) ……………………………………………. Uroleucon neocampanulae (Takahashi, 1962)
- –
- Longest setae on outer side of hind tibia 0.9–1.2 × diameter at midlength. Longest setae on ANT III 0.8–1.3 × BD III ……………………………………………………….. 12
- 12.
- ANT PT 5.0–6.2 × ANT VI BASE. ANT III 5.7–7.1 × ANT VI BASE ………………… 13
- –
- ANT PT 6.2–8.3 × ANT VI BASE. ANT III 7.0–8.0 × ANT VI BASE ………………… 14
- 13.
- Cauda with 11–14 setae. Longest setae on ABD TERG III–V are at least 2 × BD III. On unidentified Campanula. In France ……………………………………………………………………………………… Uroleucon ariegense Nieto Nafría and Pérez Hidalgo, 2013
- –
- Cauda with 14–19 setae. Longest setae on ABD TERG III–V 1 or slightly longer × BD III. On species of Adenophora. and Astrocodon kruhseanus. In Japan, Mongolia, and Russia (Transbaikalia) ……………………… Uroleucon adenophorae (Matsumura, 1918)
- 14.
- ANT III with 19–58 secondary rhinaria at a density of 20–58 per mm, extending over 0.42–0.94 of the segment. (Al. with 43–78 secondary rhinaria on ANT III and none on ANT IV.) On species of Campanula. In Europe and southwest and central Asia ………………………………………………….. Uroleucon rapunculoidis (Börner, 1939)
- –
- ANT III with 52–122 secondary rhinaria at a density of 45–84 per mm, extending over 0.77–0.97 of the segment. (Al. with 97–137 secondary rhinaria on ANT III, and usually without but sometimes with 1–7 on ANT IV.) On species of Campanula. Widely distributed in Eurasia …………………… Uroleucon nigrocampanulae (Theobald, 1928)
3.4. Key to Apterous Viviparous Females of the Known Sound-Producing Species of the Genus Uroleucon
- 1.
- Cauda pale or yellow …………………………………………………………………….. 2
- –
- Cauda dark ………………………………………………………………………………... 7
- 2.
- Abdomen with large marginal tubercles ……………….. U. phyteuma (Bozhko, 1950)
- –
- Abdomen without large marginal tubercles …………………………………………… 3
- 3.
- Abdomen without dark, well-visible scleroites at setal bases ………………………….…………………………………………………………… U. monticola (Takahashi, 1935)
- –
- Abdomen with dark, well-visible scleroites at setal bases …………………………… 4
- 4.
- Abdomen with light brown scleroites at setal bases and poorly developed and poorly visible postsiphuncular sclerites ………………………………………………... 5
- –
- Abdomen with dark brown scleroites at setal bases and dark, well-developed postsiphuncular sclerites ………………………………………………………………… 6
- 5.
- SIPH no longer than 1.2 × cauda, secondary rhinaria on ANT III distributed on about ¾ of the length of the segment ……………………... U. fuchuense (Shinji, 1942)
- –
- SIPH longer than 1.2 × cauda, secondary rhinaria on ANT III distributed only to ½ of the length of the segment ……………….. U. capsicum Rezwani and Lampel, 1990
- 6.
- Abdomen with few scleroites at setal bases, cauda with 13–18 setae ………………….…………………………………………………………………….. U. remaudierei sp. nov.
- –
- Abdomen with many scleroites at setal bases, cauda with 30–45 setae ………………..………………………………………………………………... U. cirsicola (Holman, 1962)
- 7.
- SIPH not more than 1.5 × cauda ………………………………………………………… 8
- –
- Siph more than 1.5 × cauda ……………………………………………………………… 9
- 8.
- Abdomen with antesiphuncular sclerites absent ………………………………………………………………………………………………. U. adenophorae (Matsumura, 1918)
- –
- Abdomen with antesiphuncular sclerites present ……………………………………………………………………………………………….. U. campanulae (Kaltenbach, 1843)
- 9.
- Hind tibiae uniformly dark ………………………………… U. jaceae (Linnaeus, 1758)
- –
- Hind tibiae at least with some lighter part …………………………………………… 10
- 10.
- Abdominal scleroites in spinal and pleural area all of the same size ……………………………………………………………………… U. carthami (Hille Ris Lambers, 1948)
- –
- Abdominal scleroites in the spinal area are larger than those in the pleural area ….. 11
- 11.
- Abdominal spinal setae as long as or shorter than the scleroites width, scleroites on ABD VII are fused into larger sclerites ………………………. U. minor (Börner, 1940)
- –
- Abdominal spinal setae longer than the scleroites width, scleroites on ABD VII not fused into larger sclerites ……………………………………………………………….. 12
- 12.
- Abdomen with many pleural scleroites, mesothoracic furca sesille …………………………………………………………………………………. U. riparium (Stroyan, 1955)
- –
- Abdomen with few pleural scleroites, mesothoracic furca with elongate stem ………………………………………………………… U. siculum Barbagallo and Stroyan, 1982
4. Comments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favret, C. Aphid Species File. Version 5.0/5.0. 2024. Available online: http://Aphid.SpeciesFile.org (accessed on 7 June 2024).
- Blackman, R.L. Aphids—Aphidinae (Macrosiphini). Handb. Identif. Br. Insects 2010, 2, 1–413. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids of the World’s Plants: An Online Identification and Information Guide. 2024. Available online: http://www.aphidsonworldsplants.info (accessed on 9 October 2024).
- Momeni Shahraki, F.M.; Minaei, K.; Barjadze, S. Checklist of Iranian aphids (Hemiptera: Stenorrhyncha: Aphidomorpha). J. Insect Biodivers. Syst. 2019, 5, 269–300. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Rakhshani, E.; Rokni, M.; Kanturski, M. A new species of the aphid genus Uroleucon Mordvilko, 1914 (Hemiptera: Sternorrhyncha: Aphididae) on Launaea acanthodes from Iran. Zootaxa 2022, 5183, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Mehrparvar, M. Aphids of Iran: Their host plants and distribution. Zootaxa 2024, 5516, 1–129. [Google Scholar] [CrossRef]
- Mehrparvar, M.; Kadyrbekov, R. Aphids associated with Lactuca (Asteraceae) in Iran with descriptions of two new species (Hemiptera: Aphididae). Zool. Anz. 2024, 313, 73–84. [Google Scholar] [CrossRef]
- Williams, C.B. Co-ordinated rhythm in insects; with a record of sound production in an aphid. Entomologist 1922, 55, 173–176. [Google Scholar]
- Eastop, V.F. A sound producing mechanism in the aphididae and the generic position of the species possesing it. Entomologist 1952, 85, 57–61. [Google Scholar]
- Eastop, V.F. A taxonomic study of Australian Aphidoidea (Homoptera). Aust. J. Zool. 1956, 14, 399–592. [Google Scholar] [CrossRef]
- Holman, J. Possible sound producing structures present in some Macrosiphini (Homoptera: Aphididae). Eur. J. Entomol. 1994, 91, 97–101. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs; John Wiley & Sons: London, UK, 2006; 1439p. [Google Scholar]
- Ilharco, F.A.; van Harten, A. Systematics. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; pp. 51–77. [Google Scholar]
- WFO: World Flora Online. Published on the Internet. 2023. Available online: http://www.worldfloraonline.org (accessed on 23 October 2023).
- Matsumura, S. New Aphidinae of Japan. Trans. Sapporo Nat. Hist. Soc. 2018, 7, 1–20. [Google Scholar] [CrossRef]
- Holman, J. Host Plant Catalog of Aphids. Palaearctic Region; Springer: Branisovska, Czech Republic, 2009; 1216p. [Google Scholar] [CrossRef]
- Holman, J. Aphids of the genus Uroleucon from Mongolia (Homoptera, Aphididae). Acta Entomol. Bohemoslov. 1975, 72, 171–183. [Google Scholar]
- Kaltenbach, J.H. Monographie der Familien der Pflanzenläuse (Phytophthires); Roschütz: Aachen, Germany, 1843; 222p. [Google Scholar]
- Hille Ris Lambers, D. On Palestine aphids, with descriptions of new subgenera and new species (Homoptera, Aphididae). Trans. R. Entomol. 1948, 99, 269–289. [Google Scholar] [CrossRef]
- Laamari, M.; Coeur d’Acier, A.; Jousselin, E. New data on aphid fauna (Hemiptera, Aphididae) in Algeria. ZooKeys 2013, 319, 223–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naumann-Etienne, K.; Remaudière, G. A commented preliminary checklist of the aphids (Homoptera: Aphididae) of Pakistan and their host plants. Parasitica 1995, 51, 1–61. [Google Scholar]
- Rezwani, A.; Lampel, G. Three new aphids from Iran belonging to the genera Uroleucon Mordv. and Aphis L. (Homoptera: Aphididae). Mitt. Schweiz. Entomol. Ges. 1990, 63, 241–253. [Google Scholar]
- Holman, J. Notes on Uroleucon species (Homoptera, Aphididae) from the Caucasus and Central Asia. Acta Entomol. Bohemoslov. 1991, 88, 299–312. [Google Scholar]
- Holman, J. Three new aphid species of the subfam. Dactynotinae from the Crimea (Homoptera). Čas. Českoslov. Spol. 1962, 59, 28–37. [Google Scholar]
- Barjadze, S.; Kanturski, M. Some new records of aphid species from Georgia and Mongolia and new aphid-plant interactions (Hemiptera, Aphididae). Spixiana 2022, 45, 73–76. [Google Scholar]
- Shinji, O. New aphids from Morioka. Dobutsugaku Zasshi 1924, 36, 342–372. (In Japanese) [Google Scholar]
- Kanturski, M.; Lee, Y. Hitherto unknown and poorly known sexual morphs of three Asiatic species of the aphid genus Uroleucon (Hemiptera: Aphididae). Bonn Zool. Bull. 2020, 69, 293–307. [Google Scholar]
- Pashtshenko, N.F. Aphids of the genus Uroleucon Mordvilko, 1914 (Homoptera, Aphididae) of the Russian Far East. II. Species of the subgenera Uroleucon s. str. and Lambersius Olive. Entomol. Obozr. 2001, 80, 73–80. [Google Scholar]
- Linnaeus, C. Systema Naturae per Regna tri Naturae, Secundum Classes, Ordines, Genera, Species, cum Characteribus, Differentiis, Synonymis, Locis; Typis Ioannis Thomae: Vienna, Austria, 1758; 824p. [Google Scholar]
- Barjadze, S.; Gabrielyan, I.; Kalashian, M.; Karagyan, G.; Stepanyan, I. Some new records of aphid species (Hemiptera Aphididae) from Armenia. Redia 2024, 107, 39–42. [Google Scholar] [CrossRef]
- Börner, C. Neue Blattläuse aus Mitteleuropa; Privately Published: Namburg (Saale), Germany, 1940; 4p. [Google Scholar]
- Kadyrbekov, R.K. Aphids (Hemiptera: Aphidoidea, Phylloxeroidea) of Kazakhstan. Almaty 2017, 108, 378–583. [Google Scholar]
- Takahashi, R. Additions to the aphid fauna of Formosa (Hemiptera), III. Philipp J. Sci. 1935, 56, 499–507. [Google Scholar]
- Miyazaki, M. A revision of the tribe Macrosiphini of Japan (Homoptera: Aphididae, Aphidinae). Insecta Mats. 1971, 34, 1–247. [Google Scholar]
- Choi, H.; Kim, H.; Lee, W.; Lee, S. The genus Uroleucon (Hemiptera: Aphididae) in the Korean Peninsula, with descriptions of two new species. J. Asia Pac. Entomol. 2019, 22, 481–486. [Google Scholar] [CrossRef]
- Bozhko, M.P. The aphid fauna of the Provalskaya Steppe. Proc. Sci. Res. Inst. Biol. Kharkiv State Univ. A.M. Gorky 1950, 14–15, 125–134. [Google Scholar]
- Holman, J. Dactynotus (Uromelan) asyneumatis sp. n. (Homoptera: Aphididae). Acta Entomol. Bohemoslov. 1969, 66, 97–99. [Google Scholar]
- Stroyan, H.L.G. Recent additions to the British aphid fauna. Part II. Trans. R. Entomol. 1955, 106, 283–340. [Google Scholar] [CrossRef]
- Heie, O.E. The Aphidoidea of Fennoscandia and Denmark VI. Aphidinae. Part 3 of Macrosiphini and Lachnidae. Fauna Entomol. Scand. 1995, 31, 1–222. [Google Scholar]
- Barbagallo, S.; Stroyan, H.L.G. Biological, ecological and taxonomic notes on the aphid fauna of Sicily. Frustula Entomol. Nuova Ser. 1982, 3, 1–182. [Google Scholar]
- Barbagallo, S.; Patti, I. Two new aphid species from Campanulaceae in Italy. Boll. Zool. Agraria Bach. 1994, 26, 165–181. [Google Scholar]
- Görür, G.; Akyıldırım, H.; Olcabey, G.; Akyürek, B. The aphid fauna of Turkey: An updated checklist. Arch. Biol. Sci. 2012, 64, 675–692. [Google Scholar] [CrossRef]
- Broughton, W.B.; Harris, K.M. First recording of the sound produced by the black citrus aphid, Toxoptera aurantii (Boy). Bull. Entomol. Res. 1971, 60, 559–563. [Google Scholar] [CrossRef]


| Character | Apterous Viviparous Female | Alate Viviparous Female |
|---|---|---|
| BL | 2.22–3.32 | 2.80–3.12 |
| HW | 0.45–0.56 | 0.48–0.53 |
| ANT | 2.34–3.06 | 2.75–3.23 |
| ANT III | 0.61–0.87 | 0.80–0.95 |
| ANT IV | 0.42–0.61 | 0.50–0.66 |
| ANT V | 0.35–0.45 | 0.38–0.51 |
| ANT VI | 0.74–0.99 | 0.81–0.85 |
| BASE | 0.15–0.16 | 0.16–0.17 |
| PT | 0.58–0.83 | 0.65–0.68 |
| URS | 0.15–0.18 | 0.17–0.18 |
| FEMORA III | 0.80–1.15 | 1.00–1.20 |
| TIBIAE III | 1.50–2.2. | 2.05–2.35 |
| HT II | 0.15–0.18 | 0.18–0.20 |
| SIPH | 0.65–0.92 | 0.85–1.07 |
| CAUDA | 0.36–0.50 | 0.35–0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanturski, M.; Barjadze, S.; Glumac, A.; Kaszyca-Taszakowska, N. Stridulating Species of Aphids of the Genus Uroleucon (Hemiptera: Aphididae) with Descriptions of a New Species from Iran. Insects 2025, 16, 68. https://doi.org/10.3390/insects16010068
Kanturski M, Barjadze S, Glumac A, Kaszyca-Taszakowska N. Stridulating Species of Aphids of the Genus Uroleucon (Hemiptera: Aphididae) with Descriptions of a New Species from Iran. Insects. 2025; 16(1):68. https://doi.org/10.3390/insects16010068
Chicago/Turabian StyleKanturski, Mariusz, Shalva Barjadze, Andżela Glumac, and Natalia Kaszyca-Taszakowska. 2025. "Stridulating Species of Aphids of the Genus Uroleucon (Hemiptera: Aphididae) with Descriptions of a New Species from Iran" Insects 16, no. 1: 68. https://doi.org/10.3390/insects16010068
APA StyleKanturski, M., Barjadze, S., Glumac, A., & Kaszyca-Taszakowska, N. (2025). Stridulating Species of Aphids of the Genus Uroleucon (Hemiptera: Aphididae) with Descriptions of a New Species from Iran. Insects, 16(1), 68. https://doi.org/10.3390/insects16010068







